Abstract
Abstract 1217
Poster Board I-239
Single autologous stem cell transplant (ASCT) is considered the standard of care after induction therapy for younger multiple myeloma (MM) patients (pts). However, it is not curative and virtually all patients will ultimately relapse. As more options are available to treat relapsed disease, the role of a second ASCT as salvage therapy is unclear.
Retrospective review of all MM pts who received a 2nd ASCT as salvage therapy at Princess Margaret Hospital.
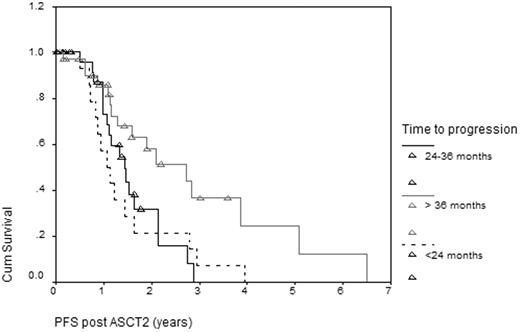
Between February 1997 and July 2009, 79 MM pts received a second ASCT for relapsed MM at our institution. Median age was 60 yrs (range 39-72) at second transplant. 48 pts (61%) were male. Immunoglobulin subtype included IgG (42), IgA (21), light chain (11), nonsecretory (3), IgM(1) and IgD (1). Transplant conditioning regimen for first transplant was high dose melphalan (MEL) 140-200 mg/m2 in 67, busulphan and cyclophosphamide in 1, and combinations of MEL with etoposide (E) or TBI in the rest. 2nd ASCT conditioning consisted of MEL alone in 76, the remaining 3 had MEL with either TBI or E. The median time to relapse after the first transplant was 2.72 years (0.81-8.26), with a median interval between transplants of 3.61 years (1.63-9.59). Response to first transplant in 78 evaluable patients was 13 CR/nCR (17%), 56 PR/VGPR (72%), 9 MR/SD (12%). Nineteen pts received maintenance therapy between transplants. Two transplant-related deaths occurred following 2nd ASCT. In 73 evaluable patients, response to second transplant was 11 CR/nCR (15%), 57 PR (78%), 6 MR/SD (8%). After 2nd ASCT, with median follow up 5.92 years (71 months), median progression-free survival (PFS) was 18.5 months and median overall survival (OS) was 4.4 years. Long term progression-free status based on the progression-free interval after 1st ASCT is summarized Table 1. PFS based on progression free interval after 1st ASCT is outlined in Figure 1.
2nd ASCT is a feasible and safe salvage therapy in relapsed MM patients. It is effective in providing a median progression free survival of 18.5 months and median overall survival of 4.4 years (52.8 months) after 2nd ASCT. This is comparable if not better than modern salvage chemotherapies. The longer the disease free interval after 1st ASCT the more effective 2nd ASCT is at extending both progression free survival and overall survival. It is reasonable, therefore, to consider a 2nd ASCT if the time to progression is greater than 2 years after first ASCT.
Outcomes Based on Time to Progression Post 1st ASCT
| Group . | # of patients . | Median PFS post 2nd ASCT (months) . | P value . | Median OS post 2nd ASCT (months) . | P value . |
|---|---|---|---|---|---|
| Entire Cohort | 79 | 18.5 | 52.8 | ||
| < 24 months | 15 | 12.7 | 42.2 | ||
| 24-36 months | 30 | 17.0 | 52.7 | ||
| >36 months | 34 | 32.6 | 0.0196 | Not reached | 0.1104 |
| Group . | # of patients . | Median PFS post 2nd ASCT (months) . | P value . | Median OS post 2nd ASCT (months) . | P value . |
|---|---|---|---|---|---|
| Entire Cohort | 79 | 18.5 | 52.8 | ||
| < 24 months | 15 | 12.7 | 42.2 | ||
| 24-36 months | 30 | 17.0 | 52.7 | ||
| >36 months | 34 | 32.6 | 0.0196 | Not reached | 0.1104 |
Chen:Celgene: Honoraria, Research Funding; Ortho Biotech: Honoraria. Trudel:Celgene: Honoraria, Speakers Bureau; Ortho Biotech: Honoraria. Kukreti:Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal