Abstract
Abstract 2268
Poster Board II-245
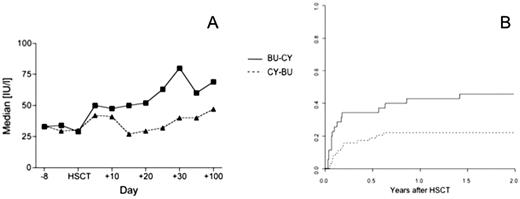
Busulfan-cyclophosphamide (BU-CY) is the established non total body irradiation based myeloablative conditioning regimen for allogeneic hematopoietic stem cell transplantation (HSCT). The introduction of intravenous busulfan has facilitated its application and reduced toxicity. Theoretical considerations and pharmacological data indicate that previous application of busulfan may trigger liver toxicity of subsequent cyclophosphamide. A reverse order of cyclophosphamide-busulfan (CY-BU) would be preferable. Recent animal data confirmed this hypothesis, showing less liver toxicity and better outcomes in mice treated with CY-BU. While CY-BU was not feasible in patients with oral busulfan, it has become a possibility with the introduction of i.v. busulfan. We were therefore interested in exploring this concept and changed the order of drug application to CY-BU in 2003 in those patients not on a multicentre standardized BU-CY protocol. We now retrospectively analyzed in this single centre cohort study liver toxicity and transplantation outcome in patients receiving BU and CY as conditioning regimen for allogeneic HSCT. We analyzed 93 consecutive patients between 1993 and 2008, 52 male (55.9%), median age 46 years (range 16 to 70) with hematological malignancies (AML 41 [44.1%], ALL 11 [11.8%], CML 12 [12.9%], myelodysplastic syndrome and myeloproliferative neoplasia 22 [23.7%], lymphoproliferative disorders 4 [4.3%]) or other diseases (3 [3.2%]), receiving an allogeneic HSCT from an HLA- identical sibling (52 [55.9%]), other family member (3 [3.2%]) or a matched unrelated donor (38 [40.9%]) after conditioning regimen with BU-CY (34 patients; 18 patients with oral, 16 patients since 2003 with i.v. busulfan) or CY-BU (59 patients). Outcomes were analyzed using a Cox regression model, adjusting for disease, stage, donor type, stem cell source, previous total body irradiation (TBI) and busulfan administration (oral vs. intravenous). Pretransplant patient characteristics were comparable in the two cohorts for age, gender, underlying disease, stem cell source, donor type and EBMT risk score, but differed in stage (advanced disease BU-CY 28 [84,8%] vs. CY-BU 40 [66.7%]) and previous TBI (BU-CY 16 [48.5%] vs. CY-BU 9 [15.0%]). Liver function as measured by levels of bilirubin and liver enzymes (aspartate amino transferase [AST], alanine amino transferase [ALT], gamma glutamyl transpeptidase [GGT] and alkaline phosphatase [AP]) was not different between the groups before starting conditioning regimen. In contrast liver function differed significantly at day 20, with higher levels of ALT (median 51.0 vs 27.0 IU/l; p=0.012) and a higher incidence of veno-occlusive disease (VOD) (5/34 vs. 1/59, p=0.036) in the BU-CY group (Figure 1A). The cumulative incidence of transplant-related mortality (TRM) at 2 years was significantly higher in patients receiving BU-CY (BU-CY 0.48, CY-BU 0.24, p=0.024; hazard ratio 4.594 for BU-CY, 95% CI 1.382-15.268, p=0.013) (Figure 1B). The cumulative incidence of TRM with BU i.v.-CY was lower (0.44) than with BU oral-CY (0.56) but still higher than CY-BU. This did translate into a higher overall survival in patients after conditioning regimen with CY-BU (hazard ratio for mortality 0.426 for CY-BU, 95% CI 0.184-0.987, p=0.047). Time to engraftment (BU-CY median 13 days vs. CY-BU median 14 days), cumulative incidence of acute GVHD and relapse were similar between patients receiving BU-CY or CY-BU. These data support the concepts derived from Sadeghi et al in their mouse model in favor of CY-BU compared to the traditional BU-CY. They form the basis for prospective controlled studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal