In this issue of Blood, McEwan and colleagues describe the structure of a complex between GPIbα and a small-molecule antagonist and elucidate a novel allosteric mechanism for targeting this receptor's interaction with von Willebrand factor.1
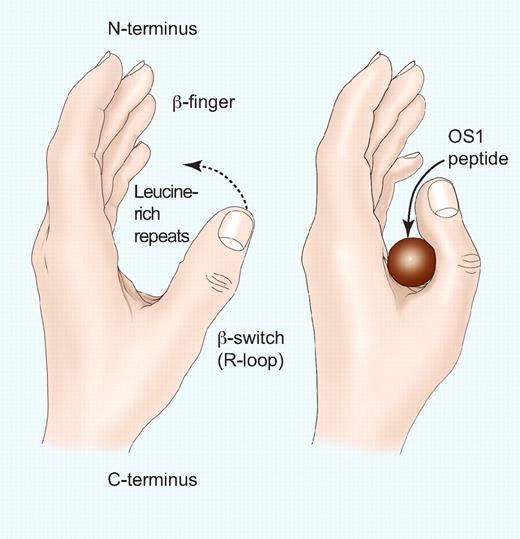
In recent years, platelet receptors have been successfully targeted by small-molecule antithrombotic drugs, with the integrin αIIbβ3 and the G-protein–coupled receptor P2Y12 being prime examples. Of the two, P2Y12 is a much more traditional small-molecule target, with drugs such as clopidogrel and prasugrel interfering with the ability of another small molecule, adenosine diphosphate, to signal to induce platelet aggregation. A priori, targeting the interaction of αIIbβ3 with either fibrinogen or von Willebrand factor (VWF) would seem much more problematic, the small molecule having to disrupt the interaction of 2 very large proteins. In this case, however, the problem is simplified by the fact that short, continuous stretches of amino acids (Arg-Gly-Asp-Ser, RGDS, in both fibrinogen and VWF, and the related HHLGGAKQAGDV γ-chain sequence in fibrinogen) make up the primary binding sites for αIIbβ3 on these ligands; this formed the basis of strategies to target the receptor. Eptifibatide, an αIIbβ3 antagonist that is based on a sequence from the pit viper protein barbourin, contains a (KGD) sequence with greater specificity for αIIbβ3 than the canonical RGD sequence.2 However, only a relatively small percentage of protein-protein interactions have such small interaction “hot spots” that contribute so much to the total binding energy of the interaction.2 The interaction between glycoprotein (GP) Ibα and VWF, which mediates the first step of platelet adhesion to sites of vessel injury, seems a particularly difficult small-molecule target, given that the interactive surface between this receptor-ligand pair covers 2600 Å,3 an enormous area for a small molecule to cover.4 Within these 2 large proteins the interaction sites reside in smaller domains, in GPIbα in a 290–amino acid sequence at the polypeptide's N-terminus, and in VWF entirely within the 186–amino acid A1 domain. In this region of GPIbα, important sequences include an N-terminal disulfide loop known as the β-finger, and a C-terminal disulfide loop called the β-switch or regulatory (R)-loop (see figure).In the complex of the 2 proteins, the R-loop forms a β-hairpin that contributes 2 strands to an 8-stranded β-sheet containing sequences from both molecules. The R-loop is also the site of gain-of-function mutations that produce the rare bleeding disorder platelet-type von Willebrand disease, where they produce mutant GPIbα molecules capable of interacting with VWF in the absence of the usual requirements of shear stress or modulators such as ristocetin.
Representation of the GPIbα N-terminus and the effect of the OS1 peptide. The fingertips represent the N-terminal β-finger, the palm represents the concave β-sheet formed by the 8 leucine-rich repeats, and the thumb represents the β–switch (R-loop). (Left) Unoccupied GPIbα with the thumb extended to interact with the A1 domain of VWF. (Right) Binding of the OS1 peptide causes the thumb to close across the palm, thereby preventing the interaction with the A1 domain of VWF. Professional illustration by Paulette Dennis.
Representation of the GPIbα N-terminus and the effect of the OS1 peptide. The fingertips represent the N-terminal β-finger, the palm represents the concave β-sheet formed by the 8 leucine-rich repeats, and the thumb represents the β–switch (R-loop). (Left) Unoccupied GPIbα with the thumb extended to interact with the A1 domain of VWF. (Right) Binding of the OS1 peptide causes the thumb to close across the palm, thereby preventing the interaction with the A1 domain of VWF. Professional illustration by Paulette Dennis.
Despite the many obstacles to developing small-molecule inhibitors of the GPIbα–VWF interaction, Benard et al were able to identify several that inhibited the interaction under static conditions and flow by screening a phage-display library of 11–amino acid cystine-constrained peptides that bind GPIbα.5 One of the optimized peptides, OS-1, bound GPIbα with very high affinity, the KD being 0.74nM.
A clue that the OS-1 peptide might disrupt the GPIbα-VWF interaction by interfering with the R-loop was provided by the finding that the peptide's affinity for GPIbα was reduced markedly in the presence of platelet-type VWD mutations.5 Now, the riddle of OS-1's mechanism of action has been solved, and the solution appears in this issue of Blood. McEwan et al determined the crystal structure of OS-1 in complex with GPIbα and found that, unlike the situation with RGD- or KGD-based inhibitors of αIIbβ3, OS-1 does not mimic the binding of VWF to GPIbα. Instead, the cyclic peptide inserts within a pocket formed by the lower part of a concave β-sheet formed by leucine-rich repeats 3 to 7 of GPIbα and the R-loop (see figure). In engaging the R-loop, OS-1 induces a tight helical structure that presumably prevents formation of the β-hairpin and the bimolecular β-sheet observed in the GPIbα-VWF complex. Thus, the mechanism of inhibition appears to have a large allosteric component, a conclusion that would have been very difficult to make without the structure. Many questions remain to be answered before OS-1 or its derivatives can be applied as antithrombotics, but the elegant structural work of McEwan et al suggests a very interesting, heretofore unrecognized, mechanism for targeting GPIbα with small molecules.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal