Abstract
To study B-cell development from bone marrow (BM), we generated recombination-activating gene 1 (Rag1)–targeted mice lacking mature lymphocytes. B-cell development can be induced in such mice by B cell–specific restoration of a functional Rag1 transcription unit. Follicular and marginal zone B cells populated the spleen when Rag1 expression was permitted. Notably, the peritoneal cavity was dominated by bona fide B-1a cells, as judged by surface markers and functional properties. These BM-derived B-1a cells exhibited a polyclonal VDJ repertoire with substantial N nucleotide insertions. Nevertheless, physiologic frequencies of phosphatidylcholine-specific B cells were detected. Importantly, the BM of young and 5-month-old mice was indistinguishable with regard to the potential to generate B-1a cells.
Introduction
On basis of anatomical location, surface markers, and function, mature B lymphocytes can be subdivided into 2 major subpopulations: B-1 and B-2. In spleen, besides immunoglobulin (Ig)MloIgDhiCD21+CD23+CD19+ follicular (FO) B-2 cells, an additional B-cell population can be distinguished residing in marginal zone (MZ) and characterized as IgMhiIgDloCD21hiCD23lo/−CD19+ MZ B cells. FO B cells, which are the dominating B-cell population in secondary lymphoid organs such as spleen, play an important role in adaptive immune responses.1 In contrast, the preactivated phenotype of splenic MZ B cells qualifies them to participate in T cell–independent immune responses via rapid antibody secretion upon antigenic stimulation.2
B-1a cells can be found in body cavities such as the peritoneal cavity, but also in spleen. In general, B-1a cells are characterized by an IgMhiIgDloCD43+ phenotype, whereas expression of CD11b is restricted to B-1a cells of the peritoneal cavity. Originally defined as CD5+ B cells, B-1 cells can now be subdivided into B-1a cells expressing moderate levels of CD5 and the CD5– B-1b sister population with distinct functions.3 Antibodies produced by B-1b cells are induced after antigenic exposure and are a requisite for long-lasting protective immunity against pathogens such as Streptococcus pneumoniae.4,5 B-1a cells are the major source of so-called natural IgM antibodies that are produced without obvious antigen challenge.5,6 Similar to MZ B cells, B-1a cells preferentially recognize T cell–independent antigens such as polysaccharides and phospholipids (eg, phosphatidylcholine [PtC]).7 Thus, both populations act in concert to mediate early responses against blood-borne particulate antigens.8
The first appearance of mature B-cell subpopulations is consistent with distinct developmental periods. During mid-to-late fetal development, B cells are generated from hematopoietic stem cells in liver, whereas after birth B-cell generation occurs in the bone marrow (BM). FO and MZ B cells become first detectable after birth and are therefore product of B-cell development in BM, whereas maturation into B-1 cells takes place already in fetal liver.9 It has been suggested that generation of B-1 cells ceases shortly after birth, and maintenance of a B-1a compartment is achieved by self-renewal.10
Reconstitution experiments indicated that donor cells from mid-gestation fetal liver efficiently reconstitute the B-1 cell population, whereas donor cells from adult BM are most efficient in generating B-2 cells.10,11 Differences have also been observed with regard to reconstitution of B-1 subpopulations: B-1a progenitor activity wanes after neonatal life, whereas substantial B-1b progenitor activity has been reported in both neonatal and adult BM.12,13 These observations provided the basis for models proposing distinct progenitors for B-1 and B-2 cells. Recently, additional support for this lineage model was provided by identifying a CD45lo/−CD19+ population that includes B-1 progenitors whose numbers peak during fetal development, but are also found in adult BM to some extent.14-16
However, there is also evidence that differences between B-1 and B-2 cells are derived solely from differences in receptor specificity.17 The premise of this induced differentiation model is that B-1 and B-2 cells arise from the same progenitor, but express Ig receptors whose antigen-binding specificity determines whether newly formed B cells differentiate into B-1 or B-2 cells. Evidence for this model came from the analysis of IgH chain transgenic mice expressing certain B-cell receptor (BCR) specificities that drive B-cell fate either into B-2 or the B-1 cell lineage.17,18 In addition, analyses of mice with alterations that affect BCR signaling have clearly shown that strength of signals provided via the BCR has a direct impact on development of B-1 cells.17,19,20 In summary, the lineage model and the induced differentiation model represent 2 alternative, not necessarily mutually exclusive concepts explaining many aspects of B-1 development.
With regard to the potential of adult BM to generate B-1a cells, contradicting results have been reported using transfers of BM cells into irradiated hosts. At least in part, these contradicting results could be attributed to differences in experimental conditions, like host strain, irradiation, and engraftment time.21 To study B-cell developmental potential of BM under more physiologic conditions, we generated a mouse mutant, in which recombination-activating gene 1 (Rag1) expression, and thereby generation of functional T and B cells, was prevented by a conditionally inactivated Rag1 gene. Using this approach in conjunction with B cell–specific activatable Cre recombinase, we established a transgenic mouse strain, in which Rag1 can be specifically activated in B-cell precursors. In this study, we describe this experimental system and apply it to study development of peripheral B-cell subsets from adult BM in otherwise nonmanipulated animals.
Methods
Generation of Indu-Rag1 mice
The BAC clone RP23-111E15 (GenBank AC984753) from the library RPCI-23 Mouse BAC II was used as source of Rag1 encoding DNA. A 9.6-kb BamHI fragment containing the entire exon 2 of Rag1 was used to develop the targeting vector. The coding sequence (CDS) was cut out by ClaI/KpnI digestion and replaced by an adapter of oligonucleotides (LoxP-i-for: 5′-TATAACTTCGTATAGCATACATTATACGAAGTTATCATATGATCGA TATGGTACCACTAGTATAACTTCGTATAATGTATGCTATACGAAGTTATAC-3′; LoxP-i-rev: 5′-CGGTATAACTTCGTATAGCATACATTATACGAAGTTATACTAGTGGT ACCATATCGATCATATGATAACTTCGTATAATGTATGCTATACGAAGTTATAGTAC-3′) that inserted new ClaI and KpnI sites in reverse order. In addition, the adapter contained 2 loxP sites in opposite orientation and a SpeI site important for the later Southern blot analysis. The excised ClaI/KpnI fragment with the CDS was ligated into the vector, resulting in an inverted and loxP-flanked Rag1 CDS. A FRT-flanked neocassette associated with a new SpeI site was inserted into the NheI site. A tandem of diphtheria toxin α cassettes mediating negative embryonic stem (ES) cell selection was inserted 5′ into the Indu-Rag1 construct. Linearized vector was electroporated into BALB/c ES cells,22 according to standard protocols. A correctly targeted ES cell clone was further transfected with a Flp-e expression plasmid (gift of T. Wunderlich, Institute for Genetics, University of Cologne, Cologne, Germany) for deletion of the neocassette. One clone with deleted neocassette was injected into C57BL/6 blastocysts to generate chimeric mice. Germline transmission was determined by coat color and confirmed by Southern blot analysis.
The Indu-Rag1 mice were bred to MerCerMer mice and afterward inter se to obtain mice homozygous for Indu-Rag1 and heterozygous for MerCreMer. Wild-type control mice were age-matched BALB/c mice (Harlan) or heterozygous Indu-Rag1 mice. Rag1-deficient mice on BALB/c background were obtained from The Jackson Laboratory and bred at the Helmholtz Centre for Infection Research animal facility.
B-cell development was induced in Indu-Rag1 mice by oral administration of 400 μL of 20 mg/mL tamoxifen (Ratiopharm) in ClinOleic (Baxter). Control mice were treated with ClinOleic only. Wild-type controls in sorting and blood kinetic experiments were treated with tamoxifen like Indu-Rag1 mice. All experiments were performed in accordance with German law on care and use of laboratory animals and were approved by the Niedersöchsisches Landesaut für Verbraucherschutz und Lebenswittelsicherheit (LAVES).
Flow cytometry, cell sorting, and adoptive transfer experiments
Monoclonal antibodies against mouse IgMa (DS-1), IgMb (AF6-78), B220 (RA3-6B2), IgDa (AMS9.1), CD21 (7G6), CD23 (B3B4), CD5 (53-7.3), Mac-1 (M1/70), CD125 (T21), CD19 (1D3), CD8α (Ly-2), CD49b (DX5), CD3ϵ (145-2C11), Gr1 (RB6-8C5), TER-119 (TER-119), CD93 (AA4.1), and CD43 (S7) were obtained from BD Biosciences or eBiosciences. Anti–c-kit (ACK-4) and anti-IgD (1.19) were homemade. Biotinylated antibodies were revealed by various streptavidin conjugates (all BD Biosciences). PtC liposomes were prepared, as described.23 Expression of cell surface markers was analyzed with FACSCalibur and CellQuest software (BD Biosciences) or with LSRII and DIVA software (BD Biosciences). Cell sorting was performed using MoFlo (DakoCytomation), FACSVantage (BD Biosciences), or FACSAria (BD Biosciences).
For adoptive transfer experiments, 2 × 105 sorted B-1a cells of CB20 (IgMb allotype) and tamoxifen-induced B-Indu-Rag1fl/fl mice (IgMa allotype) were mixed at a 1:1 ratio and injected i.p. into Rag1−/− recipient mice. After 4 weeks, total peritoneal cells were stimulated in vitro with lipopolysaccharide (LPS) and analyzed by an allotype-specific enzyme-linked immunospot (ELISPOT) assay.
LPS stimulation, ELISPOT assay, and enzyme-linked immunosorbent assay
Sorted or unsorted B-cell subpopulations were stimulated for 3 days with 50 μg/mL LPS (Escherichia coli; Sigma-Aldrich) in Iscove modified Dulbecco medium at a density of 1 × 104 to 2 × 106 cells/mL. Standard protocols were used for ELISPOTs: serial dilutions of cells were plated on plates coated with rat anti–mouse IgM (II/41; BD Biosciences). Cells were incubated overnight at 37°C in 5% CO2. After washing, biotin-conjugated rat anti–mouse IgM (LO-MM-9; AbD Serotec), mouse anti–mouse IgMa (DS-1), or IgMb (AF6-78; both BD Biosciences) were added and developed with streptavidin-horseradish peroxidase (BD Biosciences) using 3-amino-9-ethyl-carbazole (Sigma-Aldrich) in N,N-dimethylformamide (Sigma-Aldrich) diluted in 0.1M acetate solution and with H2O2 as substrate. Ig titers in serum were measured by enzyme-linked immunosorbent assay with standard methods using commercial serum as reference (Bethyl Laboratories). Plates were coated with goat anti–mouse IgG (Sigma-Aldrich) or rat anti–mouse IgM (II/41; BD Biosciences). Igs were detected with goat anti–mouse IgG-horseradish peroxidase (Jackson ImmunoResearch Laboratories) or biotin-conjugated rat anti–mouse IgM (R6-60.2; BD Biosciences) and strepatavidin-horseradish peroxidase (BD Biosciences). o-Phenylenediamine (Sigma-Aldrich) and H2O2 were used as substrate.
Amplification of VHDJHCμ chain transcripts, sequencing, and sequence analysis
RNA from sorted CD19+PtC+ peritoneal cells was isolated with TRIzol reagent (Invitrogen) and was transcribed with oligo-d(T)12-18 (Amersham) and Superscript II RNaseH− reverse transcriptase (Invitrogen). The heavy chain transcripts were amplified with primers VHcons (5′-GAGGTGCAGCTGCAGGAGTCTGG-3′) and Cμ1 (5′-ATGGCCACCAGATTCTTATCAGA-3′). Polymerase chain reaction (PCR) conditions were as follows: 94°C, 20 seconds; 50°C, 40 seconds; 72°C, 40 seconds; 35 cycles. PCR products were gel purified and cloned using the TOPO TA Cloning Kit (Invitrogen) for sequencing. Sequence analysis was done as described.24 The VBASE2 database (http://www.vbase2.org) was used for assignment of VH segments. Single-cell reverse transcription (RT)–PCR from IgM+CD5+ peritoneal cells was carried out as described.25
Results
Generation and characterization of a mouse strain allowing conditional expression of Rag1 in B-cell precursors
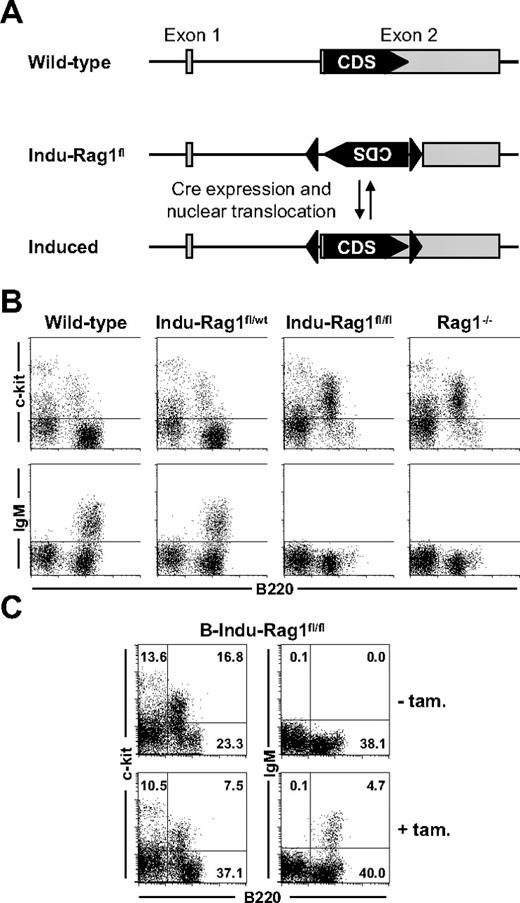
Rag1 in concert with Rag2 is indispensable for rearrangement of BCR and T-cell receptor genes, and therefore, for development of antigen receptor-expressing lymphocytes. Consequently, absence of either Rag1 or Rag2 in mice26,27 or humans28 results in a block of T- and B-cell development at an early progenitor stage and complete absence of mature B and T cells. We made use of this fact when establishing a new conditional mouse model in which B- and T-cell development is blocked, but can be induced at any given time. The general targeting strategy and screening of recombinant ES cells are described in detail in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). In brief, exon 2 of the Rag1 gene encoding the complete Rag1 protein was inverted by homologous recombination and flanked by loxP sites in opposite orientation (Indu-Rag1fl). Expression of a functional Cre recombinase should allow restoration of an intact transcription unit by reverting exon 2. As consequence, rearrangements at the BCR and T-cell receptor loci and lymphocyte development should take place (Figure 1A).
Induction of B-cell development in B-Indu-Rag1fl/fl mice. (A) Strategy of inducible Rag1 expression. Shown is the Rag1 locus of wild-type mice and of Indu-Rag1fl mice before and after restoration of the initially nonfunctional Rag1 transcription unit by B cell–specific Cre expression and activation. Filled arrowheads, loxP sites; grey boxes, Rag1 exons; CDS, coding sequence. (B) Block of B-cell development in the BM of Indu-Rag1fl/fl mice. Flow cytometric analysis of c-kit and IgM expression on B220+ BM cells from wild-type, Rag1−/−, and Indu-Rag1 mice heterozygous (fl/wt) or homozygous (fl/fl) for the inactivated allele. (C) Induction of B-cell generation in B-Indu-Rag1fl/fl mice treated with a single dose of tamoxifen at an age of 8 weeks. Flow cytometric analysis of c-kit and IgM expression on B220+ BM cells 10 days after tamoxifen administration. Untreated B-Indu-Rag1fl/fl mice were included as controls. Numbers in quadrants indicate percentages of cells.
Induction of B-cell development in B-Indu-Rag1fl/fl mice. (A) Strategy of inducible Rag1 expression. Shown is the Rag1 locus of wild-type mice and of Indu-Rag1fl mice before and after restoration of the initially nonfunctional Rag1 transcription unit by B cell–specific Cre expression and activation. Filled arrowheads, loxP sites; grey boxes, Rag1 exons; CDS, coding sequence. (B) Block of B-cell development in the BM of Indu-Rag1fl/fl mice. Flow cytometric analysis of c-kit and IgM expression on B220+ BM cells from wild-type, Rag1−/−, and Indu-Rag1 mice heterozygous (fl/wt) or homozygous (fl/fl) for the inactivated allele. (C) Induction of B-cell generation in B-Indu-Rag1fl/fl mice treated with a single dose of tamoxifen at an age of 8 weeks. Flow cytometric analysis of c-kit and IgM expression on B220+ BM cells 10 days after tamoxifen administration. Untreated B-Indu-Rag1fl/fl mice were included as controls. Numbers in quadrants indicate percentages of cells.
Mice, homozygous for the loxP-flanked Rag1 allele (Indu-Rag1fl/fl), were derived from BALB/c ES cells. To allow inducible B cell–specific inversion of the Rag1fl allele, Indu-Rag1fl/fl mice were crossed to transgenic mice expressing a Cre recombinase fused to a variant of the murine estrogen receptor (Mer) at both the N and C terminus (MerCreMer).29 B cell–specific Cre expression had been achieved by insertion of MerCreMer cDNA into the mb-1 gene. MerCreMer is held in the cytosol by chaperones and is only transferred into the nucleus in presence of estrogen or analogs thereof. For convenience, the Indu-Rag1fl/fl mice heterozygous for mb1-MerCreMer will hereafter be denoted B-Indu-Rag1fl/fl.
Flow cytometric analysis confirmed that heterozygous Indu-Rag1fl/wt mice exhibited normal B-cell development in BM (Figure 1B). In contrast, Indu-Rag1fl/fl mice (Figure 1B) and B-Indu-Rag1fl/fl mice (Figure 1C) showed the expected block in B-cell development and were indistinguishable from conventional Rag1−/− mice. They displayed an accumulation of c-kit+B220+ Pro/PreB I cells and complete absence of IgM+ cells. This is consistent with previous reports, noting that the mb1-MerCreMer recombinase has no detectable background activity, but still can be activated by tamoxifen, an estrogen analog30 (and E.H., E. Levit, M. Dautzenberg, R. Pelanda, and M.R., Conditional selection of B cells in mice with inducible B-cell development, manuscript in preparation).
Therefore, we analyzed the effect of oral administration of tamoxifen on B-cell development in BM of B-Indu-Rag1fl/fl mice. Permanent nuclear localization of Cre would result in continuous inversion (“flipping”) of the loxP-flanked DNA sequence in mutant Pro/PreB I cells. Transient nuclear localization of MerCreMer induced by tamoxifen prevents continuous recombination and stabilizes loxP-flanked DNA in one or the other orientation. A single functional Rag1 allele is obviously sufficient to mediate normal B-cell development. Therefore, it can be predicted that 75% of tamoxifen-responding B-cell precursors should have at least 1 allele in functional orientation. Such cells should be able to undergo VDJ recombination and produce IgM+ B cells. Indeed, after generation of B cells in BM of B-Indu-Rag1fl/fl mice after gavaging with tamoxifen, reduction in the percentage of c-kit+B220+ Pro/PreB I cells could be observed coinciding with appearance of surface IgM+ cells (Figure 1C).
We conclude that tamoxifen administration transiently abrogates the block in B-cell development in BM of B-Indu-Rag1fl/fl mice, and promotes generation of immature IgM+ cells by restoration of a functional Rag1 transcription unit.
Tracking populations of peripheral B cells derived from adult BM
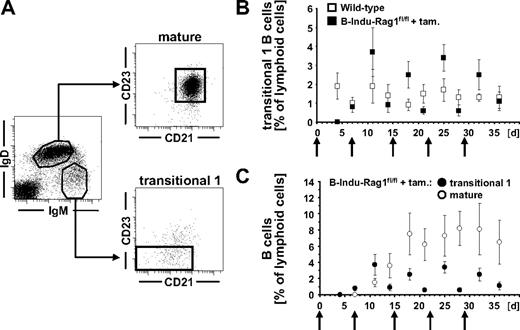
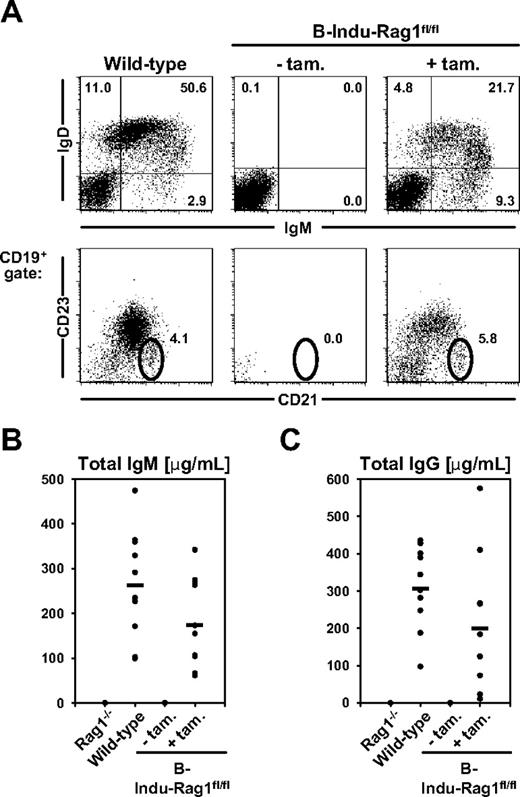
To study the potential of adult BM to reconstitute peripheral B cells, we performed kinetic experiments. We orally treated 9- to 10-week-old B-Indu-Rag1fl/fl mice and control animals weekly with tamoxifen for 5 weeks. Analysis of blood of such animals for appearance of transitional 1 (T1) and mature B cells (Figure 2A) revealed that T1 B cells with an IgMhiIgDloCD21−/loCD23− phenotype were below the level of detection in B-Indu-Rag1fl/fl at day 4 after first administration of tamoxifen. They became detectable in blood at day 7 (Figure 2B-C). During these experiments, an increase and subsequent decline of T1 B cells briefly after particular tamoxifen administrations could be consistently observed. This is in agreement with transient induction of B-cell differentiation and developmental progression of T1 B cells to the next developmental stage. Consequently, IgMloIgDhiCD21+CD23+ mature B cells became detectable in blood at day 11, with numbers increasing over time (Figure 2C). Concomitantly, 3 weekly doses of tamoxifen were sufficient to induce accumulation of CD19+IgMloIgDhi B-2 cells and CD19+CD21hiCD23lo/− MZ B cells in spleens of B-Indu-Rag1fl/fl mice, whereas mature B cells were completely absent in noninduced animals (Figure 3A). In addition, substantial amounts of both secreted IgM and IgG antibodies could be detected in sera of mice 35 days after tamoxifen administration, demonstrating the potential of newly generated B cells to participate in humoral immunity and class switching (Figure 3B).
Tracking B cells in the periphery of tamoxifen-treated B-Indu-Rag1fl/fl mice. (A) T1 (IgMhiIgD−/loCD21−/loCD23−) and mature (IgMloIgDhiCD21+CD23+) B-cell subsets in the blood of wild-type mice. (B) Percentages of T1 B cells in the blood of induced B-Indu-Rag1fl/fl (n = 9) and wild-type control (n = 12) mice. (C) Percentages of T1 and mature B cells in the blood of induced B-Indu-Rag1fl/fl mice. ↑ indicates time points of tamoxifen administration.
Tracking B cells in the periphery of tamoxifen-treated B-Indu-Rag1fl/fl mice. (A) T1 (IgMhiIgD−/loCD21−/loCD23−) and mature (IgMloIgDhiCD21+CD23+) B-cell subsets in the blood of wild-type mice. (B) Percentages of T1 B cells in the blood of induced B-Indu-Rag1fl/fl (n = 9) and wild-type control (n = 12) mice. (C) Percentages of T1 and mature B cells in the blood of induced B-Indu-Rag1fl/fl mice. ↑ indicates time points of tamoxifen administration.
Mature B-cell subsets in the spleen of induced B-Indu-Rag1fl/fl mice. (A) Flow cytometry of splenocytes from wild-type, control-treated, and tamoxifen-induced B-Indu-Rag1fl/fl mice. Mice were induced 3 times with 4-day intervals and analyzed at day 35 after the first administration. Single-cell suspensions were prepared from spleen and stained for IgM, IgD, CD21, and CD23. Numbers indicate percentages of cells in the respective quadrants and gates. (B-C) Steady-state antibody production in induced B-Indu-Rag1fl/fl mice. Sera from B-Indu-Rag1fl/fl mice (age at first induction 8-12 weeks), either treated with tamoxifen or left untreated, and from Rag1−/− and wild-type control mice (all n = 10) were collected 35 days after the first induction, and the amounts of total IgM (B) and total IgG (C) were determined by enzyme-linked immunosorbent assay.
Mature B-cell subsets in the spleen of induced B-Indu-Rag1fl/fl mice. (A) Flow cytometry of splenocytes from wild-type, control-treated, and tamoxifen-induced B-Indu-Rag1fl/fl mice. Mice were induced 3 times with 4-day intervals and analyzed at day 35 after the first administration. Single-cell suspensions were prepared from spleen and stained for IgM, IgD, CD21, and CD23. Numbers indicate percentages of cells in the respective quadrants and gates. (B-C) Steady-state antibody production in induced B-Indu-Rag1fl/fl mice. Sera from B-Indu-Rag1fl/fl mice (age at first induction 8-12 weeks), either treated with tamoxifen or left untreated, and from Rag1−/− and wild-type control mice (all n = 10) were collected 35 days after the first induction, and the amounts of total IgM (B) and total IgG (C) were determined by enzyme-linked immunosorbent assay.
Generation of B-1a cells from adult BM
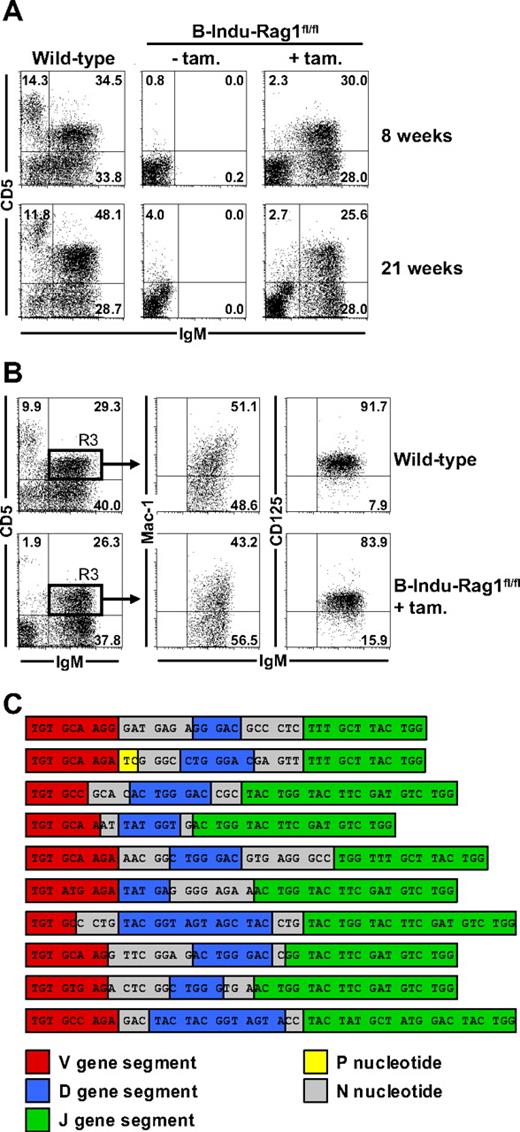
In initial experiments, we noticed that similar percentages and numbers of a recently described B-1 progenitor population (lineage marker−B220−CD19+AA4.1+) could be detected in the BM of adult B-Indu-Rag1fl/fl and Rag1−/− mice (supplemental Figure 2). To directly address the developmental potential of BM with regard to B-1 cells, we treated adult B-Indu-Rag1fl/fl with tamoxifen twice with a 4-day interval. Wild-type mice and untreated B-Indu-Rag1fl/fl were included as controls. Flow cytometric analysis of peritoneal cells confirmed the absence of lymphocytes in untreated B-Indu-Rag1fl/fl mice. In contrast, upon tamoxifen treatment of 8-week-old B-Indu-Rag1fl/fl mice, a significant population of B cells could be detected expressing high levels of surface IgM, 50% of which coexpressed CD5 (Figure 4A top panels). Similar results were obtained with mice aged 21 weeks (Figure 4A bottom panels). In addition to an IgDloCD19hiCD43+ surface phenotype (S.D., unpublished data, December 2006 and July 2008), Mac-1 and CD125 expression on such BM-derived IgMhiCD5+ cells was indistinguishable from wild-type controls (Figure 4B). Besides peritoneum, B cells with a B-1a surface marker phenotype could also be detected in spleen of induced B-Indu-Rag1fl/fl mice (S.D., unpublished data, July 2008). With regard to the B-1a and B-1b sister populations, we consistently observed a predominance of B-1a cells (supplemental Figure 3). Overall, B-cell numbers of various subpopulations in B-Indu-Rag1fl/fl mice did not reach levels of wild-type control animals at any time point analyzed (supplemental Figure 3). This is likely because tamoxifen administration results in the induction of waves rather than continuous B lymphopoiesis. This observation provided first evidence that lymphopenia-driven population expansion may not play a major role in populating B-Indu-Rag1fl/fl mice with B cells.
Generation of B-1a cells from the adult BM of B-Indu-Rag1fl/fl mice. (A) Two groups of wild-type mice and B-Indu-Rag1fl/fl mice at the age of 8 and 21 weeks were either left untreated or treated twice with tamoxifen. Flow cytometric analysis of peritoneal exudates for IgM and CD5 expression was performed 35 days after initiation of the experiment. Numbers in quadrants indicate percentages of cells. (B) Peritoneal cells from wild-type and tamoxifen-treated B-Indu-Rag1fl/fl mice were stained for IgM, CD5, Mac-1, and CD125 and analyzed by flow cytometry. Numbers in quadrants indicate percentages of cells. (C) B-1a cells of B-Indu-Rag1fl/fl mice show N nucleotide additions in all of their IgH chain CDR3. IgM+CD5+ peritoneal cells from individual induced B-Indu-Rag1fl/fl mice were sorted, and the IgH chain repertoire was analyzed by single-cell RT-PCR. Nucleotide sequences of representative CDR3 beginning with the conserved cysteine at amino acid position 92 are shown.
Generation of B-1a cells from the adult BM of B-Indu-Rag1fl/fl mice. (A) Two groups of wild-type mice and B-Indu-Rag1fl/fl mice at the age of 8 and 21 weeks were either left untreated or treated twice with tamoxifen. Flow cytometric analysis of peritoneal exudates for IgM and CD5 expression was performed 35 days after initiation of the experiment. Numbers in quadrants indicate percentages of cells. (B) Peritoneal cells from wild-type and tamoxifen-treated B-Indu-Rag1fl/fl mice were stained for IgM, CD5, Mac-1, and CD125 and analyzed by flow cytometry. Numbers in quadrants indicate percentages of cells. (C) B-1a cells of B-Indu-Rag1fl/fl mice show N nucleotide additions in all of their IgH chain CDR3. IgM+CD5+ peritoneal cells from individual induced B-Indu-Rag1fl/fl mice were sorted, and the IgH chain repertoire was analyzed by single-cell RT-PCR. Nucleotide sequences of representative CDR3 beginning with the conserved cysteine at amino acid position 92 are shown.
The efficient reconstitution of the peritoneal B-1a compartment in adult B-Indu-Rag1fl/fl mice could be due to the proliferative expansion of initially minute numbers of BM-derived B-1a cells. Therefore, we RT-PCR amplified VDJ regions of IgM+CD5+ B cells at the level of single cells from a pool of 7 tamoxifen-treated B-Indu-Rag1fl/fl mice. Sequence analysis indicated that B-1a cells from the peritoneum of induced B-Indu-Rag1fl/fl mice exhibit substantial repertoire diversity (S.D. and M.K., unpublished data, July 2006).
We extended our RT-PCR analysis to fluorescence-activated cell sorter (FACS)–purified single cells from 2 individual adult B-Indu-Rag1fl/fl mice 5 weeks after the second tamoxifen administration. Although identical VDJ rearrangements could be recovered repeatedly to some extent (mouse no. 1, 5 sequences were recovered 2, 2, 2, 4, and 6 times; mouse no. 2, 4 sequences were recovered 2, 2, 2, and 6 times), the majority of B-1a cells exhibited VDJ rearrangements with unique nucleotide sequences (mouse no. 1, 44 of 60, ie, 73.3%; mouse no. 2, 45 of 58, ie, 77.6%) that encoded distinct amino acid sequences. Consistently, nearly all of the VDJ sequences recovered from B-1a cells of the 2 individual induced B-Indu-Rag1fl/fl mice, as well as the sequences recovered from the pool of 7 mice exhibited substantial N nucleotide additions in their VH complementary-determining region 3 (CDR3; Figure 4C and S.D. and M.K., unpublished data, March 2007).
We conclude that the majority of peritoneal cavity B cells with a B-1a phenotype from B-Indu-Rag1fl/fl mice exhibit independent VDJ rearrangements, and therefore, represented individually generated B cells. In addition, the high frequency of N nucleotide insertions is consistent with an adult BM origin of B-1a cells in induced B-Indu-Rag1fl/fl mice.
Rapid IgM secretion of B-Indu-Rag1fl/fl–derived B-1 and MZ B cells upon stimulation
A prerequisite of B-1 and MZ B cells to act as first line of defense is a preactivated phenotype allowing to rapidly secrete antibodies upon antigenic challenge. This distinguishes them from B-2 cells. Therefore, we performed a comparative functional analysis of various B-cell populations from spleen and peritoneal cavity, which constituted the periphery of B-Indu-Rag1fl/fl mice after activation. Heterozygous B-Indu-Rag1fl/wt mice kept under the same hygienic conditions, and wild-type BALB/c mice were included as controls.
FACS-purified populations were stimulated with lipopolysaccharide (LPS), and numbers of IgM-secreting cells were determined by ELISPOT after various time points of culture. Without deliberate LPS stimulation, low, but significant numbers of IgM-secreting cells could be detected among the splenic B-1a populations of induced B-Indu-Rag1fl/fl mice and controls (Figure 5A top panel). IgM secretion of stimulated B-1a and B-1b cells from peritoneal cavity, and B-1a and MZ from spleen of either mice became detectable after 24 hours of stimulation. Almost no spots could be observed from B-2 cells at this time point (Figure 5A middle panel). B-1 and MZ B cells from induced B-Indu-Rag1fl/fl mice appeared to produce more IgM-secreting cells in such assays. However, the overall trend was comparable with controls. IgM-secreting cells of B-2 cells from all 3 groups became detectable after 3 days of stimulation, consistent with a resting state in vivo (Figure 5A bottom panel).
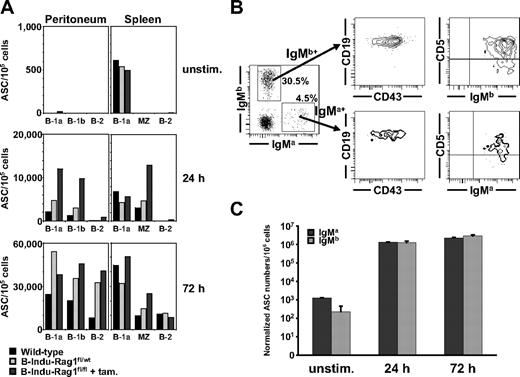
Fast LPS-induced IgM response of B-1 and MZ cells. (A) FACS-purified peritoneal B-1a, B-1b, and B-2 cells, and splenic B-1a, MZ, and B-2 cells of induced B-Indu-Rag1fl/fl mice or control-treated wild-type mice (BALB/c and B-Indu-Rag1fl/wt mice) were cultured in the absence or presence of LPS. For each condition, the number of antibody-secreting cells per 105 cells was determined by IgM-specific ELISPOT at indicated time points. For details on sorting strategies and purities, see supplemental Figure 4. (B) Peritoneal cells from 3 pooled Rag1−/− recipient mice 4 weeks after transfer were stained for IgMa, IgMb, CD43, CD19, and CD5, and analyzed by flow cytometry. Numbers in quadrants indicate percentages of cells per viable, lymphoid cells. (C) Peritoneal washout cells from Rag1−/− recipient mice were cultured in the absence or presence of LPS. The number of antibody-secreting cells per 106 cells was determined by an IgM allotype-specific ELISPOT at indicated time points and normalized with regard to the allotype-positive input at day 0.
Fast LPS-induced IgM response of B-1 and MZ cells. (A) FACS-purified peritoneal B-1a, B-1b, and B-2 cells, and splenic B-1a, MZ, and B-2 cells of induced B-Indu-Rag1fl/fl mice or control-treated wild-type mice (BALB/c and B-Indu-Rag1fl/wt mice) were cultured in the absence or presence of LPS. For each condition, the number of antibody-secreting cells per 105 cells was determined by IgM-specific ELISPOT at indicated time points. For details on sorting strategies and purities, see supplemental Figure 4. (B) Peritoneal cells from 3 pooled Rag1−/− recipient mice 4 weeks after transfer were stained for IgMa, IgMb, CD43, CD19, and CD5, and analyzed by flow cytometry. Numbers in quadrants indicate percentages of cells per viable, lymphoid cells. (C) Peritoneal washout cells from Rag1−/− recipient mice were cultured in the absence or presence of LPS. The number of antibody-secreting cells per 106 cells was determined by an IgM allotype-specific ELISPOT at indicated time points and normalized with regard to the allotype-positive input at day 0.
Next, we injected mixtures of sorted populations of B-1a cells from tamoxifen-treated B-Indu-Rag1fl/fl (IgMa) and control CB20 (IgMb) donor mice into Rag1−/− recipient mice. Analysis of recipients 4 weeks after adoptive transfer showed that B-1a cells that originate from BM of B-Indu-Rag1fl/fl can be maintained for extended periods of time in lymphopenic hosts (Figure 5B). Subsequent ELISPOT assays demonstrated that B-1a cells of B-Indu-Rag1fl/fl and CB20 controls maintained the capacity to respond to LPS stimulation in vitro, resulting in similar numbers of antibody-secreting cells among both B-1a populations (Figure 5C).
In summary, the overall response to LPS stimulation of mature B-cell populations derived from induced B-Indu-Rag1fl/fl mice is comparable with that of control animals, both in terms of kinetics and numbers of IgM-secreting cells. These findings strongly suggest the presence of true B-1 and MZ B cells as well as B-2 cells in induced B-Indu-Rag1fl/fl mice.
PtC-specific B-1a cells in induced B-Indu-Rag1fl/fl mice
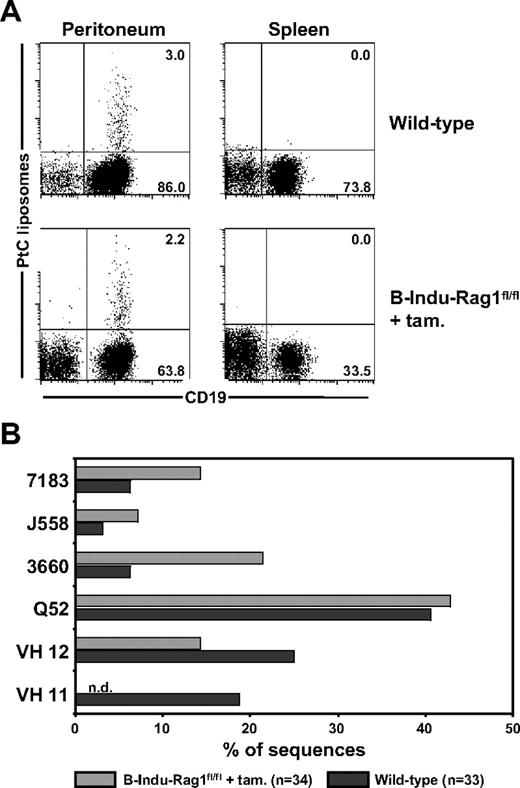
A diagnostic characteristic of peritoneal B-1a cells is their specificity for the ubiquitous membrane phospholipid PtC, which plays an important role in protection against microbial infections.31 The PtC-specific B-1a antibody repertoire is characterized by low frequencies of N nucleotides within the VH CDR3 regions.32 We therefore asked whether the high frequencies of N nucleotide insertions observed among BM-derived peritoneal B-1a cells from induced B-Indu-Rag1fl/fl mice (Figure 4C) would prevent generation of PtC-specific B-1a cells. Using fluorescein-loaded PtC liposomes to identify specific B cells, it became clear that the percentage of PtC-specific B-1a cells detectable in peritoneal cavity of induced B-Indu-Rag1fl/fl mice was comparable with that of wild-type mice (Figure 6A). Consistent with previous reports,23,25 the number of PtC-specific B-1a cells in spleen was below detection level (Figure 6A).
Detection of PtC-specific peritoneal B-1a cells derived from the BM of induced B-Indu-Rag1fl/fl mice. (A) Flow cytometry of splenic and peritoneal cells from wild-type and tamoxifen-induced B-Indu-Rag1fl/fl mice using PtC liposomes. Such PtC binders were also found to express CD5 (S.D., unpublished data, August 2006). Mice were induced twice at an age of 8 weeks and analyzed 5 weeks after initiation of the experiment. (B) PtC specificity of B cells from induced B-Indu-Rag1fl/fl mice is mediated by V genes characteristic for this specificity. CD19+ PtC-specific cells were bulk sorted from wild-type and tamoxifen-induced B-Indu-Rag1fl/fl mice, and V gene usage was determined by cloning and subsequent sequencing of RT-PCR products.
Detection of PtC-specific peritoneal B-1a cells derived from the BM of induced B-Indu-Rag1fl/fl mice. (A) Flow cytometry of splenic and peritoneal cells from wild-type and tamoxifen-induced B-Indu-Rag1fl/fl mice using PtC liposomes. Such PtC binders were also found to express CD5 (S.D., unpublished data, August 2006). Mice were induced twice at an age of 8 weeks and analyzed 5 weeks after initiation of the experiment. (B) PtC specificity of B cells from induced B-Indu-Rag1fl/fl mice is mediated by V genes characteristic for this specificity. CD19+ PtC-specific cells were bulk sorted from wild-type and tamoxifen-induced B-Indu-Rag1fl/fl mice, and V gene usage was determined by cloning and subsequent sequencing of RT-PCR products.
To investigate the IgH chain repertoire of PtC-binding B-1a cells in more detail, we used sequencing of cloned RT-PCR products after bulk sorting. A similar VH usage was observed when PtC-specific cells from induced B-Indu-Rag1fl/fl mice were compared with PtC binding B cells from wild-type mice (Figure 6B). The only exception was VH11, a family of VH genes frequently used by PtC-specific B-1a cells in normal mice. This VH family was underrepresented in the repertoire of PtC binding B-1a cells from induced B-Indu-Rag1fl/fl mice. Most likely, usage of VH11 for PtC binding is incompatible with presence of N nucleotides in the CDR3 region. Nevertheless, we conclude that BM of adult B-Indu-Rag1fl/fl mice is capable of generating PtC-specific B-1a cells despite extensive addition of N nucleotides in the VH CDR3 regions of their BCRs.
Discussion
In this study, we describe the generation and characterization of the Indu-Rag1 mouse model, in which both T and B lymphocyte development is blocked due to targeted inversion of exon 2 of the Rag1 gene flanked by opposing loxP sites. Lymphocyte development can be induced at any time by activating Cre recombinase, which leads to restoration of a functional Rag1 transcription unit.
To reliably study B-cell developmental potential of adult BM, it was required to achieve tightly controlled Cre activity without background in absence of inducer. For Indu-Rag1fl/fl mice, one could expect that even minimal Rag1 activity in absence of deliberate induction would result in accumulation of significant numbers of lymphocytes in peripheral lymphoid organs. Initially, we crossed Indu-Rag1fl/fl mice to mice expressing Cre recombinase under control of the Mx1 promoter (Mx-Cre).33 The Mx1 promoter can be induced by application of type I interferons or double-stranded RNA, but is known to exhibit moderate background activity. Analysis of Mx-Cre-Indu-Rag1fl/fl mice indicated that background activity of Mx-Cre was sufficient to result in numbers of B and T cells in peripheral lymphoid organs close to wild-type mice (S.D., unpublished data, June 2005).
Absence of detectable background recombination was achieved by crossing Indu-Rag1 mice to mice with a targeted insertion of MerCreMer cDNA into the mb-1 gene33 (and E.H., E. Levit, M. Dautzenberg, R. Pelanda, and M.R., Conditional selection of B cells in mice with inducible B-cell development, manuscript in preparation). The mb-1 gene encodes the Igα signaling subunit of the BCR,34 which is strongly expressed in the B-cell lineage beginning at an early pro-B cell stage.35 This resulted in B cell–specific, tightly controlled Cre expression activatable by tamoxifen.
Tracking B cells in blood of adult B-Indu-Rag1fl/fl mice after repeated tamoxifen administration indicated waves of B-cell development. This was reflected by a transient increase in T1 B cells shortly after individual tamoxifen doses. Similarly, numbers of mature B cells accumulated in blood after induction until they reached plateau and declined thereafter. This observation is consistent with expression of mb-1–controlled Cre in early B-cell progenitors, which are limited to only a few cycles of proliferation, unlike hematopoietic stem cells, which exhibit self-renewing capacity. Thus, developmental progression of B-cell progenitors with restored Rag1 transcription in B-Indu-Rag1fl/fl mice leads to a self-limiting wave of B-cell development.
BM-derived naive FO B cells and MZ B cells accumulated in peripheral lymphoid organs such as spleen. However, even repeated administration of tamoxifen resulted in numbers of mature B cells in blood and spleen of B-Indu-Rag1fl/fl mice below wild-type levels. Nevertheless, significant amounts of steady-state IgM and IgG antibodies could be detected in serum of tamoxifen-induced B-Indu-Rag1fl/fl mice, indicating that B cells generated in initially B cell–deficient mice can participate in humoral immunity by antibody secretion and class switch.
In addition to FO and MZ B cells in spleen, tamoxifen treatment of B-Indu-Rag1fl/fl mice resulted in efficient population of peritoneal and splenic B-cell compartments with B cells expressing high levels of IgM and low levels of IgD, half of which concomitantly expressed CD5. The CD5+IgMhiIgDlo cells were bona fide B-1a cells, as judged by expression of various surface markers and functional properties such as expression of anti-PtC–specific BCRs and rapid IgM secretion upon stimulation. Single-cell analyses of peritoneal B-1a cells indicated a largely polyclonal VDJ repertoire with substantial N nucleotide insertions, which is consistent with their origin from adult BM. This precludes proliferative expansion as a mechanism that might have dominantly contributed to replenishment of the peritoneal B-1a compartment in B-Indu-Rag1fl/fl mice.
B-1 cells spontaneously secrete natural IgM, which plays a critical role in host immune defense. It is well established that the preactivated phenotype of MZ B cells and B-1 cells leads to faster IgM secretory responses upon LPS stimulation compared with naive B-2 cells.36,37 For B-1a cells, antibody secretion at low levels has been described even in absence of deliberate stimulation.38 In this study, we could demonstrate that B-cell subpopulations in induced B-Indu-Rag1fl/fl mice resemble not only phenotypically their counterparts from normal mice, but also show typical functional characteristics. It appears that B-1 and MZ B cells of B-Indu-Rag1fl/fl mice respond faster to LPS stimulation by antibody secretion than the corresponding populations of wild-type mice. One possible explanation for this observation is that recently generated cells can more rapidly activate their secretory apparatus, perhaps due to an enhanced preactivated state in a lymphopenic environment.
In steady state, B cells with antigen receptors of a broad spectrum of antigen specificities are generated. Such specificities include BCRs against self-antigens like PtC. Anti-PtC antibodies are mainly produced by B-1a cells and mediate protection against certain bacterial pathogens.7 Our observation that the percentage of peritoneal PtC liposome-binding CD19hiCD5+ cells in tamoxifen-treated B-Indu-Rag1fl/fl mice was comparable with control animals indicated that adult BM-derived B cells can be efficiently selected into the PtC-specific B-1a compartment. Of note, the repertoire of BM-derived PtC-specific B-1a cells from B-Indu-Rag1fl/fl mice exhibited VH usage with overrepresentation of Q52 and VH12 family members. This is also characteristic for B-1a cells from normal mice of fetal origin. Strikingly, the VH11 family was clearly underrepresented among BCRs from PtC-specific B-1a cells in the peritoneum of B-Indu-Rag1fl/fl mice. This is reminiscent of loss of T15 anti-phosphorylcholine antibodies by forced addition of N nucleotides in terminal deoxynucleotidyltransferase transgenic mice.39 Thus, it is reasonable to speculate that the striking underrepresentation of VH11 in PtC-specific B-1a cells is due to presence of substantial N nucleotides in all IgH chains of B-Indu-Rag1fl/fl mice.
Whether B-1a cells are derived from a distinct progenitor has been hotly debated over years.40 Identification of an early B-cell progenitor that preferentially differentiates into B-1 cells14 and its further characterization15,16 lent support to the lineage model of B-1 generation. This model was initially based on adoptive transfer experiments, in which reconstitution of irradiated mice with adult BM cells often failed to reconstitute the B-1a compartment in several studies, whereas the preferential generation of B-2 cells, and in some cases B-1b cells, could be observed.9 Contrary, at least in some reports, BM reconstitution resulted in significant numbers of B-1a cells.41,42 These contradicting results could in part be explained by differences in recipient strains, irradiation conditions, and engraftment time.21 Compared with BM reconstitution of irradiated hosts, the B-Indu-Rag1fl/fl model allows analyzing B-cell developmental potential of adult BM upon tamoxifen administration in otherwise unmanipulated mice. Thus, conditions could be considered more physiologic. Our results clearly demonstrate that BM of aged, 5-month-old mice is indistinguishable from that of 8-week-old mice with regard to B-1a cell generation upon Rag1 activation. This result appears somewhat unexpected considering previous observations that adult BM contains only minute numbers of B-1 progenitor that mainly differentiate into B cells with a B-1b phenotype.14 However, the fact that B-Indu-Rag1fl/fl mice are initially devoid of any lymphocytes allows studying the full potential of BM with regard to generation of B-cell subsets in absence of any competing preexisting cells. Our finding that adult BM is able to generate significant numbers of B-1a cells, many of which are characterized by unique VDJ rearrangements, is consistent with BM transfers that reconstituted B-1a cells.41,42
Analysis of many transgenic and knockout mice has demonstrated that fate decisions of B-cell subsets are strongly influenced by both BCR specificity and signal strength.17 Hence, in the BCR transgenic model specific for the self-antigen Thy-1, variations in expression of soluble self-antigen resulted in a BCR signal strength-dependent preferential generation of B-1, MZ, or FO B cells.43 In addition, transcription factors may also be involved in guiding fate decisions. Thus, gene-targeted abrogation of PU.1 expression resulted in reduced levels of B-2 cells, whereas B-1–like cells increased dramatically. These changes are probably caused by forced reprogramming during B-cell development.44
Research over the past years has indicated that neither the lineage model nor the induced differentiation model alone can fully explain the generation of B-1, and especially B-1a cells. In this study, we could demonstrate that BM of aged mice has the potential to produce substantial numbers of bona fide B-1a cells, indicating that previously unappreciated developmental pathways may contribute to the adult B-1a compartment. This raises the question as to whether adult BM is contributing to the B-1 cell pool under steady-state conditions. The contribution might be minor and therefore might have been missed to date. This would lead to the question as to why the contribution is low because in our B-Indu-Rag1fl/fl mice significant numbers of B-1 cells can be generated from adult and even aged BM. Recent studies on molecules like SiglecG have shown that such molecules might inhibit proliferation or generation of B-1 cells.45 Thus, B-1 cell generation in adult BM might be blocked by negative feedback. The Indu-Rag1fl/fl mouse represents a suitable model system to study such aspects of B-cell development, homeostasis, and function. In addition, using mice with temporally and spatially controlled Cre function in hematopoietic stem cells or early T-cell precursors will allow to extend this model to future studies on T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to S. zur Lage and R. Lesch for expert technical assistance.
This work was supported in part by Deutsche Forschungsgemeinschaft to S.W. via WE817/6 and SFB 621-A5, the Deutsche Krebshilfe, the Ministry for Education and Research (Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie), and the Hannover Biomedical Research School.
Authorship
Contribution: S.D. designed and performed research and wrote the paper; M.H. designed research; M.K., S.L., and B.R. performed research; E.H. and M.R. provided recombinant mice; T.B. and A.W. supervised the ES cell targeting work and provided reagents; K.K. conceived the research and wrote the paper; and S.W. conceived the research, guided its design, analysis, and interpretation, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandra Düber, Molecular Immunology, Helmholtz Centre for Infection Research, Inhoffenstr 7, D-38124 Braunschweig, Germany; e-mail: Sandra.Dueber@helmholtz-hzi.de.
References
Author notes
K.K. and S.W. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal