Abstract
Abstract 1212
Poster Board I-234
The use of sirolimus (rapamycin) as primary immunosuppression for allogeneic blood and marrow transplantation is logical given its favorable anti-tumor and tolerance inducing properties.
After IRB approval, we analysed long term survival and predictive correlates in 92 patients receiving sirolimus based non-ablative alternative donor hematopoietic transplantation for hematological malignancies. All received conditioning with a purine nucleotide and cyclophosphamide 1mg/m2 (d-7, -6), 5mg/m2 methotrexate days 1, 3, 6 and combination sirolimus and tacrolimus each dosed at 6-10 ng/ml levels. Where feasible tacrolimus was discontinued as early as day 50, but sirolimus was continued until at least 9 months unless discontinued for toxicity or relapse. Median age was 57 years (range 23-74). Diagnoses were ALL – 4, AML – 44, CLL – 7, CML – 4, HD – 3, MDS – 4, MM – 2, MPD – 4, NHL – 19, AA-1. Only 32/92 patients were in complete remission (CR) at the time of transplant and only 16 in CR1. Median number of prior chemotherapy regimens was 3 (0-7). CIBMTR risk scores for outcome were: high in 43, intermediate in 28, and low in 20. 11 patients had received prior autologous transplantation (12%). Donors were unrelated (URD) in 80 (87%) patients and 5/6 antigen matched related in 12 (13%). 57 of 80 URD recipients were transplanted with cells matched at HLA A,B,C and DRB1, while the other 23 received cells mismatched at least 1 locus. 86/92 patients received transplants of G-CSF mobilized blood cells, the other 6/92 received bone marrow (all URD). Hospital discharge was generally day 1 post transplant and many engrafted as out patients.
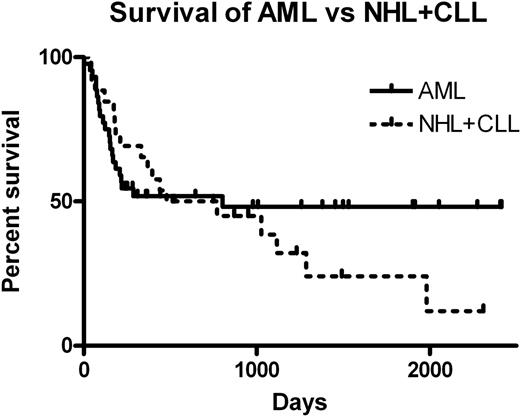
Patients were hospitalized a median of only 9 of the initial 100 days post transplant (range 1-66 days). Median follow-up of the 39 surviving patients was 1025 days (214-2414) from transplantation. Primary graft failure (< 10% donor engraftment in survivors at day 30) occurred in 8/92 patients, and secondary graft failure developed in 3/92. In 44/92 (47.8%) tacrolimus was discontinued prior to day 100. Graft versus host disease (GVHD) developed by day 100 in 45/85 patients followed at least 100 days. Median overall survival (OS) was 480 days. OS for AML (44 patients) and NHL(19) +CLL(7) patients is shown in the figure below. Of pre-transplant features, only stage (CR vs other) predicted for OS (p=0.01). Donor chimerism at 100 days was also predictive for OS (HR=0.33, p=0.02). Increased chimerism was correlated with HLA matched grafts (p=0.02) and with increased numbers of infused donor CD34+ve cells (p=0.04). Treatment related mortality was 9/92 (9.8%) at 100 days and 18/85 (21%) at 1 year.
Sirolimus treatment with early discontinuation of tacrolimus provides effective GVHD prophylaxis and acceptable long-term survival in a group of older, poor prognosis patients receiving non-ablative allogeneic transplants from alternative donors for hematological malignancies. Measures to further improve day 100 donor chimerism, including improved HLA matching and higher infused numbers of CD34 +ve cells, may yield further improvements.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal