Abstract
Abstract 515
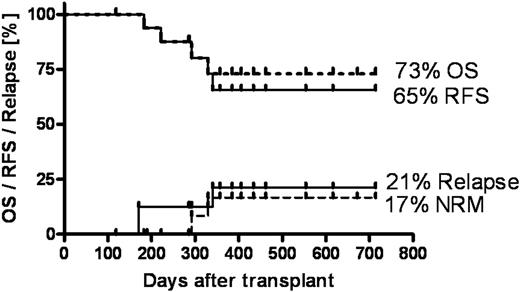
We established a highly efficient GMP-grade, ex-vivo selective allodepletion process where host-activated donor T cells are eliminated based on their preferential retention of the photosensitizer 4,5-dibromorhodamine 123 (TH9402) and exposure to visible light (Kiadis Pharma, The Netherlands). As relapse of disease largely impairs the overall success of allogeneic stem cell transplantation we aimed to improve this outcome by using selectively T cell depleted allografts in order to reduce post transplant immunosuppression and thereby enhance graft versus malignancy effects. To determine the appropriate level of post transplant immunosuppression we designed a three sequential de-escalation stage trial with grade III-IV acute GvHD as the primary endpoint involving 17 patients per study cohort. Here we report on the first completed study cohort of NIH trial 07-H-0136 where seventeen patients (median age 44 (28-68) years) with hematological malignancies received a CD34-selected (Miltenyi, Germany) stem cell allograft together with 5 × 106/kg selectively depleted donor T cells following an age-adapted, radiation-based preparative regimen (FluCyTBI). Eleven patients had high risk disease (including ALL (Ph+, CR>1), refractory NHL, AML/MDS, AML with chloroma and blast crisis CML). Low-dose cyclosporine was used as sole immunosuppression for 90 days post transplant in the absence of GvHD. At a median follow-up of 385 (119-714) days actuarial probabilities (±SEM) of acute GvHD were 35±12% for grade II-IV and 0% for grade III-IV. Non-relapse mortality (NRM) was low with 17±11%. Overall survival (OS) was 73±12% and relapse-free survival (RFS) was 65±13% with a relapse probability of 21±11% (Figure). A low relapse incidence in a high-risk population suggests functionality of selectively allodepleted T cells. The absence of severe GvHD reflects the efficacy of the allodepletion process. Based on these findings we have initiated recruitment for the next study cohort where post transplant immunosuppression will be limited to 45 days only. Ultimately the aim is to achieve further reduction in immunosuppression in the absence of severe GvHD in order to enhance graft versus malignancy effects and thereby improve the outcome after allogeneic stem cell transplantation especially for patients at high risk for relapse.
Mielke:Kiadis Pharma, The Netherlands: Research Funding, The current trial is supported under a clinical trial agreement between NHLBI and Kiadis.. Savani:Kiadis Pharma Inc., The Netherlands: Consultancy. Barrett:Kiadis Pharma, The Netherlands: The current trial is supported under a clinical trial agreement between NHLBI and Kiadis..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal