Abstract
Abstract 267
The tumor cells of HL, the Hodgkin and Reed-Sternberg (H-RS) cells, derive from germinal center B-cells with a deranged B-cell transcription program due to epigenetic silencing and acquired genetic lesions. Tissue H-RS cells are surrounded by a preponderant infiltrate of mixed non-malignant reactive cells, including B-lymphocytes, which provide essential signals for their survival and proliferation. Small percentages of B-cells, clonally related to H-RS cells, were found in the blood of HL patients (pts), suggesting they may represent putative HL-initiating cells (Jones, 2009). Since the serum free light chain (sFLC) assay detects and quantifies monoclonal and polyclonal B-cell populations expanding in lymphohemopoietic tissues, we exploited the sFLC testing to further explore the biologic significance of B-lymphocytes in HL.
Frozen (-80°C) serum samples from 119 untreated cHL pts (48% males), with normal renal function and serum immunochemistry, were assayed by immunonephelometry (Freelite, The Binding Site, Ltd., UK). After quantization of free κ and λ concentrations (normal ranges, κ: 3.3-19.4 mg/L; λ: 5.71-26.3 mg/L), sFLC κ/λratio was calculated (reference range 0.26-1.65). The median age was 31 years (r, 15–70), with 22% aged ≥ 45 years. Histology was nodular sclerosis in 85 pts (71.4%) and 72 (60.5%) were in stage I–II. According to GHSG criteria, 16% had early favorable, 29% early unfavorable and 55% advanced disease. The International Prognostic Score (IPS) was of 0-2 and ≥ 3 in 66.4% and 33.6% of pts, respectively. Following ABVD (4-6 courses ± RT), the median Event-free survival (EFS) was 78 mo.s [95% CI, 68-88] for the entire population and 75 mo.s [95% CI, 60-90] and 64 mo.s [95% CI, 50-77] for pts in early and advanced stage, respectively.
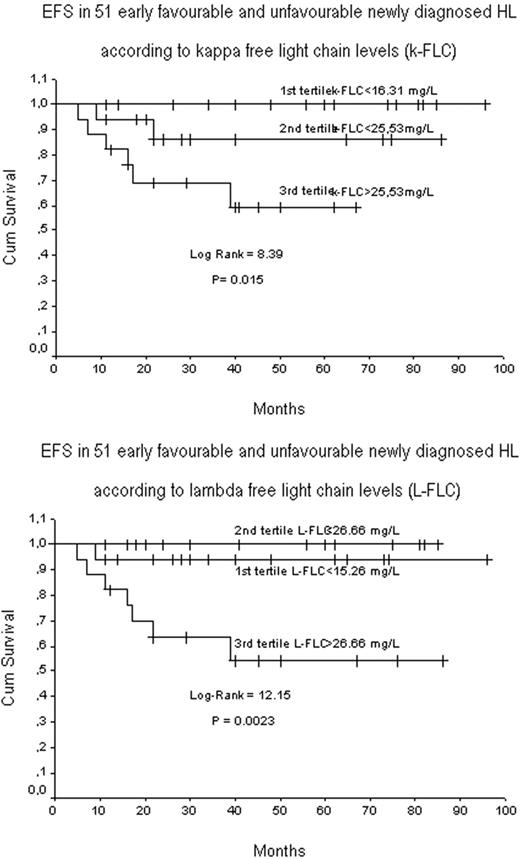
Elevated κor λsFLC concentrations were found in 47% (median 29.55 mg/L; r, 19.44 - 64.70) and 29.4% (median 32.70 mg/L; r, 26.60 - 79.77) of pts, respectively. In 30 pts (25.2%) levels of polyclonal κ and λ sFLC were concurrently elevated. The sFLC κ/λratio was abnormal in only 7.5% of pts (clonal λ: 8/119; clonal λ: 1/119). The presence of high sFLC levels correlated with lymphopenia (< 0.6 × 109/L; p= 0.04), leukocytosis (WBC > 15 × 109/L; p=0.03), ESR (> 50; p=0.04) and unfavorable IPS (≥ 3; p=0.03), but not with EBV status and risk factors such as stage, B symptoms, bulky and extra nodal disease, LDH and albumin. The association with leukocytosis may in part result from inhibition of spontaneous neutrophils apoptosis exerted by FLC (Cohen, 2003). Most interestingly, while we found no significant association with response rate, baseline elevation in sFLC predicted for EFS in 51 evaluable pts with early stage disease. Patients were divided into tertiles and best break point value for predicting EFS coincided with the upper limit of the highest tertile for both κ (>25.53 mg/L) or λsFLC (>26.66 mg/L) (Figure 1). The pts in the top tertile had the worst outcome compared with the 2 lower tertiles (κ, p=0.015; λ, p=0.002). Outcomes for the 2 lower tertiles were comparable. Data remained significant after stratification for the IPS. In contrast, baseline sFLC levels, κ or λ, were not predictive for EFS in pts with advanced disease. Interestingly, sFLC levels in a control group of 30 pts in continuous CR from 2 years, were within the normal ranges.
We have shown that about 50% of cHL pts displays elevated levels of sFLC mirroring the presence of a consistent polyclonal B-cell expansion at diagnosis. The role of reactive B-cells in HL is poorly understood. While some data indicate that high intratumoral B-cell counts may predict for a better outcome, the activity of rituximab in cHL, regardless of CD20 expression on H-RS cells, was suggested to result by depletion from the HL microenvironment of normal B lymphocytes required for tumor cell growth (Younes, 2003). Our study support that elevated sFLC levels may reflect an increased polyclonal B cell activity in cHL microenvironment which appears to negatively influence the outcome of pts in early stage disease. This effect is lost in advanced disease, suggesting that rituximab might result more active for pts in early than advanced stages. That putative HL-initiating small B-cells may emerge from the expanded polyclonal B-cell population present from the early phases of disease development, is an intriguing possibility.
Marchei:Radim, Italy: Employment. Amoroso:The Binding Site, Ltd: Consultancy.
[RDF and FR contributed equally to the work]
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal