Abstract
Nilotinib has a higher binding affinity and selectivity for BCR-ABL with respect to imatinib and is an effective treatment of chronic myeloid leukemia (CML) after imatinib failure. In a phase 2 study, 73 early chronic-phase, untreated, Ph+ CML patients, received nilotinib at a dose of 400 mg twice daily. The primary endpoint was the complete cytogenetic response (CCgR) rate at 1 year. With a median follow-up of 15 months, the CCgR rate at 1 year was 96%, and the major molecular response rate 85%. Responses were rapid, with 78% CCgR and 52% major molecular response at 3 months. During the first year, the treatment was interrupted at least once in 38 patients (52%). The mean daily dose ranged between 600 and 800 mg in 74% of patients, 400 and 599 mg in 18% of patients, and was less than 400 mg in 8% of patients. Dose interruptions were mainly due to nonhematologic and biochemical side effects. Myelosuppression was irrelevant. One patient progressed to blastic crisis after 6 months; one went off-treatment for lipase increase grade 4 (no pancreatitis). Nilotinib is safe and very active in early chronic-phase CML. These data support a role for nilotinib for the frontline treatment of CML. This study was registered at ClinicalTrials.gov as NCT00481052.
Introduction
The development of imatinib (IM), a small molecule targeting the protein tyrosine kinase (PTK) that is coded by the BCR-ABL fusion gene on the Philadelphia chromosome (Ph), has revolutionized the treatment of Ph+ chronic myeloid leukemia (CML) and has radically modified the prognosis and the outcome of the disease.1-4 The patients who are treated with IM 400 mg daily achieve a complete cytogenetic response (CCgR) in 65% to 85% of cases, and a major molecular response (MMolR) in 40% to 60% of cases. Responses are stable, and 5- to 7-year survival free from progression or relapse ranges between 65% and 85%.5-11 However, 15% to 20% of patients are or become resistant to IM, and most patients continue to harbor detectable, though minimal, residual disease.3,4,10,12-17 Although mostly in a quiescent state, Ph+ stem cells are detectable even in optimal responders, and BCR-ABL kinase domain (KD) point mutations may be also found both in patients who are protein tyrosine kinase (PTK) inhibitors (PTKI) naive patients, as well as in optimal responders.18-23 Other BCR-ABL targeting agents were rapidly developed, such as nilotinib, dasatinib, bosutinib, and inno-406,24-28 all having different degrees of potency and selectivity, and different pharmacokinetic and pharmacodynamic properties. Nilotinib and dasatinib have already been registered for the treatment of IM-resistant and IM-intolerant patients, to whom they offer a significant therapeutic benefit.29-32 Nilotinib is more selective for BCR-ABL than any other PTKIs.25 Moreover, the concentrations of nilotinib that are required to inhibit the in vitro growth of Ph+ cells are many fold lower than those required for IM.24-26,28 The mean trough blood concentration of nilotinib (889 ng/mL, for a dose of 400 mg twice daily) is much higher than the concentrations required to inhibit the growth of Ph+ cells carrying most of known BCR-ABL KD point mutations, with the exception of T315.33 The characteristics of these second generation PTKIs are such that they may represent an important advance also as a frontline therapy. For these reasons, the GIMEMA CML Working Party designed and conducted a study of nilotinib in the frontline therapy of previously untreated, early chronic-phase Ph+ CML. A preliminary report of this study was presented at the 2008 Meeting of the American Society of Hematology.34
Methods
Study protocol (ClinicalTrial.gov NCT00514488)
Patients were eligible for the study if they were 18 years of age or older, had received a diagnosis of chronic-phase Ph+ CML within less than 6 months before study entry, and were untreated or treated only with hydroxyurea or anagrelide. The screening procedures included medical history, physical examination, electrocardiogram, echocardiogram, chest x-ray, hepatitis C virus (HCV)/hepatitis B virus (HBV)/HIV serology, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin and creatinine, blood counts and differential, bone marrow aspirate, cytogenetics, and the determination of BCR-ABL transcript type and level. Women who were breastfeeding, pregnant, or of childbearing potential without a negative pregnancy test were not enrolled. Patients were excluded if their WHO performance status was 2 or more, they had other uncontrolled serious medical conditions, or they had received prior treatment with any investigational agent. The study protocol was approved by the ethic committees of all participating centers and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent.
Once the screening procedures were completed, all patients were assigned to receive nilotinib 400 mg twice daily, after at least 1 hour of fasting condition. No dose escalation was allowed. The core trial time was 1 year, during which the treatment was continued at the assigned dose. In case of adverse events (AE), the dose was adapted, or treatment was discontinued (Table 1). After 1 year of treatment, all patients were allowed to continue on nilotinib.
Protocol guidelines for nilotinib dose adaptation according to hematologic and nonhematologic toxicity
| Guidelines for nilotinib dose adaptation . | |
|---|---|
| For hematologic toxicity | |
| Grade 1 and 2: ANC > 1.0 × 109/L Platelets > 50 × 109/L | No dose reduction |
| Grade 3 and 4: 1st and 2nd time | Discontinue nilotinib and resume (800 mg daily) when grade < 3 |
| Grade 3 and 4: 3rd time | Discontinue nilotinib and resume (400 mg daily) when grade < 3, and 800 mg after 1 wk |
| Grade 3 and 4: 4th time | Discontinue nilotinib and resume (400 mg daily) when grade < 3, and 800 mg after 1 mo |
| Grade 3 and 4: 5th time | Discontinue nilotinib |
| For nonhematologic toxicity, noncardiac | |
| Grade 1 | No dose reduction |
| Grade 2 and 3: 1st and 2nd time | Discontinue nilotinib and resume (800 mg daily) when grade < 2 |
| Grade 2 and 3: 3rd time | Discontinue nilotinib and resume (400 mg daily) when grade < 2 and 800 mg after 1 wk |
| Grade 2 and 3: 4th time | Discontinue nilotinib and resume (400 mg daily) when grade < 2 |
| Grade 4 | Discontinue nilotinib permanently* |
| For nonhematologic toxicity, cardiac | |
| QTc prolongation up to 499 msec | Discontinue nilotinib and resume (400 mg daily) when QTc < 450 msec |
| QTc prolongation > 500 msec | Discontinue nilotinib permanently |
| Guidelines for nilotinib dose adaptation . | |
|---|---|
| For hematologic toxicity | |
| Grade 1 and 2: ANC > 1.0 × 109/L Platelets > 50 × 109/L | No dose reduction |
| Grade 3 and 4: 1st and 2nd time | Discontinue nilotinib and resume (800 mg daily) when grade < 3 |
| Grade 3 and 4: 3rd time | Discontinue nilotinib and resume (400 mg daily) when grade < 3, and 800 mg after 1 wk |
| Grade 3 and 4: 4th time | Discontinue nilotinib and resume (400 mg daily) when grade < 3, and 800 mg after 1 mo |
| Grade 3 and 4: 5th time | Discontinue nilotinib |
| For nonhematologic toxicity, noncardiac | |
| Grade 1 | No dose reduction |
| Grade 2 and 3: 1st and 2nd time | Discontinue nilotinib and resume (800 mg daily) when grade < 2 |
| Grade 2 and 3: 3rd time | Discontinue nilotinib and resume (400 mg daily) when grade < 2 and 800 mg after 1 wk |
| Grade 2 and 3: 4th time | Discontinue nilotinib and resume (400 mg daily) when grade < 2 |
| Grade 4 | Discontinue nilotinib permanently* |
| For nonhematologic toxicity, cardiac | |
| QTc prolongation up to 499 msec | Discontinue nilotinib and resume (400 mg daily) when QTc < 450 msec |
| QTc prolongation > 500 msec | Discontinue nilotinib permanently |
Notify treatment advisory committee.
Treatment and response monitoring required a physical examination, blood counts, and differential and blood chemistry every 15 days for 3 months, then monthly for 1 year, a bone marrow aspirate with cytogenetics after 3, 6, and 12 months, and a quantitative determination of BCR-ABL transcripts level in peripheral blood after 1, 2, 3, 6, 9, and 12 months. An electrocardiogram was required twice during the first month, then at month 2, 3, 5, 7, and 12. An echocardiogram was required at 12 months. All data were collected through electronic case report forms, but all sites were monitored directly by trained personnel for data quality and consistency.
Definition of phase, risk, and response
The accelerated (AP) and blast phase (BP) were identified according to European LeukemiaNet criteria.3 In particular, BP was identified by a percentage of blast cells (≥ 30%), or blast cells and promyelocytes (≥ 50%), respectively, or by any extramedullary blast involvement, excluding spleen and liver. The relative risk (RR) was calculated and defined according to Sokal et al35 and Hasford et al.36 The hematologic response (HR) and the cytogenetic response (CgR) were defined according to ELN criteria.3 The molecular response was defined as major (MMolR) if the BCR-ABL:ABL ratio was less than 0.10% on the international scale, in which 0.10% corresponds to a 3-log reduction from a standard baseline level as defined by the results of the IRIS study.3,6,37 Treatment failures were defined, according to the ELN criteria,3 as absence of HR at 3 months, less than CHR or no CgR at 6 months, less than PCgR at 12 months, less than CCgR at 18 months, loss of a CHR, or loss of a CCgR.
Cytogenetics
Cytogenetic studies were performed by chromosome banding analysis of at least 20 marrow cell metaphases, after short-term culture (24 and/or 48 hours) with standard G or Q banding techniques. If marrow cells could not be obtained, or the number of metaphases was less than 20, fluorescence in situ hybridization of interphase cells was performed with BCR-ABL extra-signal, dual-color, dual-fusion probes (Vysis LSI BCR-ABL Translocation Probe; Abbott Molecular), counting at least 200 nuclei. The response was classified as complete only when BCR-ABL+ nuclei were 0 or 1 (< 1%).38,39
BCR-ABL transcript level
BCR-ABL transcript level assessment was performed by real-time quantitative polymerase chain reaction according to suggested procedures and recommendations.37,40 ABL was used as a control gene. All analyses were performed on an ABI PRISM 7900 Sequence Detection System (Applied Biosystems). BCR-ABL transcripts level was expressed as a percentage according to the international scale, taking advantage of the ongoing international initiatives that allow to standardize the quantitation of BCR-ABL transcripts through the use of a conversion factor and consequently to express their results according to the scale.41-43 The lower detection limit of the assay is 10−4. In patients with undetectable (< .0001) BCR-ABL transcript levels by real-time quantitative polymerase chain reaction, a nested reverse transcription–polymerase chain reaction was run with a lower detection limit of 10−6. The reference laboratory that performed all molecular analyses on this study was located in Bologna (conversion factor, 0.6), and the data were checked and validated (10 samples at 3, 6, 9, and 12 months) in Orbassano and Naples.
Statistics
The primary endpoint of the study was the CCgR rate at 1 year. This is a binary variable in which each patient is classified at 1 year as a CCgR or not, according to the intention-to-treat principle. Based on the expectation that the CCgR rate after 12 months of standard IM treatment would range between 50% and 70%, and using a Fleming single-stage design (62), we set p0 (as the proportion of responses below which the treatment would be considered of no interest) at 50% and p1 (as the proportion of responses above which the treatment would be considered of interest) at 70%. With a one-side alpha error of 0.025 and a power 1-beta of 90%, the number of patients to be enrolled was 6544 and was adjusted to 70 to account for dropouts and withdrawals. No subgroup analysis was planned.
Results
A total of 76 patients were screened; 2 patients refused and 1 did not fit the eligibility criteria because of severe coronary hearth disease. A total of 73 patients fit the eligibility criteria and were enrolled over an 8-month period, between June 2007 and February 2008, in 18 GIMEMA clinical centers. This report is based on the data collected up to April 2009, with a median follow-up of 15 months (range, 12-24 months). Main baseline data are reported in Table 2. Hematologic and cytogenetic responses are shown in Table 3. The CHR rate was 100% at 3 months, 99% at 6 months, and 97% at 12 months. The CCgR rate was 78% at 3 months, and 96% at 6 and 12 months. Only 1 patient so far, who had achieved a CCgR after 3 months from treatment start, relapsed and progressed to BP at month 6, with a T315I BCR-ABL KD mutation.
Characteristics of patients
| Characteristics . | . | Value . | . |
|---|---|---|---|
| Total no. of patients | 73 | ||
| Median age, y (range) | 51 (18-83) | ||
| Patients 60 y or older, n (%) | 26 (36%) | ||
| Males | 37 (51%) | ||
| Relative risk | Sokal35 | Hasford36 | |
| Low, n (%) | 33 (45%) | 29 (40%) | |
| Intermediate, n (%) | 30 (41%) | 43 (59%) | |
| High, n (%) | 10 (14%) | 1 (1%) | |
| Variant translocations | 10 (14%) | ||
| Clonal chromosome abnormalities in Ph+ cells, n (%) | 3 (4%) | ||
| Del9q+, n (%) | 7 (10%) |
| Characteristics . | . | Value . | . |
|---|---|---|---|
| Total no. of patients | 73 | ||
| Median age, y (range) | 51 (18-83) | ||
| Patients 60 y or older, n (%) | 26 (36%) | ||
| Males | 37 (51%) | ||
| Relative risk | Sokal35 | Hasford36 | |
| Low, n (%) | 33 (45%) | 29 (40%) | |
| Intermediate, n (%) | 30 (41%) | 43 (59%) | |
| High, n (%) | 10 (14%) | 1 (1%) | |
| Variant translocations | 10 (14%) | ||
| Clonal chromosome abnormalities in Ph+ cells, n (%) | 3 (4%) | ||
| Del9q+, n (%) | 7 (10%) |
All patients were enrolled within less than 6 months from diagnosis (median 1 month). Fifty-three patients (73%) received a short course of hydroxyurea before nilotinib.
Hematologic response, cytogenetic response, discontinuation for AE, and failure
| . | 3 mo,n (%) . | 6 mo, n (%) . | 12 mo, n (%) . |
|---|---|---|---|
| No. of patients | 73 | 73 | 73 |
| HR | |||
| Complete | 73 (100) | 72 (98) | 71 (97) |
| Less than complete or lost | 0 | 1† (1) | 1† (1) |
| Nonevaluable | 0 | 0 | 1‡ (1) |
| CgR | |||
| Complete* | 57 (78) | 70 (96) | 70 (96) |
| Partial (Ph+ 1%-35%) | 5 (7) | 2 (3) | 1 (1) |
| Minor (Ph+ 36%-65%) | 5 (7) | 0 | 0 |
| Minimal (Ph+ 66%-95%) | 3 (4) | 0 | 0 |
| None or lost | 0 | 1† (1) | 1† (1) |
| Nonevaluable | 3 (4) | 0 | 1‡ (1) |
| Discontinued for AEs | 0 | 0 | 1‡ (1) |
| Failure | 0 | 1† (1) | 1† (1) |
| . | 3 mo,n (%) . | 6 mo, n (%) . | 12 mo, n (%) . |
|---|---|---|---|
| No. of patients | 73 | 73 | 73 |
| HR | |||
| Complete | 73 (100) | 72 (98) | 71 (97) |
| Less than complete or lost | 0 | 1† (1) | 1† (1) |
| Nonevaluable | 0 | 0 | 1‡ (1) |
| CgR | |||
| Complete* | 57 (78) | 70 (96) | 70 (96) |
| Partial (Ph+ 1%-35%) | 5 (7) | 2 (3) | 1 (1) |
| Minor (Ph+ 36%-65%) | 5 (7) | 0 | 0 |
| Minimal (Ph+ 66%-95%) | 3 (4) | 0 | 0 |
| None or lost | 0 | 1† (1) | 1† (1) |
| Nonevaluable | 3 (4) | 0 | 1‡ (1) |
| Discontinued for AEs | 0 | 0 | 1‡ (1) |
| Failure | 0 | 1† (1) | 1† (1) |
All rates are calculated on all 73 patients, according to the intention-to-treat principle.
HR indicates hematologic response; and CgR, cytogenetic response.
The assessment of CCgR was based on CBA of marrow cell metaphases in 90%, 85% and 80% of patients. It was based on I-FISH in 10%, 15%, and 20% of patients.
This patient was in CHR and CCgR at 3 months, but developed sudden BC (lymphoid, with the T315I mutation) and died at 9 months.
This patient discontinued nilotinib after 9 months, for persistent lipase increase, grade 3/4 (no pancreatitis).
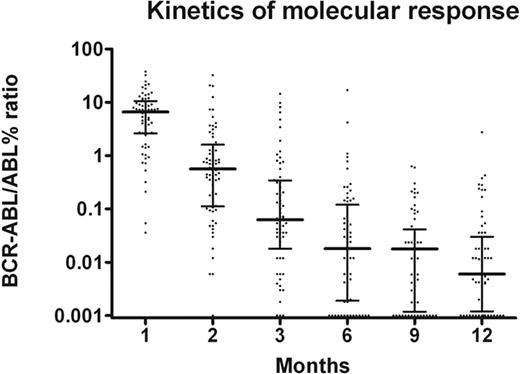
The molecular response is shown in Figure 1. Two patients achieved a MMolR after 1 month of treatment, and 15 (21%) after 2 months of treatment. The MMolR rate was 52% at 3 months, 66% at 6 months and reached 85% at 12 months, when the median BCR-ABL:ABL ratio was 0.006%. At month 12, 9 of 10 patients not in MMolR had a BCR-ABL:ABL ratio between 0.1% and 1.0%, and 1 patient had a BCR-ABL:ABL ratio above 1%. Five patients (7%) tested negative with nested PCR.
Kinetics of molecular response. The proportion of patients in MMolR was 3%, 20%, 52%, 66%, 73%, and 85%, at 1, 2, 3, 6, 9, and 12 months. The median BCR-ABL transcript level was 4.704, 0.456, 0.063, 0.018, 0.018, and 0.006, at 1, 2, 3, 6, 9, and 12 months.
Kinetics of molecular response. The proportion of patients in MMolR was 3%, 20%, 52%, 66%, 73%, and 85%, at 1, 2, 3, 6, 9, and 12 months. The median BCR-ABL transcript level was 4.704, 0.456, 0.063, 0.018, 0.018, and 0.006, at 1, 2, 3, 6, 9, and 12 months.
The nonhematologic AEs are listed in Tables 4 and 5. Skin rash, with pruritus, and bone/muscle/joint pain were more common, being grade 3 in 5% and 4% of patients (Table 4). Peripheral edema was rare, 4% overall and never grade 3. Biochemical laboratory abnormalities were more frequent, particularly bilirubin increase, 53% all grades and 16% grade 3 (Table 5). Pancreatic enzymes were increased in 29% (lipase) and 18% (amylase) of patients. No patient reported clinical symptoms or had any evidence of pancreatitis. Lipase elevation reached grade 4 in 3 patients, and one of these patients discontinued nilotinib after 9 months for this reason. This patient, who was in CCgR and MMolR at time of nilotinib discontinuation, is currently on imatinib 400 mg daily, as a second-line treatment, maintaining the CCgR and the MMolR (without any evidence of lipase increase). Hyperglycemia was not frequent, 12% all grades and 3% grade 3; all the episodes of hyperglycemia were transitory and clinically irrelevant. Anemia, neutropenia, and thrombocytopenia were exceedingly rare (Table 6), and this was confirmed by the careful review of all blood counts and differentials that were due every 2 weeks during the first quarter of therapy.
Nonhematologic adverse events
| . | This study (n = 73) . | Kantarjianet al (n = 280)32 . | |
|---|---|---|---|
| All grades, % . | Grade 3, % . | Grade 3/4, % . | |
| Skin rash | 42 | 5 | 8 |
| Bone/muscle/joint pain | 41 | 4 | 2 |
| Headache | 30 | 2 | |
| Dry eye/conjunctivitis | 23 | NR | |
| Fatigue | 22 | 1 | |
| Pruritus | 21 | 4 | 1 |
| Gastric pain | 19 | NR | |
| Nausea/vomiting | 11 | 1 | |
| Fever | 11 | NR | |
| Abdominal pain | 8 | NR | |
| Diarrhea | 7 | 2 | |
| Peripheral edema | 4 | NR | |
| . | This study (n = 73) . | Kantarjianet al (n = 280)32 . | |
|---|---|---|---|
| All grades, % . | Grade 3, % . | Grade 3/4, % . | |
| Skin rash | 42 | 5 | 8 |
| Bone/muscle/joint pain | 41 | 4 | 2 |
| Headache | 30 | 2 | |
| Dry eye/conjunctivitis | 23 | NR | |
| Fatigue | 22 | 1 | |
| Pruritus | 21 | 4 | 1 |
| Gastric pain | 19 | NR | |
| Nausea/vomiting | 11 | 1 | |
| Fever | 11 | NR | |
| Abdominal pain | 8 | NR | |
| Diarrhea | 7 | 2 | |
| Peripheral edema | 4 | NR | |
The frequency of grade 3 nonhematologic AEs in these 73 early CP patients is compared with the data previously reported in 280 IM-resistant or intolerant patients who were treated with nilotinib in late CP.32
NR indicates not reported.
Biochemical laboratory abnormalities
| . | This study (n = 73) . | Kantarjian et al (n = 280)32 . | |
|---|---|---|---|
| All grades, % . | Grade 3, % . | Grade 3/4, % . | |
| Bilirubin (total) | 53 | 16 | 9 |
| ALT (GPT) | 42 | 8 | 4 |
| γ -GT | 36 | 7 | NR |
| AST (GOT) | 29 | 3 | 1 |
| Alkaline phosphatase | 11 | NR | |
| Lipase | 29 | 8* | 14 |
| Amylase | 18 | 4 | NR |
| Hyperglycemia | 12 | 3 | 12 |
| Hypophosphatemia | 10 | 3 | 15 |
| Creatinine | 7 | NR | |
| Hypocalcemia | 5 | NR | |
| . | This study (n = 73) . | Kantarjian et al (n = 280)32 . | |
|---|---|---|---|
| All grades, % . | Grade 3, % . | Grade 3/4, % . | |
| Bilirubin (total) | 53 | 16 | 9 |
| ALT (GPT) | 42 | 8 | 4 |
| γ -GT | 36 | 7 | NR |
| AST (GOT) | 29 | 3 | 1 |
| Alkaline phosphatase | 11 | NR | |
| Lipase | 29 | 8* | 14 |
| Amylase | 18 | 4 | NR |
| Hyperglycemia | 12 | 3 | 12 |
| Hypophosphatemia | 10 | 3 | 15 |
| Creatinine | 7 | NR | |
| Hypocalcemia | 5 | NR | |
The frequency of grade 3 nonhematologic AEs in these 73 early CP patients is compared with the data previously reported in 280 IM-resistant or intolerant patients who were treated with nilotinib in late CP.32
NR indicates not reported.
Percentages: 4% grade 3 and 4% grade 4.
Hematologic adverse events
| . | Grade 2, % . | Grade 3, % . | Grade 4, % . |
|---|---|---|---|
| Anemia | 16 | ||
| Neutropenia | 10 | 3 | 1 |
| Thrombocytopenia | 1 | 1 | 1 |
| . | Grade 2, % . | Grade 3, % . | Grade 4, % . |
|---|---|---|---|
| Anemia | 16 | ||
| Neutropenia | 10 | 3 | 1 |
| Thrombocytopenia | 1 | 1 | 1 |
Overall, the number of patients with at least one AE grade 3/4 was 5 (7%), and the number of patients with at least on AE grade 2, 3, or 4, was 21 (29%). All the hematologic AEs were identified during the first quarter of therapy. Their identification was based on 431 blood counts which were due out of 438 counts (per protocol, blood counts and differentials were due every 15 days during the first quarter). The frequency of all grade 3/4 AEs reported in 280 CP patients who were treated with nilotinib second line32 was higher (29% neutropenia and thrombocytopenia, 10% anemia).
Biochemical laboratory abnormalities and, less frequently, nonhematologic AEs, led to temporary dose interruption in 38 patients (52%; Table 7). The median cumulative duration of dose interruptions was 19 days (range, 3-169 days). Overall, 74% of patients received a mean daily dose ranging between 600 and 800 mg, while 16% of patients received 400 to 600 mg and 8% less than 400 mg. All 584 electrocardiograms were checked and reviewed; transient and clinically irrelevant abnormalities were noticed in 16 patients (22%). In 2 patients, the duration of QTc was prolonged to more than 450 msec, but in no case was QTc longer than 500 msec.
Dose reductions and interruptions
| Mean daily dose . | No. of patients . | |||
|---|---|---|---|---|
| Overall . | 1st quarter . | 2nd quarter . | 2nd half . | |
| 600-800 mg, n (%) | 54 (74) | 60 (82) | 55 (75) | 51 (71) |
| 400-599 mg, n (%) | 13 (18) | 8 (11) | 14 (19) | 15 (21) |
| Less than 400 mg, n (%) | 6 (8) | 5 (7) | 4 (5) | 6 (8) |
| Patients with dose interruption, n (%) | 38 (52) | 27 (37) | 18 (25) | 16 (22) |
| No. of interruptions | 86 | 41 | 25 | 27 |
| Median cumulative duration of interruptions, days (range) | 19 (3-169) | 13 (1-48) | 12 (5-48) | 20 (5-99) |
| Mean daily dose . | No. of patients . | |||
|---|---|---|---|---|
| Overall . | 1st quarter . | 2nd quarter . | 2nd half . | |
| 600-800 mg, n (%) | 54 (74) | 60 (82) | 55 (75) | 51 (71) |
| 400-599 mg, n (%) | 13 (18) | 8 (11) | 14 (19) | 15 (21) |
| Less than 400 mg, n (%) | 6 (8) | 5 (7) | 4 (5) | 6 (8) |
| Patients with dose interruption, n (%) | 38 (52) | 27 (37) | 18 (25) | 16 (22) |
| No. of interruptions | 86 | 41 | 25 | 27 |
| Median cumulative duration of interruptions, days (range) | 19 (3-169) | 13 (1-48) | 12 (5-48) | 20 (5-99) |
The percentage of patients who received a different cumulative median daily dose of nilotinib in the first quarter, in the second quarter, in the second half, and overall is shown. At 12 months, the number of patients receiving the full dose of 800 mg daily was 51 (71%), the number of patients with a permanent dose reduction to 400 mg was 18 (25%), and the number of patients receiving less than 400 mg was 3 (4%).
No subgroup analysis had been planned, and no subgroup analysis was possible, also because of the high response rate. However, it may be noted that the CCgR rate of the 10 high-Sokal-risk patients was 50% at 3 months and that the only one patient who failed and developed BP was high risk. It should also be noted that 5 patients took a mean daily dose of less than 400 mg in the first quarter of therapy and that at the end of this quarter “only” 2 of them were in CCgR, while 1 was in PCgR and 2 were in less than PCgR. However, after 12 months of therapy, all these 5 patients were in CCgR.
Discussion
This study provides important new information on the early therapeutic effects of nilotinib, 400 mg twice daily, in previously untreated, early chronic phase Ph+ CML patients. The results are straightforward, because after 1 year of treatment all but 2 patients were in CCgR and 62 of 73 patients (85%) were in MMolR. Only 1 patient discontinued the treatment due to an AE (persistent and recurrent lipase elevation grade 3/4, without any clinical consequence), and only 1 patient failed. Moreover, it was shown that both the CgR and the MolR were achieved very rapidly, with 78% of patients already in CCgR and 52% of patients already in MMolR, after only 3 months of therapy. All these responses were durable through study duration.
A high and rapid response rate was not unexpected, because the phase-2 studies had already shown that in IM-resistant, late chronic-phase patients with nilotinib a major (complete plus partial) CgR could be achieved in approximately 50% of patients and that the median time to response was less than 3 months.32,45 However, the magnitude and rapidity of the response in this frontline study of nilotinib was greater than expected. It cannot be explained by a single-center effect, because the patients were enrolled competitively in 18 clinical centers. However, all centers were experienced in the management of CML with PTKIs, including nilotinib second-line. It should also be noted that patients distribution by risk was shifted, in that the proportion of high-risk patients was less than expected. This was likely due to chance, as a result of the relatively low number of patients enrolled because no selection occurred. High-risk (Sokal) patients were 10; one of them progressed to BP, and the remaining 9 were in CCgR at 12 months. An independent, single-center study of nilotinib frontline in ECP CML has provided similar cytogenetic response rates.46
Nilotinib is more potent and more selective toward BCR-ABL than IM.24-28 The mean trough blood level that is achieved with 400 mg twice daily is many fold higher than the concentration that is required to inhibit the growth of 50% of cultured Ph+ cells, wild-type or mutated.33 The flux of nilotinib into CML cells is not dependent upon transporter proteins, as in the case of IM.47,48 Therefore, although a single-arm study of a relatively small number of patients cannot yet provide sufficient evidence and cannot allow comparisons with other therapies, the results are such that they raise the legitimate expectation that a prospective randomized study of nilotinib versus IM may confirm that in the short-term nilotinib is superior to IM. One such study is ongoing (ClinicalTrials.gov NCT00471497). A superiority of nilotinib in the short term could contribute to a better long-term outcome, because on IM therapy, most failures occur during the first 3 years of treatment.7-9
In this study, the frequency and the degree of nonhematologic and biochemical AEs was as expected from phase 2 studies,32,45 with few minor and irrelevant differences (Tables 4 and 5), which may be easily explained by the fact that all patients were in ECP and previously untreated. Only grade 3/4 hyperglycemia and hypophosphatemia were significantly less frequent than previously reported32,45 (3% vs 12% and 3% vs 15%, respectively; Table 5). Most dose discontinuations, interruptions, and reductions were guided either by clinically relevant side effects, mainly skin and bone-muscle-joint pain, or more frequently by clinically uneventful biochemical abnormalities, mainly bilirubin and pancreatic enzymes elevation. We were surprised that the degree of hematopoietic toxicity (anemia, neutropenia, and thrombocytopenia) was lower than expected from the earlier results of phase 2 studies32,45 (Table 6). The rarity of hematologic AEs may be explained again by the fact that all patients were previously untreated and in ECP. We also think that the dose interruptions and reductions, which were due to biochemical abnormalities, prevented the occurrence of hematologic toxicity. As a matter of fact, the treatment was interrupted at least once in 52% of patients, and 26% of patients took a mean daily dose lower than 600 mg. Because all patients but 2 achieved a CCgR, the question may be asked if a dose of 400 mg twice daily is necessary, suggesting to explore if a lower dose can be as effective and more easily manageable, particularly in low-risk patients.
We conclude that nilotinib 400 mg twice daily is safe and highly effective, in ECP, previously untreated Ph+ CML patients, provided that the dose is properly adjusted to account for nonhematologic and biochemical side effects. The results obtained within the frame of a controlled trial need confirmation in the clinical practice. The response rates are such that nilotinib is expected to be more effective and more rapid than IM in the short term. Because it has been shown that with IM most primary and secondary failures occur during the first 2 years of therapy,7-9 it is possible that a more rapid response may help to reduce failures and also improve the late outcome of therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Nilotinib was provided free of charge by Novartis Oncology Italy. We particularly acknowledge the cooperation of D. Alberti, F. Bartucci, and G. Boano. We strongly acknowledge the skilled help of Patrizia Beccari and Maddalena Fiorentino (CRO, IONsrl, Bologna) who monitored the study, Katia Vecchi and Lucia Toni who contributed to data collection, and Antonio de Vivo who supervised all informatic issues.
This work was supported by grants from The Italian Association Against Leukemia-Lymphoma and Myeloma (BolognAIL), The Fondazione del Monte di Bologna e Ravenna, The Italian Ministry of Education (PRIN 2005, No. 20 050 63732_003, and PRIN 2007, No. 2007F7 AE7B_002), The University of Bologna, and The European Union (European LeukemiaNet, 6th Framework Program, Contract No. 503216).
Authorship
Contribution: G.R., M. Baccarani, G.A., G.S., F. Pane, and G.M. designed and supervised the trial; G.R. and M. Baccarani drafted the manuscript; G.R., F.C., M. Baccarani, and G.G. analyzed the data; F. Palandri, F.C., M. Breccia, L.L., G.G., A.C., M.C., C.F., G.R.C., T.I., F.S., and M.T. enrolled the study patients and collected clinical data; M.A., A.P., and S.S. performed molecular analysis; N.T. and S.L. performed cytogenetic analysis; and all authors gave final approval for submission.
Conflict-of-interest disclosure: G.R. is a consultant with Novartis and serves on the speakers' bureaus of Novartis and Bristol-Myers Squibb. M. Baccarani receives research support from Novartis, Bristol-Myers Squibb, and Wyeth-Lederle; is a consultant for Novartis, Bristol-Myers Squibb, and Wyeth-Lederle; and serves on the Novartis speaker's bureau. G.M. serves on the speakers' bureaus of Novartis, Bristol-Myers Squibb, and Wyeth-Lederle. F. Pane receives research support from Novartis; is a consultant for Novartis and Bristol-Myers Squibb; and serves on the Novartis speaker's bureau. G.S. is a consultant for Novartis and Bristol-Myers Squibb and serves on the speakers' bureaus of Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
For a complete list of GIMEMA CML Working Party participants, see the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Gianantonio Rosti, MD, Institute of Hematology, L. and A. Seràgnoli, University of Bologna, S. Orsola-Malpighi Hospital, Via Massarenti, 9, 40138 Bologna, Italy; e-mail: gianantonio.rosti@unibo.it.