Abstract
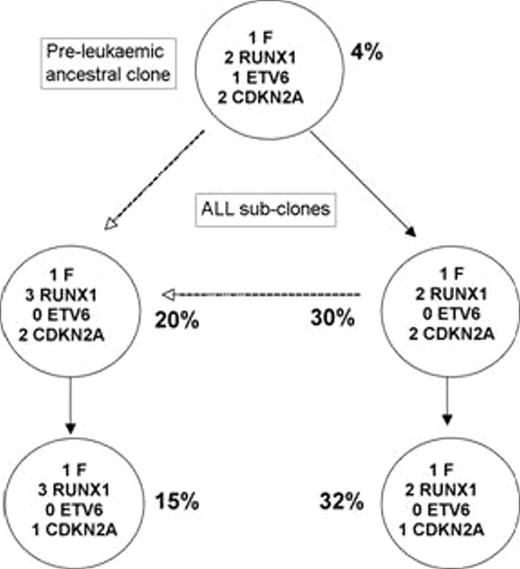
Intra-clonal, mutational complexity is a hallmark feature of cancer and provides the substrate for sub-clonal selection, progression and therapeutic resistance. There is limited insight however into detailed sub-clonal, genetic architecture in cancers and their propagating ’stem’ cells. Childhood acute lymphoblastic leukaemia (ALL) genotypes are characterized by chimeric fusion genes (or hyperdiploidy) coupled with recurrent, copy number alterations (CNA), primarily in genes regulating cell cycle or differentiation. For ETV6-RUNX1-positive cases of B precursor ALL, the temporal sequence of events is known with the fusion gene usually arising as a pre-natal, initiating event. Recurrent CNA, presumed to be functional or ’driver’ mutations, are secondary to gene fusion and probably post-natal in origin. We have interrogated clonal architecture by multiplexed FISH analysis of single cells using probes labelled with three or four distinct fluorochromes. We pre-selected diagnostic samples from 30 ETV6-RUNX1-positive cases that we found to have deletion of ETV6 and CDKN2A or PAX5 or combinations of these. The application of multiple FISH probes allowed us to score all cells (200 per patient sample) for up to six genetic lesions: ETV6-RUNX1 fusion (and multiple copies of the fusion), deletion of the unrearranged ETV6 allele, extra copies of chromosome 21q (via RUNX1 signals), and one or two copy deletions of PAX5 and CDKN2A. The genetic classification of individual cells using this method allowed a designation of sub-clones and the assembly of putative ancestral, or evolutionary, trees. Although sub-clones were previously known to exist at diagnosis of ALL, the extent of sub-clonal diversity revealed by this study was unanticipated and very marked. Sub-clones, of varying sizes (1–90% of total cells), numbered 3 to 14 per case. This must be an under-estimate of genetic complexity as only selected mutations were included in the screen. The common CNA (ETV6, CDKN2A and PAX5 deletions, chromosome 21q gains) arose independently and recurrently in sub-clones and in no preferential order. Matched relapse and diagnosis pairs were available on 5 patients. In all of these, the clones at relapse could be matched to individual sub-clones at diagnosis, with relapse originating from a major or minor subclone at diagnosis. Importantly, the relapse clone often diversified further, reiterating the evolution pathway of the primary clones. These data indicate that sub-clonal diversification does not arise via linear, clonal succession but rather has a complex, branching architecture reminiscent of Charles Darwin’s 1837 evolutionary speciation model. The ancestral trees so revealed in ALL are single time point snapshots and therefore disguise temporal or sequential dynamics. We show that sub-clonal architecture changes in the months leading up to a diagnosis of ALL and is re-ordered by treatment and subsequent relapse. An important prediction derived from this pattern of sub-clonal architecture is that leukaemia propagating or ’stem’ cells in ALL (and other cancers) should themselves be genetically diverse. Preliminary transplantation experiments in NOD/SCID/IL2Rgamma(null) mice show that this is the case, since multiple, genetically distinct sub-clones regenerated in vivo (Figure1). The complexity of genetic architecture in ALL has substantial implications for the cancer stem cell concept and for the efficacy of generic or targeted treatments.
No relevant conflicts of interest to declare.
Sub-clones present in diagnostic sample before intratibial injection into NOD/SCID/IL2Rgamma(null) mice, assayed by 3 colour FISH for the ETV6-RUNX1 gene fusion, ETV6 deletion, RUNX1 gain and CDKN2A deletion. All four leukaemic subclones were present in the mice after leukaemic engraftment at 10 -12 weeks, but with the CDKN2A deleted sub-clones predominant.
Sub-clones present in diagnostic sample before intratibial injection into NOD/SCID/IL2Rgamma(null) mice, assayed by 3 colour FISH for the ETV6-RUNX1 gene fusion, ETV6 deletion, RUNX1 gain and CDKN2A deletion. All four leukaemic subclones were present in the mice after leukaemic engraftment at 10 -12 weeks, but with the CDKN2A deleted sub-clones predominant.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal