Abstract
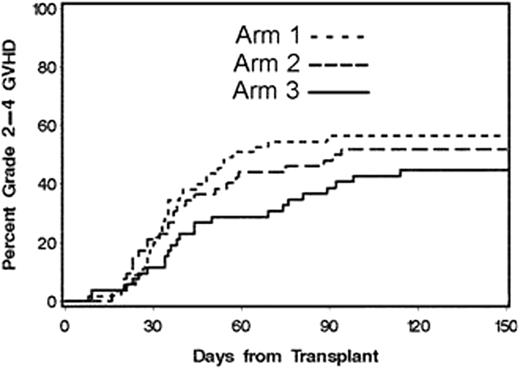
We previously reported results of 3 sequential trials of GVHD prophylaxis with mycophenolate mofetil (MMF) BID/TID and cyclosporine (CSP) BID with various taper schedules in patients (pts) with advanced hematologic malignancies given unrelated G-CSF-mobilized peripheral blood stem cell (PBSC) grafts after fludarabine 90 mg/m2 and 2 Gray total body irradiation. Cumulative incidences of grades II-IV acute GVHD in the 3 trials were 52, 53 and 77%, respectively. The goal of the current protocol was to evaluate, in a phase II randomized 3-arm study, which drug combination or schedule was most promising in preventing acute GVHD. Tacrolimus (Tac) was used in place of CSP and each of the 3 arms used MMF TID until day 30 and then BID, but the subsequent duration of MMF varied. In Arm1, pts received Tac until day 180 and MMF until day 96. In Arm2, Tac was given until day 150 and MMF until day 180. In Arm3, Tac was given until day 150 and MMF until day 180 with the addition of rapamycin from days -3 through 80. One hundred seventy-five pts ineligible for myeloablative conditioning were enrolled on this multi-institutional study between Jan/05 and Aug/09, and results on the first 159 pts (Arm1 n=56; Arm2 n=51; Arm3 n=52) are reported here with a median follow-up of 18.4 months for surviving pts. The median age of pts was 60 (range 13-75) yrs. Sixty-six (42%) had previous autologous (n=55) or allogeneic (n=11) HCT. All pts were matched for HLA-DRB1 and -DQB1 at the allele level: 16 had single allele mismatches at HLA-A, -B or –C and the remainder (n=143) were fully HLA-matched. Diagnoses included AML (n=72), NHL (n=36), MM (n=19), ALL (n=10), CLL (n=9), MDS (n=8), HL (n=4), and CML (n=1). Randomization was based upon transplant center (FHCRC vs other), number of prior chemotherapy treatments (0-2 vs 3+), and age (<55 vs 55+ years). The pts received PBSC grafts containing a median of 7.9 ×106 CD34 and 2.8 × 108 CD3 cells/kg. Sustained donor engraftment occurred in 99.4% of pts. The day-150 cumulative incidences of grades II-IV (figure 1) and III-IV acute GVHD were as follows: Arm1: 56%, 9%; Arm2: 52%, 12%; and Arm3: 45%, 10%, respectively. Chronic GVHD requiring therapy was as follows: Arm1: 44%, Arm2: 35%, and Arm3: 55% of pts. The 6-month nonrelapse mortality was 6% in Arm1, 8% in Arm2, and 2% Arm3. The 2-year Kaplan-Meier estimates of relapse and nonrelapse mortality (figure 2) were as follows: Arm1: 27%, 24%; Arm2: 39%, 19%; and Arm3: 30%, 15%, respectively (overall 32% and 20%, respectively). The 2-year overall and progression-free survivals were as follows: Arm1: 49%, 41%; Arm2: 42%, 37%; Arm3: 55%, 41%, respectively (overall 48% and 40%, respectively). The addition of rapamycin to MMF and Tac (Arm3) resulted in the lowest incidence of grades II-IV acute GVHD (p=0.09 compared to reference Arm1), without a significant difference in chronic GVHD. While the phase II design of the study was not powered to show statistical differences between the 3 arms, the lower incidence of grades II-IV acute GVHD combined with the low morbidity and nonrelapse mortality in Arm3 using MMF, Tac and rapamycin is encouraging and warrants further study.
Off Label Use: Fludarabine - conditioning prior to HCT. Mycophenolate mofetil - immunosuppression after HCT. Tacrolimus - immunosuppression after HCT. Rapamycin - immunosuppression after HCT..
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal