Abstract
Abstract 645
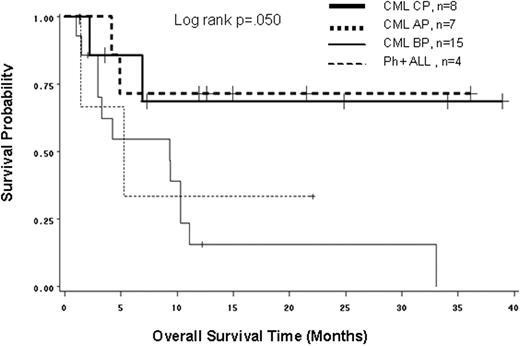
Background: The development of a BCR-ABL T315I mutation is associated with a poor prognosis and limited therapeutic options. The impact of the mutation on the outcome of stem cell transplantation (SCT) is unknown. Aim: To describe the overall survival (OS) of CML patients in any phase and Ph+ ALL patients who received an allogeneic SCT after developing a T315I mutation after exposure to tyrosine kinase inhibitors (TKI). Methods: We conducted a retrospective, multi-center observational study of 222 CML and de novo Ph+ ALL patients who developed a T315I mutation between 1999 and 2008. Data from the medical records of 33 patients (15% of all patients in the registry) from 9 countries (USA, France, Italy, Germany, Denmark, Singapore, and the UK) who received an allogeneic SCT after T315I mutation detection were included in this study. Results: At the time of diagnosis, the median age was 39 years (range, 16-67); 70% were male; 26 patients were in CML CP, 1 in CML AP, 2 in CML BC, and 4 had Ph+ ALL. The median time between diagnosis and TKI treatment start was 3 months (range, 0-125), between diagnosis and T315I mutation detection was 28 months (range, 3-131), and between TKI treatment start and T315I mutation detection was 19 months (range, 2-64). Five (15%) patients had TKIs as frontline therapy. At the time of T315I detection, 10 patients were in CML CP, 7 in CML AP, 12 in CML BC, and 4 had Ph+ ALL. Hydroxyurea (alone or combined with other treatments) was the most common 1st line treatment (55%) after T315I mutation detection. The median time from T315I mutation detection to SCT was 3 months (range, 0.3-28). At the time of transplant, the median age was 42 years (range, 22-68); 8 patients were in CML CP, 7 in CML AP, 14 in CML BC and 4 had Ph+ ALL; 32 patients received 1 SCT and 1 received 2 SCTs after T315I mutation detection. The source of stem cells was peripheral blood (53%), bone marrow (35%), cord blood (6%), and unknown (6%). 82% were matched donor and 18% were unmatched. The median follow-up time from SCT was 7 months and 15 (55%) patients had died by their last follow-up. The OS of CML CP and CML AP patients was much better than CML BP and Ph+ ALL patients (Fig. 1; logrank, p=.050). The 1-yr OS rates (95% CI) from SCT were 69% (21-91%) for CML CP, 71% (26-92%) for CML AP, 16% (3-39%) for CML BC, and 33% (1-77%) for Ph+ ALL; and the 3-yr OS rates (range) was 69% (21-91%) for CML CP, 71% (26-92%) for CML AP, 0 for CML BC, and 0 for Ph+ ALL. Conclusion: These results suggest that the survival of patients harboring a T315I mutation and treated with allogeneic SCT is dependent on the disease phase at the time of SCT. SCT is the treatment of choice for these CML patients, particularly those in CP and AP.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2009 by The American Society of Hematology
2009

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal