Abstract
Tandem pleckstrin homology domain proteins (TAPPs) are recruited to the plasma membrane via binding to phosphoinositides produced by phosphoinositide 3-kinases (PI3Ks). Whereas PI3Ks are critical for B-cell activation, the functions of TAPP proteins in B cells are unknown. We have identified 40 potential interaction partners of TAPP2 in B cells, including proteins involved in cytoskeletal rearrangement, signal transduction and endocytic trafficking. The association of TAPP2 with the cytoskeletal proteins utrophin and syntrophin was confirmed by Western blotting. We found that TAPP2, syntrophin, and utrophin are coexpressed in normal human B cells and B-chronic lymphocytic leukemia (B-CLL) cells. TAPP2 and syntrophin expression in B-CLL was variable from patient to patient, with significantly higher expression in the more aggressive disease subset identified by zeta-chain–associated protein kinase of 70 kDa (ZAP70) expression and unmutated immunoglobulin heavy chain (IgH) genes. We examined whether TAPP can regulate cell adhesion, a known function of utrophin/syntrophin in other cell types. Expression of membrane-targeted TAPP2 enhanced B-cell adhesion to fibronectin and laminin, whereas PH domain–mutant TAPP2 inhibited adhesion. siRNA knockdown of TAPP2 or utrophin, or treatment with PI3K inhibitors, significantly inhibited adhesion. These findings identify TAPP2 as a novel link between PI3K signaling and the cytoskeleton with potential relevance for leukemia progression.
Introduction
The importance of phosphoinositide 3-kinase (PI3K) enzymes in leukocyte signal transduction and transformation is well established.1,2 PI3Ks phosphorylate membrane phosphatidylinositol lipids on the D3 position of the inositol ring to generate the 3-phosphoinositides PI(3)P, PI(3,4)P2, or PI(3,4,5)P3. These PI3K lipid products are increased upon cell stimulation and oncogenic transformation,3 and activating mutations of the alpha isoform of PI3K have recently been found in human cancers.4 In B lymphocytes, PI3K is rapidly activated upon ligation of the antigen receptor (B-cell antigen receptor [BCR])5,6 and these enzymes are critical for BCR signaling and B-cell survival.7,8 Signaling via specific antigen-reactive BCRs is thought to be an important mechanism driving survival and expansion of leukemic B cells.9 B cell–derived leukemias and lymphomas have high constitutive activity of PI3Ks, and activity of this pathway may be important for aberrant survival and malignant properties of these cells.10,11 Acute cross-linking of BCR on circulating chronic lymphocytic leukemia (CLL) cells induces activation of PI3K and its target Akt, and sustained activation of this pathway promotes CLL cell survival.12
Recruitment of pleckstrin homology (PH) domain–containing signal transduction proteins to the plasma membrane through binding to 3-phosphoinositide second messengers represents the major mechanism for transmission of signals initiated by PI3K enzymes.13-18 Perhaps the best-described PH domain–containing target of PI3K is the serine-threonine kinase Akt/protein kinase B, which is known to phosphorylate key substrates in cascades promoting cell survival, metabolic activity, and translation of cap-dependent mRNAs.19,20 However evidence in animal models indicates that active Akt alone is insufficient for cancer development,21 suggesting that other PH domain–containing targets of 3-phosphoinositides likely contribute important functions of this signaling pathway. We and others have previously described the PH domain adaptor proteins tandem pleckstrin homology domain protein 1 (TAPP1) and TAPP2,22 which bind specifically to PI(3,4)P2 in vitro.23 In B cells, TAPP1 and TAPP2 proteins are recruited to the plasma membrane in vivo in a manner paralleling PI(3,4)P2 production,18,24 indicating that these proteins are specific effectors of PI(3,4)P2 signaling.
TAPP1 and TAPP2 have distinctive expression patterns, with TAPP2 being more abundantly expressed in human lymphoid tissues and lymphocyte cell lines. The sequence intervening between the C-terminal PH domain and the postsynaptic density-95/disks large/zonula occludens-1 (PDZ)–binding motif of these proteins is largely distinct and may specify unique functions of TAPP1 and TAPP2. TAPP1 was shown to bind to PDZ domain proteins multi-PDZ domain protein 1 and protein tyrosine phosphatase L1 in HEK293 cells.24,25 TAPP1 was also found to associate with the PDZ domains of multiple syntrophin isoforms in yeast 2-hybrid and in vitro pull-down assays, and colocalized with γ1-syntrophin in membrane ruffles of platelet-derived growth factor–stimulated NIH-3T3 cells.26 Syntrophins are scaffold proteins that interact with the dystrophin family of cytoskeletal proteins, known to function in cell polarity and adhesion.27
Here we used a mass spectrometry approach to identify TAPP2-associating proteins in lymphocytes, and have identified multiple potential interaction partners including β2-syntrophin and the syntrophin-binding protein utrophin. We provide evidence that TAPP2 is coexpressed with syntrophin and utrophin in B-CLL cells and promotes B-cell adhesion to extracellular matrix. The ability of TAPP2 to bind PI(3,4)P2 via its PH domain is critical for this function. Our data provide new insight into the function of TAPP adaptors, suggesting that they can participate in an adhesion-promoting complex containing utrophin and syntrophin.
Methods
Reagents and constructs
Anti-TAPP2 antibody was generated by immunizing rabbits with a synthetic peptide corresponding to the unique TAPP2 protein sequence CKAPSVASSWQPWTPVPQ, followed by affinity purification of peptide-binding antibodies (Affinity Bioreagents). Anti-myc tag antibody 9E10 was from Upstate. Mouse antisyntrophin (clone 1351) was from Affinity Bioreagents. Rabbit antiutrophin (H-300, used for immunoprecipitation and immunofluorescence staining) and mouse antiutrophin (8A4, used for Western blotting) were from Santa Cruz Biotechnology. Mouse anti–zeta-chain–associated protein kinase of 70 kDa (ZAP70; clone 2F3.2) was from Chemicon Inc. F(ab)2 goat anti–human IgM antibodies used for cell stimulation and all horseradish peroxidase (HRP)–labeled secondary antibodies were from Jackson ImmunoResearch Laboratories. Stromal-derived factor 1 (SDF1)/CXC ligand 12 chemokine was purchased from PeproTech. Alexa 488 anti–rabbit and Alexa 568 anti–mouse secondary antibodies were from Molecular Probes. N-hydroxysuccinimidobiotin and phorbol myristate acetate (PMA) were from Sigma-Aldrich. Plasmid constructs encoding TAPP2 were generated by cloning the cDNA into pcDNA3.0 expression vector, and R218L PH domain mutants were generated using the QuikChange method (Stratagene). Membrane-targeted TAPP2 was generated by addition of an N-terminal myristoylation signal sequence derived from the Src kinase Lyn (amino acids 1-16). C-terminal myc-tagged TAPP2 was generated by cloning the TAPP2 cDNA into pcDNA3.1 myc/his vector (Invitrogen). All constructs were verified by sequencing before use.
Cell cultures and transfections
BJAB (Burkitt lymphoma), JVM-3 (chronic lymphocytic leukemia; purchased from DSMZ), and Nalm-6 (pre-B acute lymphocytic leukemia) were cultured in RPMI 1640 medium (Invitrogen) containing 10% fetal calf serum and penicillin-streptomycin. Transfections were carried out with an ElectroSquarePorator ECM 830 (BTX) set at 310 V using a single 10-ms pulse. Cells were resuspended at 2.5 × 107 cells/mL in cold RPMI medium and 0.4 mL cells was mixed with 20 μg of linearized plasmid vectors in a GenePulser Cuvette (Bio-Rad) with 0.4-cm electrode gap. The cuvettes were then placed on ice for 10 minutes before transferring to 20 mL of medium and culturing overnight. G418 (2 mg/mL) was then added and cells were cultured for an additional 10 to 14 days before cloning by limiting dilution to generate stable transfectant clones.
Immunoprecipitation and TAPP2 elution
Cells were washed once and resuspended at 2 × 106 per milliliter in prewarmed serum-free RPMI medium. Cells were incubated for 1 hour in a 37°C water bath and in some cases then stimulated with 10 μg/mL F(ab)2 anti-IgM for 10 minutes. For small-scale immunoprecipitation (IP) 2 × 107 cells were pelletted and lysed in 0.5 mL of cold Nonidet P-40 (NP-40) lysis buffer containing 1% NP-40, 50mM of tris(hydroxymethyl)aminomethane, 150mM NaCl, and 5mM ethylenediaminetetraacetic acid and complete protease inhibitor cocktail (Roche). For large-scale preparative IPs, 2 × 108 cells were pelleted, resuspended in 0.75 mL of lysis buffer lacking NP-40 to disperse the cells, then a further 0.75 mL of 2% NP-40 lysis buffer was added and mixed thoroughly. Samples were incubated for 30 minutes at 4 degrees with continuous rocking. Nuclei and insoluble material were removed by centrifuging for 10 minutes at 14 000g. The supernatants were precleared with protein G sepharose beads (Amersham Pharmacia) for 1 hour and then centrifuged. Anti-TAPP2, rabbit IgG control, anti-myc, or mouse IgG1 control antibodies were added into the supernatants (2 μg for small scale, 10 μg for large scale) and incubated for 2 hours on ice. Prewashed protein G beads (20 μL for small scale, 50 μL for large scale) were added and samples were rocked for 30 minutes at 4°C. The immunoprecipitates were washed twice with 1.2 mL of NP-40 lysis buffer and once with phosphate-buffered saline (PBS; both containing protease inhibitors). To elute TAPP2 complex from specific antibody, immunoprecipitates were incubated at room temperature for 1 hour with the indicated concentration of epitope peptide dissolved in PBS. Laemmli sample buffer (Sigma-Aldrich) was added and samples were boiled for 5 minutes before loading onto sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels.
Antibody biotinylation and Western blotting
N-hydroxysuccinimidobiotin–biotin (Pierce) was dissolved in dimethyl sulfoxide (DMSO) and conjugated with rabbit anti-TAPP2 antibody according to the manufacturer's protocol. Biotinylated antibody was dialyzed against PBS overnight with several changes of buffer. For Western blotting, samples were separated with 10% SDS-PAGE gels followed by semidry protein transfer to polyvinylidene fluoride membranes (Bio-Rad). Membranes were washed with TBST 3 times and then incubated in TBST with 5% skim milk overnight. The next day the membrane was blotted with 5 μg/mL rabbit anti-TAPP2 antibody (Ab) or 1 μg/mL mouse anti-myc Ab for 2 hours, followed by incubation with 0.1 μg/mL HRP-conjugated anti–mouse or anti–rabbit antibody (Jackson ImmunoResearch Laboratories) for 1 hour. The biotinylated anti-TAPP2 antibody was visualized by incubating with 0.1 μg/mL HRP-conjugated streptavidin. Blots were then incubated with enhanced chemiluminescence substrate (Amersham Biosciences) and signals were detected with a Fluorchem 8800 chemiluminescence imager (Alpha Innotech).
Gel electrophoresis, in-gel digestion, and protein identification
Immunoprecipitated samples were boiled in Laemmli buffer for 5 minutes before loading onto 7% SDS-PAGE gels. Gels were run at 4°C for 3 to 4 hours at 250 V with constant stirring, then washed in deionized water and stained with GelCode Blue or Silver Snap stain (Pierce). Candidate protein bands were excised from the gel and washed 3 times at room temperature as follows. NH4HCO3 (100mM) was added into the gel piece for 10 minutes, followed by exchange of buffer with 1:1 solution of acetonitrile (ACN)/100mM NH4HCO3 for another 10 minutes, and finally with pure ACN for additional 5 minutes. Proteins were reduced by incubation with 10mM of dithiothreitol dissolved in 100mM NH4HCO3 (45 minutes at 57°C). Protein alkylation was performed by incubation with 55mM iodoacetamide dissolved in 100mM NH4HCO3 (45 minutes at room temperature). Gel pieces were then subjected to the ACN/NH4HCO3 wash cycle once and allowed to shrink with ACN. Trypsin (5 ng/μL) dissolved in 50mM NH4HCO3 was added into the gel to digest the peptide overnight at 37°C. After addition of 0.01% trifluoroacetic acid to stop the digestion, peptides were extracted by sonicating in 0.01% trifluoroacetic acid/50% ACN solution for 10 minutes in cold water. ACN was added to shrink the gel for an additional 5 minutes. Sample supernatants collected after the digestion step were concentrated by speed-vacuum before analysis by liquid chromatography tandem mass spectrometry (LC-MS/MS) on an ABI Qstar Pulsar instrument with an LC Packings nano LC system (Applied Biosystems). The resulting spectra were submitted to The Global Proteome Machine (http://human.thegpm.org/tandem/thegpm_tandem.html) for protein identification. Identifications were prioritized by the software confidence score and by their absence in corresponding control precipitation samples.
Immunofluorescence staining and Western blot analyses of primary human B cells and B-CLL
Peripheral blood samples were obtained from CLL patients or healthy age-matched persons after informed consent in accordance with the Declaration of Helsinki, with approval from the Research Ethics Board at the University of Manitoba. Mononuclear cells were isolated from the buffy coat of CLL patients or healthy donors using a Ficoll-Paque density gradient. For patients with lymphocyte counts of less than 40 × 109cells/L and for control blood donors, B cells were isolated using the RosetteSep B cell enrichment Cocktail (StemCell Technologies) to remove contaminating T cells and monocytes. B-cell purity was determined by staining with fluorescein isothiocyanate–labeled anti-CD20, and was routinely greater than 95% for CLL patients and greater than 85% for controls. NP-40 protein extracts were generated as described under “Immunoprecipitation and TAPP2 elution” and protein concentrations were determined using a Bradford assay kit according to the manufacturers protocol (Bio-Rad). Protein (5 μg/sample) was loaded for TAPP2, ZAP70, or syntrophin detection, whereas 1 μg was loaded for actin detection.
For immunofluorescence staining, isolated cells were fixed with 2% paraformaldehyde for 1 hour on ice, washed twice with PBS, and resuspended at 106/mL, and 50 μL of cells was centrifuged onto glass slides using a Cytospin 3 (ThermoShandon). Before staining, cells were permeabilized and blocked by 15-minute incubation with PBS containing 5% goat serum and 0.l% TritonX. After a brief PBS rinse, primary antibodies were added at 5 to 10 μg/mL in a 100-μL volume of PBS plus 5% goat serum and slides were incubated for 2 hours in a humidified chamber at room temperature. Slides were washed several times with PBS containing 1% goat serum and 0.01% TritonX, and then further rinsed with PBS. Secondary antibodies were added at 2 μg/mL, incubated for 1 hour, and washed several times with PBS. After air-drying, slides were mounted with ProLong Gold antifade mounting medium containing DAPI (4,6 diamidino-2-phenylindole; Molecular Probes). Microscopy was performed using an Olympus FV500 confocal microscope, with a 60×/NA 1.4 objective. Image acquisition and analysis were performed using Fluo-View software.
siRNA treatment
Transfection with synthetic siRNA was carried out essentially as described.28 Briefly, BJAB or JVM-3 cells were washed and resuspended at 3 × 106/mL in serum-free, antibiotic-free RPMI medium and plated in 48-well plates. Specific siRNAs (Invitrogen) were mixed with RNAiFECT lipofection reagent (QIAGEN) and added to the cells according to the manufacturer's recommendations. Ten percent serum was added the next day, and the cells were incubated a further 24 hours before assay. Specific siRNA sequences used were as follows: control-CUCCUGUCCAAGUAGUAUUCUCUAC; TAPP1-UUGAAAUGUAGGUAAGCUUAAUGGC; TAPP2-CCCUGUCUCAGAGAUAUUUCCUUCA; utrophin-CCAUCAGAACCAGCUAGAAAUAUUU.
Lentiviral transduction
The human TAPP2 gene sequence, GCAAGATCACCGTTCCCAAAG, or a nonsilencing control sequence targeting firefly luciferase gene, GTGCGTTGTTAGTACTAATCCTATTT, was cloned as a shRNA template into the pSIH1-H1-copGFP shRNA expression lentivector (System Biosciences). Lentivirus was packaged through cotransfection of shRNA expression lentivectors, pCMV-dR8.2 dvpr29 (Addgene plasmid 8455) and pCMV-VSV-G29 (Addgene plasmid 8454), into 293T human embryonic kidney cells using lipofectamine 2000 (Invitrogen), essentially following the supplier's transfection protocol. Three days after transfection virus-containing supernatants were harvested and viral titers were measured according to a previously published protocol.30 Lentiviral transduction of Nalm-6 cells was performed following the spin protocol as described previously31 with slight modifications. Briefly, 5 × 104 Nalm-6 cells were suspended in 250 μL of viral supernatant (or dilutions with Iscove modified Dulbecco medium) at multiplicity of infection of 20 in the presence of 8 μg/mL polybrene (Sigma-Aldrich), plated in 24-well plates, and centrifuged at 2000 rpm for 1 hour at room temperature. Viral supernatant was then replaced by Nalm-6 culture media. After 1 week, transduction efficiency, as indicated by the percentage of green fluorescent protein–positive cells, was usually more than 90%.
Adhesion assay
Plates (Costar no. 3369) were coated overnight with fibronectin or laminin (50 μg/mL diluted in carbonate coating buffer), washed once with PBS, and blocked for 30 minutes at room temperature with Hanks balanced salt solution plus 2% fetal bovine serum. Cells were resuspended at 106/mL in prewarmed blocking medium and mixed with the indicated stimuli in the coated plates (100 μL per well). Plates were incubated for 1 hour (BJAB cells) or 15 minutes (JVM-3 or Nalm-6 cells) at 37°C in a humidified CO2 incubator. Plates were gently inverted to remove nonadherent cells and medium. After addition of PBS, plates were covered with sealing tape, inverted, and centrifuged at 100g for 3 minutes. After removal of PBS wash, adherent cells were fixed with 1% glutaraldehyde solution for 10 minutes at room temperature, fixative was removed, and cells were stained for 20 minutes with 0.1% crystal violet (in ddH2O). After complete removal of crystal violet solution, stained cells were dissolved in 1% SDS solution. Crystal violet absorbance was read at 570 nm using an enzyme-linked immunosorbent assay plate reader (Molecular Devices).
Results
Isolation of TAPP2-associated proteins
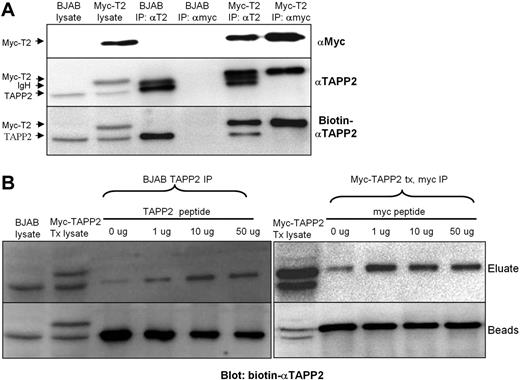
Affinity-purified rabbit anti-TAPP2 antibodies were generated against a conserved peptide sequence within the intervening region between the C-terminal PH domain and the PDZ-binding motif of TAPP2. These affinity-purified antibodies were effective for TAPP2 immunoprecipitation and detection by Western blot (Figure 1A). The anti-TAPP2 Ab also efficiently immunoprecipitated C-terminal myc-tagged TAPP2 expressed in the human B-lymphoma line BJAB. On 10% SDS-PAGE gels, endogenous TAPP2 runs only slightly below the Ig heavy chain of the immunoprecipitating Abs (close to its predicted molecular weight of 47 kDa), necessitating the use of biotin-labeled Ab to eliminate detection of IgH by the secondary antibody (Figure 1A).
Immunoprecipitation and elution of TAPP2. Protein extracts from BJAB B-lymphoma cells or BJAB cells expressing myc-tagged TAPP2 were immunoprecipitated and blotted as indicated. (A) Rabbit anti-TAPP2 can immunoprecipitate (IP) and blot endogenous TAPP2 or myc-tagged TAPP2. IP antibodies are indicated above each lane and blotting antibodies are indicated on the right of each blot. Note the first 2 lanes are whole-cell lysates from the indicated cell line (106 cells per lane), whereas IP lanes represent proteins precipitated from 10 × 106 cells per lane. (B) Elution of TAPP2 using epitope peptide competition. (Left) BJAB extracts were subjected to immunoprecipitation with anti-TAPP2 Ab followed by incubation with various concentration of TAPP2 epitope peptide to elute endogenous TAPP2 from the Ab. (Right) Extracts from BJAB cells expressing myc-tagged TAPP2 were subjected to immunoprecipitation with anti-myc Ab followed by incubation with various concentration of c-myc peptide to elute myc-TAPP2 from the anti-myc Ab. After elution, IP beads were boiled in Laemmli buffer to remove remaining proteins. Eluates and their corresponding bead-bound fractions were blotted with biotinylated anti-TAPP2.
Immunoprecipitation and elution of TAPP2. Protein extracts from BJAB B-lymphoma cells or BJAB cells expressing myc-tagged TAPP2 were immunoprecipitated and blotted as indicated. (A) Rabbit anti-TAPP2 can immunoprecipitate (IP) and blot endogenous TAPP2 or myc-tagged TAPP2. IP antibodies are indicated above each lane and blotting antibodies are indicated on the right of each blot. Note the first 2 lanes are whole-cell lysates from the indicated cell line (106 cells per lane), whereas IP lanes represent proteins precipitated from 10 × 106 cells per lane. (B) Elution of TAPP2 using epitope peptide competition. (Left) BJAB extracts were subjected to immunoprecipitation with anti-TAPP2 Ab followed by incubation with various concentration of TAPP2 epitope peptide to elute endogenous TAPP2 from the Ab. (Right) Extracts from BJAB cells expressing myc-tagged TAPP2 were subjected to immunoprecipitation with anti-myc Ab followed by incubation with various concentration of c-myc peptide to elute myc-TAPP2 from the anti-myc Ab. After elution, IP beads were boiled in Laemmli buffer to remove remaining proteins. Eluates and their corresponding bead-bound fractions were blotted with biotinylated anti-TAPP2.
Because nonspecifically precipitated contaminant proteins are a common feature of immunoprecipitation, we tested whether TAPP2 and its associated proteins could be specifically eluted from the immunoprecipitates by incubation with excess epitope peptides. We found that the TAPP2 peptide used for rabbit immunization was able to release a portion of precipitated TAPP2 (Figure 1B). Similarly, the myc epitope peptide was able to release a portion of myc-TAPP2 precipitated with 9E10 anti-myc antibody (Figure 1B right panel). These results indicated that our rabbit anti-TAPP2 reagent provides an opportunity to isolate untagged TAPP2 and associated proteins with similar efficiency as an epitope tag system, avoiding potential disruption of protein interactions by the tag.
Identification of TAPP2-associated proteins
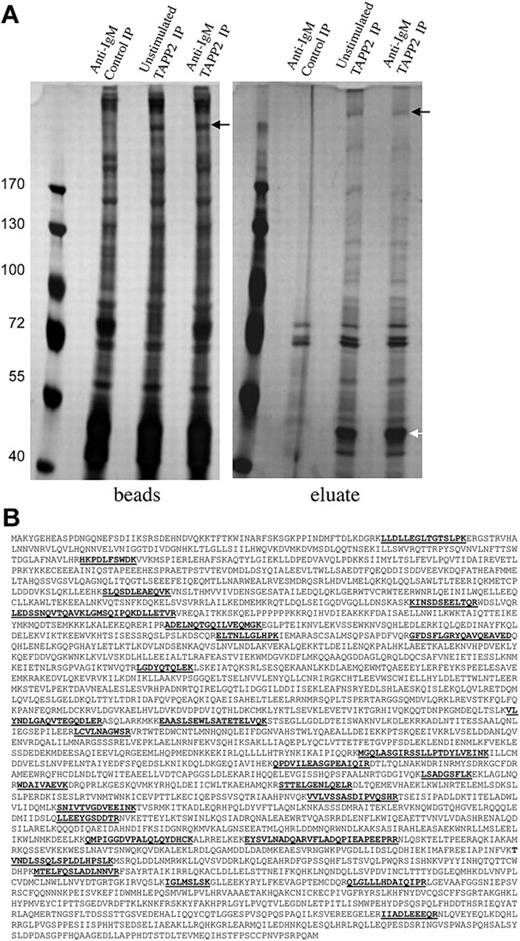
BJAB cells overexpressing untagged TAPP2 were stimulated with anti-IgM antibody to activate PI3K and trigger membrane recruitment of TAPP2,18 and TAPP2 immunoprecipitations were performed. TAPP2 and associated proteins were eluted by incubation with the epitope peptide, and eluted proteins or remaining bead-bound proteins were separated by SDS-PAGE. After gel staining with mass spectrometry–compatible silver stain, several distinct bands were visible in eluates from TAPP2 IP, but not control IP (Figure 2A). Noneluted proteins removed from the precipitation beads by boiling in Laemmli buffer were visually similar in control and TAPP2 IP lanes, with the exception of a more than 200-kDa protein present only in TAPP2 IP lanes (black arrows in Figure 2A). This band was observed repeatedly in independent experiments (in both eluate and bead-bound fractions of TAPP2 IPs) and was thus excised along with the corresponding gel fragment from the control IP lane. Gel samples were processed for in-gel protein digestion, and peptides were analyzed by LC-MS/MS as described in “Gel electrophoresis, in-gel digestion, and protein identification.” The cytoskeletal protein utrophin was identified with high confidence in all TAPP2 IP samples, but not the corresponding control IP samples, based on 27 unique peptide identifications (Figure 2B).
Proteins coimmunoprecipitated and coeluted with TAPP2. (A) Protein extract from 160 × 106 BJAB cells overexpressing untagged TAPP2 were used in scaled-up TAPP2 immunoprecipitations, followed by elution with 10 μg/mL TAPP2 epitope peptide. Where indicated, cells were stimulated for 10 minutes with 10 μg/mL anti-BCR before protein isolation. Bead-bound and eluted fractions were separated on 7% SDS-PAGE gels and stained with SilverSnap mass spectrometry–compatible protein stain. The white arrow indicates a 47-kDa band in TAPP2 eluates (obscured by the heavy chain of the IP antibody in the bead fractions). This band was excised and identified as TAPP2 by mass spectrometry. indicates a frequently observed high-molecular-weight band present only in TAPP2 IP lanes and eluting with TAPP2 epitope peptide. (B) The band indicated by the was excised, destained, processed with trypsin, and identified by mass spectrometry peptide fingerprinting as the cytoskeletal protein utrophin. The figure shows the amino acid sequence of utrophin, indicating the 27 unique tryptic peptides identified, spanning the protein sequence.
Proteins coimmunoprecipitated and coeluted with TAPP2. (A) Protein extract from 160 × 106 BJAB cells overexpressing untagged TAPP2 were used in scaled-up TAPP2 immunoprecipitations, followed by elution with 10 μg/mL TAPP2 epitope peptide. Where indicated, cells were stimulated for 10 minutes with 10 μg/mL anti-BCR before protein isolation. Bead-bound and eluted fractions were separated on 7% SDS-PAGE gels and stained with SilverSnap mass spectrometry–compatible protein stain. The white arrow indicates a 47-kDa band in TAPP2 eluates (obscured by the heavy chain of the IP antibody in the bead fractions). This band was excised and identified as TAPP2 by mass spectrometry. indicates a frequently observed high-molecular-weight band present only in TAPP2 IP lanes and eluting with TAPP2 epitope peptide. (B) The band indicated by the was excised, destained, processed with trypsin, and identified by mass spectrometry peptide fingerprinting as the cytoskeletal protein utrophin. The figure shows the amino acid sequence of utrophin, indicating the 27 unique tryptic peptides identified, spanning the protein sequence.
To perform a global search for additional TAPP2-interacting proteins, a scaled-up TAPP2 IP and peptide elution was performed. After separation on SDS-PAGE, the entire TAPP2 IP and control IP lanes were separated into 28 fractions for separate LC-MS/MS analyses. This approach resulted in more than 200 candidate protein identifications, including TAPP2 itself. By excluding contaminant proteins identified in the control IP lane and proteins identified with low confidence scores, we narrowed the list of potential interaction partners to 40. This list includes utrophin and the known utrophin-interacting protein β2-syntrophin, as well as other proteins involved in signal transduction, cytoskeletal regulation, and endocytosis (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The utrophin/syntrophin complex associates with TAPP2
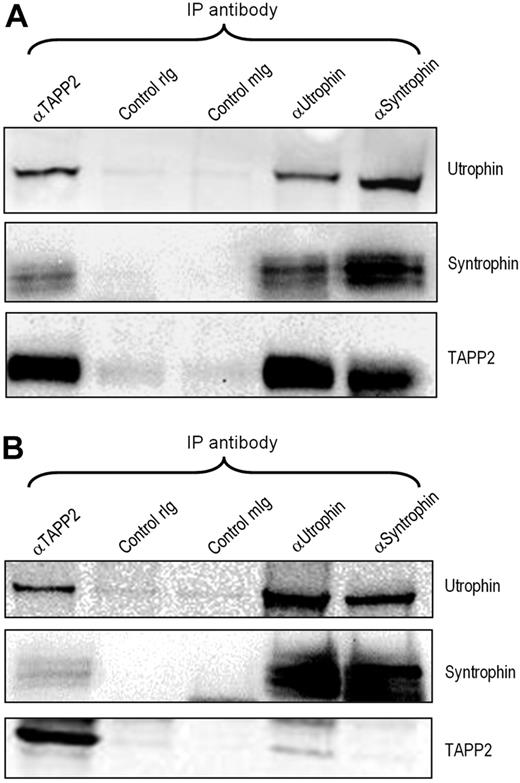
The repeated high-confidence identification of utrophin in TAPP2 immunoprecipitates, together with the identification of β2-syntrophin, prompted us to investigate the potential significance of a TAPP2/utrophin/syntrophin complex in lymphocytes. Utrophin is a broadly expressed homologue of dystrophin, an extensively studied muscle-specific protein known to be mutated in Duchene muscular dystrophy.32 Coimmunoprecipitation and Western blotting confirmed that TAPP2 associates with utrophin and syntrophins in BJAB B-lymphoma cells, and also confirmed that utrophin forms a complex with syntrophins in lymphocytes (Figure 3A). Similar results were obtained in JVM-3 chronic lymphocytic leukemia cells (Figure 3B) as well as Nalm-6, MEC-2, and Ramos B-cell lines (data not shown). These results confirm the mass spectrometry identifications and indicate that TAPP2 can associate with the syntrophin/utrophin complex.
TAPP2 associates with the utrophin/syntrophin complex. NP-40 extracts from BJAB (A) or JVM-3 (B) cells were immunoprecipitated and blotted as indicated, demonstrating that the utrophin-syntrophin complex forms in lymphocytes and associates with TAPP2. rIg indicates rabbit IgG control; mIg, mouse IgG control.
TAPP2 associates with the utrophin/syntrophin complex. NP-40 extracts from BJAB (A) or JVM-3 (B) cells were immunoprecipitated and blotted as indicated, demonstrating that the utrophin-syntrophin complex forms in lymphocytes and associates with TAPP2. rIg indicates rabbit IgG control; mIg, mouse IgG control.
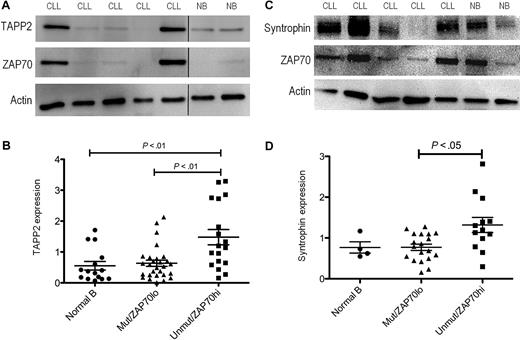
Coexpression of TAPP2, syntrophin, and utrophin in B leukemia cells: association of expression level with ZAP70 expression and IgH mutation status
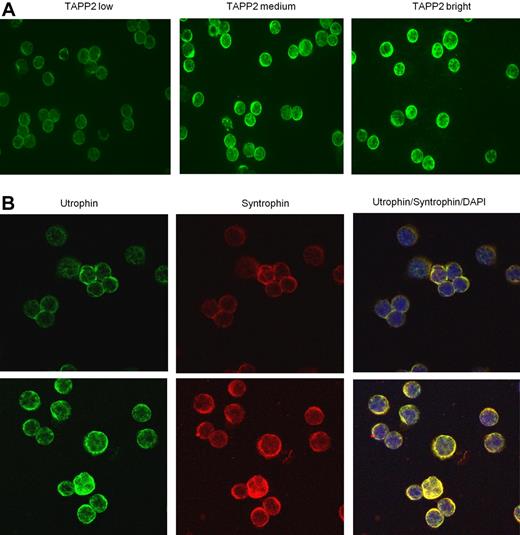
Because expression of the syntrophin/utrophin complex in lymphocytes has not been previously reported, we investigated its expression in normal and leukemic B cells. We found that all human and mouse B-cell lines examined, as well as freshly isolated mouse or human B cells, express TAPP2, utrophin, and syntrophin. Leukemic B cells isolated from B-CLL patients all showed detectable TAPP2 expression, however expression levels varied markedly from patient to patient (Figure 4A). Utrophin and syntrophin were also readily detectable in freshly isolated B-CLL cells, showing prominent colocalization in the cytoplasm (Figure 4B). Quantitative Western blot analysis confirmed variable TAPP2 expression among B-CLL patients, with a subset showing higher expression than B cells from healthy donors (Figure 5A). We also assessed expression of ZAP70 in these samples, which is a known prognostic marker of the more aggressive B-CLL subset with unmutated IgH genes.33 An apparent correlation between high TAPP2 expression and high ZAP70 expression was noted by Western blotting (Figure 5A). We thus further compared the levels of TAPP2 protein in ZAP70+/IgH unmutated versus ZAP70−/IgH mutated CLL subgroups and found that TAPP2 expression was significantly higher in the former subset (Figure 5B). A similar analysis of syntrophin protein levels also indicated variable expression with higher expression associated with the ZAP70+/IgH unmutated group (Figure 5C-D). Detailed CLL patient information and the corresponding TAPP2, syntrophin, and ZAP70 expression levels can be found in supplemental Figure 2. Together these results indicate that the TAPP2/syntrophin/utrophin complex is most highly expressed in the more aggressive subset of B-CLL cells.
TAPP2, utrophin, and syntrophin are coexpressed in freshly isolated B chronic lymphocytic leukemia cells. B-CLL cells were isolated, fixed, and centrifuged onto glass slides. (A) Samples were stained with rabbit anti-TAPP2, followed by Alexa 488 anti–rabbit secondary antibodies. Note the membrane/cytoplasmic staining of TAPP2 is variable from patient to patient (3 representative patients are shown). (B) Coexpression of utrophin and syntrophin in B-CLL. In the righthand panels, yellow color indicates colocalization of utrophin and syntrophin staining, and blue indicates nuclear staining with DAPI. Two independent patient samples are shown in top and bottom panels. No significant signal was seen when primary antibodies were omitted (controls not shown).
TAPP2, utrophin, and syntrophin are coexpressed in freshly isolated B chronic lymphocytic leukemia cells. B-CLL cells were isolated, fixed, and centrifuged onto glass slides. (A) Samples were stained with rabbit anti-TAPP2, followed by Alexa 488 anti–rabbit secondary antibodies. Note the membrane/cytoplasmic staining of TAPP2 is variable from patient to patient (3 representative patients are shown). (B) Coexpression of utrophin and syntrophin in B-CLL. In the righthand panels, yellow color indicates colocalization of utrophin and syntrophin staining, and blue indicates nuclear staining with DAPI. Two independent patient samples are shown in top and bottom panels. No significant signal was seen when primary antibodies were omitted (controls not shown).
Elevated TAPP2 and syntrophin expression in the ZAP70+, IgH unmutated subset of B-CLL patients. Protein extracts of B-CLL (CLL) or normal human B cells (NB) were made using NP-40 lysis buffer, and 5 μg of protein per sample was run on SDS-PAGE gels. (A) TAPP2, ZAP70, or actin expression was detected by Western blot. Blots are representative of > 40 samples analyzed (each sample examined in at least 2 independent experiments). (B) Chemiluminescence signals for TAPP2, ZAP70, and actin blots were quantified using a Fluorchem instrument. Data are normalized to the actin signal for each sample. Graphs represent the normalized expression of 41 CLL and 15 normal B-cell samples, and CLL samples were divided into IgH unmutated/ZAP70+ and IgH mutated/ZAP70− groups. P values were calculated using a Mann-Whitney test comparing the indicated groups. (C) Representative Western blot showing syntrophin expression in CLL and normal B cells. (D) Quantitative analysis of syntrophin expression in normal B cells, and unmutated/ZAP70+ and IgH mutated/ZAP70− CLL groups.
Elevated TAPP2 and syntrophin expression in the ZAP70+, IgH unmutated subset of B-CLL patients. Protein extracts of B-CLL (CLL) or normal human B cells (NB) were made using NP-40 lysis buffer, and 5 μg of protein per sample was run on SDS-PAGE gels. (A) TAPP2, ZAP70, or actin expression was detected by Western blot. Blots are representative of > 40 samples analyzed (each sample examined in at least 2 independent experiments). (B) Chemiluminescence signals for TAPP2, ZAP70, and actin blots were quantified using a Fluorchem instrument. Data are normalized to the actin signal for each sample. Graphs represent the normalized expression of 41 CLL and 15 normal B-cell samples, and CLL samples were divided into IgH unmutated/ZAP70+ and IgH mutated/ZAP70− groups. P values were calculated using a Mann-Whitney test comparing the indicated groups. (C) Representative Western blot showing syntrophin expression in CLL and normal B cells. (D) Quantitative analysis of syntrophin expression in normal B cells, and unmutated/ZAP70+ and IgH mutated/ZAP70− CLL groups.
TAPP2 and utrophin regulate lymphocyte adhesion
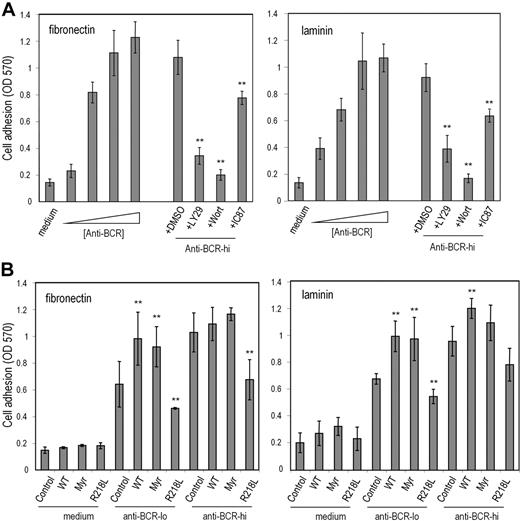
One function of utrophin/syntrophin complexes is to provide a submembrane scaffolding that stabilizes cell-matrix adhesive interactions.34,35 We therefore examined whether TAPP2 can regulate cell adhesion to matrix proteins. We established adhesion assay conditions that can distinguish substantial activation-induced increases in B-lymphoma cell adhesion (Figure 6A). BCR-induced increases in adhesion to fibronectin- or laminin-coated wells were reduced when cells were treated with chemical inhibitors of PI3K enzymes (Figure 6A), consistent with a role for this signaling pathway in “inside-out” activation of cell adhesion in this model. The inhibitor compound IC87114, which selectively blocks the p110δ isoform of PI3K,36 had a significant effect on adhesion, but was less potent than the pan-PI3K inhibitors wortmannin or LY294002 (Figure 6A). BJAB cells were transfected with expression vectors encoding wild-type TAPP2, myristoylated TAPP2, or PH mutant TAPP2 and assessed for alterations in adhesion function. Transfectants expressing wild-type or membrane-targeted TAPP2 showed increased adhesion compared with control BJAB cells, whereas transfectants expressing PH mutant TAPP2 showed significantly reduced adhesion to both fibronectin and laminin substrates (Figure 6B). Similar results were observed in independent stable transfectants (data not shown). These results suggested that membrane-localized TAPP2 promotes adhesion, whereas TAPP2 unable to associate with membranes via the PH domain (R218L mutant) can antagonize adhesion, presumably by sequestering TAPP-associated proteins.
TAPP2 regulates cell adhesion to extracellular matrix proteins. BJAB cells were incubated in fibronectin- or laminin-coated wells under the indicated conditions. After 1 hour nonadhered cells were removed, and then plates were fixed and stained with crystal violet. Stained cells were dissolved in SDS buffer and the optical density at 570 nm was read on an absorbance plate reader. (A) BCR cross-linking induces a PI3K-dependent increase in B-lymphoma cell adhesion to laminin and fibronectin. (Right bars) BJAB cells were stimulated with increasing doses of anti-BCR or in medium alone during the 1-hour adhesion assay. The triangle indicates increasing concentrations of anti-BCR stimulus (0.01, 0.1, 1, or 10 μg/mL). (Left bars) Cells were stimulated with 10 μg/mL anti-BCR during adhesion assay together with addition of PI3K inhibitors or DMSO diluent containing no inhibitor (DMSO indicates 0.5% DMSO with no inhibitor; LY29, 50μM LY294002; Wort, 50 nM wortmannin; and IC87, 50μM IC87114). (B) BJAB transfectants expressing the indicated TAPP2 proteins were assessed for BCR-induced adhesion (Control indicates untransfected BJAB; WT, BJAB expressing wild-type TAPP2; Myr, expressing myristoylated TAPP2; and R218L, expressing PH mutant TAPP2). Anti–BCR-lo denotes stimulation with 0.1 μg/mL concentration, whereas anti–BCR-hi denotes stimulation with 1 μg/mL. Note that at 0.1-μg/mL dose, all TAPP2 transfectants showed significantly altered adhesion compared with control BJAB cells (**P < .05 by Student t test). Results shown represent average and SE obtained from 3 to 5 independent experiments, and are representative of data obtained with at least 2 independent stable transfectants.
TAPP2 regulates cell adhesion to extracellular matrix proteins. BJAB cells were incubated in fibronectin- or laminin-coated wells under the indicated conditions. After 1 hour nonadhered cells were removed, and then plates were fixed and stained with crystal violet. Stained cells were dissolved in SDS buffer and the optical density at 570 nm was read on an absorbance plate reader. (A) BCR cross-linking induces a PI3K-dependent increase in B-lymphoma cell adhesion to laminin and fibronectin. (Right bars) BJAB cells were stimulated with increasing doses of anti-BCR or in medium alone during the 1-hour adhesion assay. The triangle indicates increasing concentrations of anti-BCR stimulus (0.01, 0.1, 1, or 10 μg/mL). (Left bars) Cells were stimulated with 10 μg/mL anti-BCR during adhesion assay together with addition of PI3K inhibitors or DMSO diluent containing no inhibitor (DMSO indicates 0.5% DMSO with no inhibitor; LY29, 50μM LY294002; Wort, 50 nM wortmannin; and IC87, 50μM IC87114). (B) BJAB transfectants expressing the indicated TAPP2 proteins were assessed for BCR-induced adhesion (Control indicates untransfected BJAB; WT, BJAB expressing wild-type TAPP2; Myr, expressing myristoylated TAPP2; and R218L, expressing PH mutant TAPP2). Anti–BCR-lo denotes stimulation with 0.1 μg/mL concentration, whereas anti–BCR-hi denotes stimulation with 1 μg/mL. Note that at 0.1-μg/mL dose, all TAPP2 transfectants showed significantly altered adhesion compared with control BJAB cells (**P < .05 by Student t test). Results shown represent average and SE obtained from 3 to 5 independent experiments, and are representative of data obtained with at least 2 independent stable transfectants.
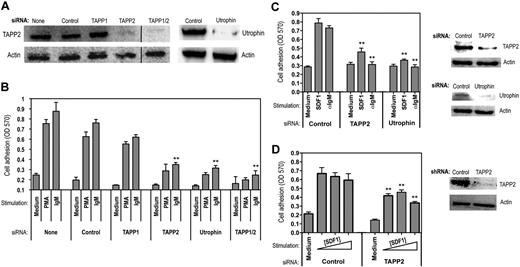
As an alternate approach for assessing TAPP function, we used siRNA knockdown and achieved substantial reduction of TAPP2 proteins levels in BJAB cells (Figure 7A). Consistent with the results using expression of PH mutant TAPP2, cells with reduced TAPP2 protein levels showed reduced adhesion to fibronectin (Figure 7B). Similar results were observed for adhesion to laminin (data not shown). Use of an siRNA predicted to specifically knockdown TAPP1 had little effect on adhesion; however, the combination of TAPP1 and TAPP2 siRNA was more effective in reducing adhesion than TAPP2 alone (Figure 7B). Knockdown of utrophin also led to substantially decreased adhesion (Figure 7B), indicating that this molecule is required for lymphocyte adhesion. To determine whether the requirement for TAPP2 in adhesion responses can be generalized to other B-lineage cancers and other receptor systems, we applied TAPP2 or utrophin siRNA to JVM-3 chronic lymphocytic leukemia cells, which also respond to the chemokine SDF1/CXC ligand 12. TAPP2 or utrophin knockdown significantly decreased adhesion of JVM-3 triggered by either BCR or SDF stimulation (Figure 7C). Lastly, we examined the effect of TAPP2 knockdown on SDF1-induced adhesion of Nalm-6 pre-B leukemia cells. Using lentiviral vectors expressing TAPP2-specific short hairpin RNA, we were able to achieve substantial inhibition of TAPP2 expression in these cells, resulting in significantly blunted adhesion responses (Figure 7D). These results provide the first evidence defining a specific biologic function for TAPP adaptors, and suggest that they may exert this function by modulating cytoskeletal reorganization events involving utrophin.
siRNA knockdown of TAPP2 or utrophin inhibits lymphocyte adhesion. (A) BJAB cells were transfected with synthetic siRNAs using a lipid transfection vehicle and harvested after 48 hours. Cell viability was similar under all conditions. TAPP2 expression in the indicated siRNA-treated populations was then assessed by Western blot. (Right panels) Utrophin expression in BJAB cells treated with control or utrophin-specific siRNAs, as determined by utrophin immunoprecipitation and Western blot. (B) Various siRNA-treated populations (indicated by bottom labels) were assessed for adhesion to fibronectin-coated plates in the presence of anti-IgM (1 μg/mL) or PMA (50 ng/mL) stimulation. Results represent the average and SD of triplicate wells from a representative experiment (1 of 5). **P < .05 in a Student t test comparing the indicated specific siRNA-treated groups to the corresponding IgM-stimulated control siRNA group. (C) JVM-3 cells were transfected with control, TAPP2, or utrophin siRNAs using the method described in panel A. Cells were then assayed for adhesion in the presence of anti-IgM (1 μg/mL) or SDF1 (0.5 μg/mL). Results indicate the average and SEM of 2 independent experiments (6 replicate wells). **P < .05 in a Student t test comparing the specific siRNA-treated groups to the correspondingly stimulated control siRNA group. Right panels show Western blot analysis of TAPP2 and utrophin in the siRNA-treated cells. (D) Nalm-6 cells were transduced with lentiviral vectors expressing control or TAPP2-specific shRNAs and then assayed for adhesion in the presence of increasing SDF1 concentrations (0.2, 0.5, 1 μg/mL), indicated by a triangle. Results indicate the average and SEM mean of 2 independent experiments (6 replicate wells). **P < .05 in a Student t test comparing the TAPP2 shRNA-transduced group to the correspondingly stimulated control shRNA group. Right panel shows Western blot analysis of TAPP2 in the cells transduced with shRNA-lentivirus.
siRNA knockdown of TAPP2 or utrophin inhibits lymphocyte adhesion. (A) BJAB cells were transfected with synthetic siRNAs using a lipid transfection vehicle and harvested after 48 hours. Cell viability was similar under all conditions. TAPP2 expression in the indicated siRNA-treated populations was then assessed by Western blot. (Right panels) Utrophin expression in BJAB cells treated with control or utrophin-specific siRNAs, as determined by utrophin immunoprecipitation and Western blot. (B) Various siRNA-treated populations (indicated by bottom labels) were assessed for adhesion to fibronectin-coated plates in the presence of anti-IgM (1 μg/mL) or PMA (50 ng/mL) stimulation. Results represent the average and SD of triplicate wells from a representative experiment (1 of 5). **P < .05 in a Student t test comparing the indicated specific siRNA-treated groups to the corresponding IgM-stimulated control siRNA group. (C) JVM-3 cells were transfected with control, TAPP2, or utrophin siRNAs using the method described in panel A. Cells were then assayed for adhesion in the presence of anti-IgM (1 μg/mL) or SDF1 (0.5 μg/mL). Results indicate the average and SEM of 2 independent experiments (6 replicate wells). **P < .05 in a Student t test comparing the specific siRNA-treated groups to the correspondingly stimulated control siRNA group. Right panels show Western blot analysis of TAPP2 and utrophin in the siRNA-treated cells. (D) Nalm-6 cells were transduced with lentiviral vectors expressing control or TAPP2-specific shRNAs and then assayed for adhesion in the presence of increasing SDF1 concentrations (0.2, 0.5, 1 μg/mL), indicated by a triangle. Results indicate the average and SEM mean of 2 independent experiments (6 replicate wells). **P < .05 in a Student t test comparing the TAPP2 shRNA-transduced group to the correspondingly stimulated control shRNA group. Right panel shows Western blot analysis of TAPP2 in the cells transduced with shRNA-lentivirus.
Discussion
TAPP adaptor proteins are known for their specific binding to the PI3K product PI(3,4)P2, leading to their membrane recruitment with distinct kinetics and regulation.18,23 In contrast, the protein associations and function of TAPP adaptors are not well understood. We discovered the TAPP proteins via their homology to B-cell adaptor molecule of 32 kDa/dual adaptor for phosphotyrosine and phosphoinositide, a PI(3,4)P2-binding PH domain adaptor protein whose expression is limited to specific immune cell subsets and is known to be important in B-cell signaling and receptor endocytosis,17,37,38 thus it seems reasonable to speculate that TAPP adaptors may share some signaling functions in lymphocytes. Here we have developed conditions for isolation of TAPP2 and associated proteins and identified more than 30 potential interaction partners, including several proteins involved in signal transduction, cytoskeletal regulation, and endocytosis. Although most of these interactions remain to be confirmed, we have here confirmed that the actin-binding proteins utrophin associates with TAPP2. We also found that the PDZ domain–containing protein β2-syntrophin, a known utrophin-binding protein, was present in TAPP2-eluted material. This result is consistent with a report indicating that TAPP1 can associate with multiple syntrophins in nonhematopoietic cells.26
We found that TAPP2, utrophin, and syntrophin are coexpressed in B cells and B-CLL cells, suggesting that this complex may be involved in orchestrating the cytoskeleton in lymphocytes. In nonlymphoid cells, utrophin/syntrophin complexes are known to play important roles in cell-matrix adhesive interactions.34,35,39 We found that both TAPP2 and utrophin were required for efficient B-cell adhesion to matrix proteins, indicating that this complex may have a related function in lymphocytes. PH mutant TAPP2 acted as a dominant negative to inhibit cell adhesion, possibly due to sequestration of the syntrophin/utrophin complex from membrane-associated adhesion complexes. Given the established linkage of TAPP adaptors to the PI3K signaling pathway, which is highly active in antigen-stimulated B cells and B-CLL cells, we hypothesize that these adaptors may organize signaling-induced cytoskeletal changes affecting cell motility and/or interactions with matrix and other cells. Intriguingly, we find that TAPP2 and syntrophins have significantly increased expression within the subset of IgH-unmutated B-CLL expressing ZAP70, compared with IgH mutated, ZAP70-low/negative CLL or normal B cells. These results suggest this complex could potentially contribute to cytoskeletal changes contributing to the more rapid disease progression of this CLL subset.
TAPP1 was previously shown to bind to PDZ domain proteins multi-PDZ domain protein 1 and protein tyrosine phosphatase L1 in HEK293 cells.24,25 We have been unable to verify expression of these proteins in BJAB lymphoma cells, perhaps explaining why they were not identified in our mass spectrometry analysis (supplemental Figure 1). However, other notable protein identifications in the TAPP2-eluted material included clathrin heavy chains 1 and 2 and 1 clathrin-associated adaptor subunit (adaptor protein 2 [AP-2]), suggesting that TAPPs may regulate receptor internalization via clathrin-coated pits. Inspection of TAPP1 and TAPP2 sequences reveals sequence motifs near the C-terminus with very good match to consensus “clathrin-box” binding sequences, suggesting a potential site of direct interaction. It is interesting to note that we previously found that the TAPP-related protein Bam32/DAPP1 can regulate BCR internalization.37,40 One study found that AP-2 but not AP-1 can directly interact with PI(3,4)P2 and PI(3)P41 consistent with a potential membrane-proximal functional interaction between TAPPs and specific clathrin/AP complexes. Among the signaling molecules identified, growth factor receptor-bound protein 2 is an interesting candidate that could potentially directly bind via the putative Src homology 3 domain–binding proline motif in the TAPP2 unique region,22 and is also known to regulate receptor endocytosis.42 Several monomeric GTPases were also identified, such as Rac2, Rab1B, and Rab7, all of which are known regulators of the cytoskeleton and endocytic trafficking. An important future goal will be to map the respective binding sites of TAPP2-associated proteins and determine which interaction partners are shared with TAPP1.
Our studies implicating utrophin and syntrophins in lymphocyte biology suggest these molecules may have a whole range of additional functions beyond those currently described. Utrophin and dystrophin are spectrin-family scaffold proteins known to function in cell signaling, adhesion, and polarization.43 Syntrophin association with utrophin/dystrophin is thought to be critical for their functions in muscle.44 In muscle cells, dystrophin and utrophin predominantly associate with a nonintegrin adhesion complex known as the dystroglycan complex; however, utrophin likely associates with other types of adhesion complexes in nonmuscle tissues.45 To our knowledge, our siRNA results provide the first evidence for a function of utrophin in lymphocytes. Although expression of the utrophin homologue dystrophin is generally considered to be muscle specific, gene-profiling experiments identified dystrophin as one of a short list of genes (including ZAP70) that is selectively overexpressed in IgH unmutated B-CLL.46 The correlation of dystrophin protein levels with patient survival has recently been confirmed.47 These intriguing data are consistent with the general hypothesis that rewiring of cytoskeletal regulators may contribute to CLL progression.
Leukocyte cell-cell and cell-matrix adhesion is a highly regulated, integrin-mediated process generally requiring both inside-out activation of integrins triggered by stimulation through various receptors including immunoreceptors and chemokine receptors, and outside-in signaling via integrins themselves.48,49 A series of studies have implicated utrophin as well as a differentially spliced short form of dystrophin in integrin-mediated platelet aggregation.34,50,51 In adhering platelets, utrophin was found to localize in plasma membrane patches and focal adhesions, and coprecipitated with focal adhesion–associated proteins such as α-actinin and focal ahesion kinase.34 Integrin-mediated platelet activation is known to be PI3K dependent and shares many signaling mechanisms in common with lymphocytes.52,53 Intriguingly, one study found that integrin activation of platelets leads to preferential production of PI(3,4)P2,54 suggesting that PI(3,4)P2 effector proteins such as the TAPPs may be involved. TAPP2 may also be particularly critical for inside-out activation of cell adhesiveness in B cells, because they abundantly express the phosphoinositide phosphatase Src homology 2 domain-containing 5′ inositol phosphatase 1, which converts most of the PI(3,4,5)P3 produced by PI3K into PI(3,4)P2.55 Together our results indicate that the TAPP adaptors provide a means of specifically linking PI(3,4)P2 to increased B-cell adhesiveness through interaction with utrophin and syntrophins.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kading Cheng for help with mass spectrometry analysis, Brenda Kuschak for help in isolating CLL cells and determining IgH mutation status, and Dr Monther Al-Alwan for helpful discussions. CLL samples came from the Manitoba Tumor Bank, a member of the Canadian Tumor Repository Network.
This work was supported by the Canadian Cancer Society, with additional support from the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, and the translational research program of the Manitoba Institute for Cell Biology. A.J.M. is supported by a Canada Research Chair.
Authorship
Contribution: J.L.C. and S.M.S.C. designed and performed research; S.H. and H.L. performed research; S.K.K. provided vital reagents and technical expertise; J.B.J. provided vital clinical samples; J.A.W. provided vital analytical tools; S.B.G. provided vital reagents and samples; and A.J.M. designed research, supervised students and the technician, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.L.C. is Manitoba Institute for Cell Biology/Biochemistry and Cell Biology, CancerCare Manitoba, Winnipeg, MB. The current affiliation for S.M.S.C. is Department of Microbiology and Immunology, University of Alberta, Edmonton, AB.
Correspondence: Aaron Marshall, Departments of Immunology, Biochemistry and Medical Genetics, University of Manitoba, 415 Apotex Centre, 750 McDermot Ave, Winnipeg, MB, R3E-0T5; e-mail: marshall@ms.umanitoba.ca.
References
Author notes
J.L.C., S.M.S.C., and S.H. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal