Abstract
Abstract 4572
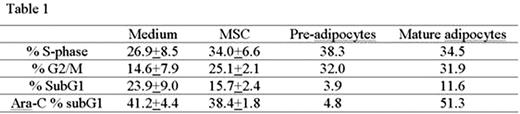
Bone marrow stromal cells (MSCs) from elderly subjects have a reduced capacity to differentiate into osteoblasts and an increased capacity to differentiate into adipocytes, which leads to progressive accumulation of fat in the bone marrow space with increasing age. Adipocytes are the prevalent stromal cell type in adult BM that play an important role in the leukemic bone marrow microenvironment (Tabe et al., Blood 2004 103:1815-22). In this study, we examined the role of BM-derived adipocytes at different stages of differentiation on proliferation and apoptosis of AML cells. U937 cells were co-cultured with BM-derived MSC, MSC-differentiated pre-adipocytes (containing few small lipid vesicles), and mature adipocytes (with multiple hypertrophic lipid vesicules). Under serum-starved conditions, MSC and premature/mature adipocytes induced cell cycle progression of U937 cells with increase in the proportion of cells in S- and G2/M-phase fractions, and inhibited spontaneous cell death with decrease in subG1 fractions. However, only pre-adipocytes inhibited Ara-C-induced cell killing (Table 1).
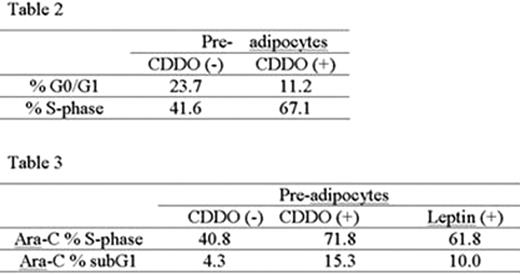
We next focused on lepin and plasminogen activator inhibitor 1(PAI-1) as potential mediators of these effects by adipocytes. Leptin mRNA and protein levels were upregulated during adipocytic differentiation (mRNA relative expression to GAPDH (PCR): MSC 0, premature adipocyte 2.0±0.5, mature adipocyte 123.3± 35.0; leptin secretion: MSC 23.1±2.9, premature adipocyte 49.3±11.3, mature adipocyte 110.0±4.6 pg/mL (ELISA). PAI-1 mRNA levels were increased in mature adipocyte (relative expression to GAPDH: MSC 314.9±46.5, premature adipocyte 215.1, mature adipocyte 3766.1±656.2). Since PPARÿ activation is known to promote maturation and re-generation of fat-derived adipocytes, we next examined the potential of the synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) on the BM adipocytes, leptin and PAI-1 production and the survival of the leukemic cells. CDDO induced adipocyte re-generation with significant increase of the number of Oil-Red(+) small sized lipid vesicles without apoptosis induction. This resulted in a markedly enhanced leptin release from adipocytes (10-fold and 23-fold increase at 0.5 μM and 1.0 μM CDDO, respectively, at 72 hrs) without change in leptin mRNA transcription. On the contrary, PAI-1 mRNA levels were significantly decreased by CDDO (6 fold decrease in MSC, 4 fold decrease in premature adipocyte, 6 fold decrease in mature adipocyte). Co-culture of U937 cells with CDDO-primed premature adipocytes and mature adipocytes resulted in increased spontaneous apoptosis of U937 cells compared to adipocytes not exposed to CDDO (% specific apoptosis, U937 co-cultured with CDDO-primed premature adipocytes 26.9 %, mature adipocytes 20.9 %). At the same time, CDDO-primed premature adipocytes induced significant cell cycle progression with decreased proportion of G0/G1-phase and increase in S-phase fractions in U937 cells (Table 2). Co-culture with CDDO-primed premature adipocytes or with premature adipocytes co-treated with recombinant leptin increased subG1- and S-phase fractions in Ara-C-treated U937 cells compared to U937 cells co-cultured with premature adipocytes (Table 3).
In mature adipocytes, which already produce high levels of leptin, CDDO or leptin treatment failed to modulate anti-apoptotic or proliferative effects of AraC on U937 cells. In contrast, human recombinant PAI-1 effectively inhibited spontaneous and Ara-C induced apoptosis of U937 cells (decreased % of Annexin V: spontaneous apoptosis 11.2±1.1%, Ara-C induced apoptosis 15.1± 1.7%).
In summary, these results suggest that BM pre-adipocytes support proliferation and survival of myeloid leukemia cells in part through complementary effects of leptin and PAI-1. Our findings indicate that secretion of leptin during MSC differentiation or through PPARg ligation promotes cell cycle progression, while PAI-1 primarily inhibits apoptosis of AML cells. It is conceivable that increased adipocyte content of BM in elderly AML patients may negatively affect the responsiveness of AML cells to chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal