Abstract
In persons with hemophilia, life expectancy is now approaching that of the general male population, at least in countries that can afford regular replacement therapy with coagulation factor concentrates. The new challenges for comprehensive treatment centers are thus to provide optimal health care for this aging population of patients, who often present not only with the comorbidities typically associated with hemophilia (arthropathy, chronic pain, blood-borne infections), but also with common age-related illnesses such as cardiovascular disease and cancer. There are no evidence-based guidelines for the management of these conditions, which often require drugs that interfere with hemostasis, enhance the bleeding tendency, and warrant more intensive replacement therapy. At the moment, elderly patients with hemophilia affected by other diseases should be managed like their age-group peers without hemophilia, provided replacement therapy is tailored to the heightened risk of bleeding associated with the need for invasive procedures and drugs that further compromise the deranged hemostasis. More detailed advice is provided on the schedules of replacement therapy needed to tackle cardiovascular diseases, such as acute coronary syndromes and nonvalvular atrial fibrillation, because these conditions will become more and more frequent challenges for the comprehensive treatment centers.
Introduction
Starting in the early 1970s, hemophilia, previously a life-threatening and crippling condition, became a gratifying example of successful secondary prevention of a chronic disease.1 Because of the increasing availability of coagulation factor concentrates (CFCs), at least in high-income countries, life expectancy increased in persons with hemophilia (PWHs) from less than 30 years to more than 60 years.2-7 This favorable trend was temporarily halted in the last 2 decades of the 20th century by the devastating impact of infections with the human immunodeficiency virus (HIV) and the hepatitis C virus (HCV). After the development of efficacious methods to inactivate blood-borne viruses in plasma-derived CFC and the availability of factor VIII (FVIII), factor IX (FIX), and factor VIIa produced by recombinant DNA technology, new blood-borne infections are no longer considered a threat, and preexisting infections can be controlled (HIV) or cured (HCV) with antiviral therapies in a substantial proportion of affected patients.8,9
A major problem is still the acquired occurrence of alloantibodies that inhibit the activity of coagulation factors. Inhibitors develop in 20% to 30% of patients with severe hemophilia A, whereas this complication is much rarer in severe hemophilia B10,11 and in patients with mild hemophilia A, who develop inhibitors predominantly in adulthood or even at an old age.12-14 Products that bypass the coagulation defect, such as activated prothrombin complex coagulation factors and recombinant factor VIIa (rFVIIa), are usually effective to control bleeding episodes, and alloantibodies can be eradicated in at least 50% to 60% of patients with inhibitors by immune tolerance therapy based on the prolonged administration of large doses of recombinant or plasma-derived CFC.13,14 On the whole, in the first decade of the third millennium, safe and effective treatment is available for PWHs, whose life expectancy approaches that of the general population.5-7
No rose is without thorns. As a consequence of aging, the number of PWHs affected with one or more age-related diseases is increasing and newer clinical concerns are emerging.15-17 At the same time, PWHs born before the 1960s, who did not receive adequate replacement therapy throughout a great part of their lives, now have the long-term comorbidities typically associated with hemophilia, such as arthropathy and chronic viral infections. The purpose of this article is to advise on the management of the most frequent age-related comorbidities occurring in the elderly PWH. Particular emphasis is put on cardiovascular diseases and cancer, which often require therapeutic procedures and drugs that further compromise the already deranged hemostasis of PWHs. Because of the current paucity of data, our recommendations are not evidence-based but stem from the available literature and the clinical experience gained in 2 large hemophilia treatment centers.
Hemophilia-associated comorbidities in the elderly person with hemophilia
Arthropathy is the epitome of hemophilia-associated comorbidities. Joint damage increases with aging in an almost linear fashion, not only in severely affected patients but also in moderate hemophilia.18,19 A study carried out in a cohort of 39 elderly PWHs, ranging in age from 65 to 78 years, established that more than half of them had severe damage in all 6 joints more frequently affected by bleeding (knees, elbows, and ankles).17 There was also a high rate of joint instability and balance dysfunction, so that a large proportion of elderly PWHs (70%) had a high risk of falling, spontaneously or after tripping on obstacles.17 In turn, falls probably cause an increasing number of serious injuries and fractures, also because of osteoporosis and osteopenia.20-22 An adverse influence on the course of arthropathy may be also the result of a more sedentary lifestyle, overweight, and obesity, which are commonly associated with both hemophilia and aging.23,24
On the whole, there will be an increasing need for emergency and elective orthopedic operations with the aging of PWHs. Ankle surgery probably matches knee surgery in frequency, and there will be more revision operations (with a higher risk of bleeding) in PWHs who had their first joint prosthesis implanted 10 to 15 years earlier. More and more elderly patients with inhibitors will also need joint replacement, which is now possible and safe but at exorbitant costs using bypassing agents.25-27 Table 1 shows some schedules of replacement or bypassing therapy recommended for these operations in PWHs without and with inhibitors.
Prophylaxis schedule for indications other than cardiovascular diseases in elderly persons with severe hemophilia
| Indication . | Dosage . | Duration . |
|---|---|---|
| Frequent bleeding, ie, ≥ 2×/mo | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | 1 y, then stop and restart if bleeding frequency increases again |
| Rehabilitation after severe bleeding, for severe arthropathy or after orthopedic surgery | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | During the whole period of rehabilitation |
| Medications increasing the underlying bleeding tendency | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | During the medication period |
| Platelet count < 30 000/μL | Daily 10 U/kg FVIII; every other day 20 U/kg FIX | Until platelet count > 30 000/μL |
| Major surgery (especially orthopedic surgery) in patients without inhibitors | ||
| Day of surgery | FVIII or IX 50 U/kg, followed by bolus injections 2×/d 25 U/kg, or continuous infusion with 3-4 U/kg/h aiming at trough levels > 60 U/dL, followed by prophylaxis aiming at trough levels of 25 U/dL | 1 wk, trough > 60 U/dL, followed by 1 wk, trough > 25 U/dL |
| Postsurgical prophylaxis | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | 1-2 wk, depending on the type of surgery |
| Major surgery (especially orthopedic surgery) in patients with inhibitors | Day of surgery, rFVIIa 100 μg/kg every 2 h for 24-48 h, then every 3 h until day 5, then every 4 h until days 7-10, then every 6 h until days 14-21 | ≥ 2-3 wk, depending on the type of surgery |
| Indication . | Dosage . | Duration . |
|---|---|---|
| Frequent bleeding, ie, ≥ 2×/mo | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | 1 y, then stop and restart if bleeding frequency increases again |
| Rehabilitation after severe bleeding, for severe arthropathy or after orthopedic surgery | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | During the whole period of rehabilitation |
| Medications increasing the underlying bleeding tendency | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | During the medication period |
| Platelet count < 30 000/μL | Daily 10 U/kg FVIII; every other day 20 U/kg FIX | Until platelet count > 30 000/μL |
| Major surgery (especially orthopedic surgery) in patients without inhibitors | ||
| Day of surgery | FVIII or IX 50 U/kg, followed by bolus injections 2×/d 25 U/kg, or continuous infusion with 3-4 U/kg/h aiming at trough levels > 60 U/dL, followed by prophylaxis aiming at trough levels of 25 U/dL | 1 wk, trough > 60 U/dL, followed by 1 wk, trough > 25 U/dL |
| Postsurgical prophylaxis | 3×/wk 15 U/kg FVIII; 2×/wk 30 U/kg FIX | 1-2 wk, depending on the type of surgery |
| Major surgery (especially orthopedic surgery) in patients with inhibitors | Day of surgery, rFVIIa 100 μg/kg every 2 h for 24-48 h, then every 3 h until day 5, then every 4 h until days 7-10, then every 6 h until days 14-21 | ≥ 2-3 wk, depending on the type of surgery |
When major orthopedic operations are carried out in the elderly, venous thromboembolism is a frequent event, so that thromboprophylaxis with anticoagulants, such as low molecular weight heparins, should be routinely implemented also in PWHs.28 In them, orthopedic surgeons tend to skip thromboprophylaxis on the assumption that the coagulation defect confers protection from venous thromboembolism. This assumption is not supported by evidence, and venous thromboembolism does occur in patients with congenital coagulation defects,29-31 particularly in older patients. Rather than preoperatively, low-molecular-weight heparins should be preferably started 6 to 12 hours postoperatively to minimize the risk of bleeding (Table 2). Mechanical methods of prophylaxis are advised in patients at particularly high risk of perioperative and postoperative bleeding, such as those undergoing major orthopedic surgery with bypassing agents that are less hemostatically effective than CFC. In arthroscopic orthopedic surgery, much less invasive and challenging for hemostasis than joint replacement surgery, early mobilization is usually sufficient in the absence of additional thromboembolism risk factors that, when present, demand the same schedules recommended for joint replacement. Locoregional anesthesia can be used, provided the PWH is treated with CFC at the time of this procedure that may carry a risk of spinal hematoma.
Antithrombotic prophylaxis in persons with hemophilia undergoing orthopedic surgery
| Patient characteristic . | Recommended methods . |
|---|---|
| Without inhibitors, undergoing joint arthroplasty | Subcutaneous low-molecular-weight heparin, started 6-12 h after the end of the operation (eg, enoxiparin 40 mg), continued daily for no less than 4 wk |
| With inhibitors, undergoing joint arthroplasty and treated with bypassing agents | Mechanical methods of thromboprophylaxis (graduated compression stocking or intermittent pneumatic compression), for no less than 30 days postoperatively |
| With or without inhibitors, undergoing arthroscopic surgery | Early mobilization, as the only method of thromboprophylaxis |
| Patient characteristic . | Recommended methods . |
|---|---|
| Without inhibitors, undergoing joint arthroplasty | Subcutaneous low-molecular-weight heparin, started 6-12 h after the end of the operation (eg, enoxiparin 40 mg), continued daily for no less than 4 wk |
| With inhibitors, undergoing joint arthroplasty and treated with bypassing agents | Mechanical methods of thromboprophylaxis (graduated compression stocking or intermittent pneumatic compression), for no less than 30 days postoperatively |
| With or without inhibitors, undergoing arthroscopic surgery | Early mobilization, as the only method of thromboprophylaxis |
A few studies, all small and retrospective, have evaluated the effects of secondary prophylaxis based on 2 to 3 infusions per week of CFC in adult PWHs, including very few patients older than 65 years.32-34 As expected, secondary prophylaxis markedly reduced the frequency of bleeding (total bleeds from 35.8 to 4.2 and joint bleeds from 32.4 to 3.3 after 5 years),33 but, although orthopedic status in adolescents improved, in adults the worsening of arthropathy was not halted.33 Even though the consumption of CFC for replacement therapy was much increased by the prophylactic regimen, quality of life improved, and pain and need for analgesics decreased.33 Prophylaxis may also decrease the risk of intracranial hemorrhage that (because of hypertension and traumas after falling) increases as the PWH gets older35 and is one of the most frequent causes of death.
On the whole, prophylaxis in elderly PWHs is not a fully established indication in terms of health technology assessment and cost-effectiveness. However, when resources are available, prophylaxis is indeed recommended because it does dramatically improve the quality of life, particularly in frequent bleeders and during rehabilitation (Table 1). Moreover, continuous prophylaxis is going to be needed more and more frequently in elderly PWHs because of cardiovascular disease and cancer that entail patient exposure to drugs and procedures enhancing the baseline risk of bleeding (see “Cancer” and “Cardiovascular disease”).
Pain control
Chronic pain is prevalent in the elderly PWH, and drug addiction is not rare. The widely used paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) have adverse effects that may become more clinically significant with aging: gastroduodenal toxicity, paracetamol-associated liver dysfunction (particularly with excessive alcohol consumption and liver disease), hypertension, and renal insufficiency.36 Selective cyclooxygenase (COX) 2 inhibitors are better tolerated in the gastrointestinal tract than nonselective NSAIDs, but the risk of cardiovascular disease may increase.37 On the other hand, traditional NSAIDs do inhibit, albeit transiently, COX1 and platelet function and may cause in the elderly PWH a greater need for replacement therapy not only because of more musculoskeletal bleeding but also because of bleeding in dangerous sites, such as the central nervous system.35
Upper gastrointestinal bleeding was reported to occur in 42 of 2285 PWHs (incidence, 1.3 per 100 patient-years) and the use of NSAIDs was associated with an increased bleeding risk (hazard ratio = 3.7), whereas the use of COX2 inhibitors was not.38 Hence, we prefer COX2 inhibitors to NSAIDs when paracetamol, still the drug of first choice, is not effective to control pain. An example of a pain control schedule, based on sequential steps of more and more aggressive treatment in case of failure, is given in Table 3.39 For optimal management, the involvement of pain control teams in comprehensive hemophilia treatment centers may become necessary.
Steps for the use of analgesics in patients with hemophilic arthropathy
| Guideline . | Medication . | Administration . |
|---|---|---|
| 1 | Paracetamol | 500-1000 mg orally, ≤ 6×/d is the initial medication of choice; if not effective, go to Guideline 2. |
| 2 | Celecoxib (COX2 inhibitor) | 100-200 mg orally, 1-2×/d; if not effective, go to Guideline 3. |
| 3 | Paracetamol + codeine orParacetamol + tramadol | 10-20 mg orally, no more than 6×/d, or50-100 mg orally, 3-4×/d; if not effective, go to Guideline 4. |
| 4 | Morphine | Use a slow-release product, starting with 20 mg 2×/d, with an escape to a rapid-release product 10 mg 4×/d. Increase the slow-release product if the rapid-release product is used more than 4×/d. |
| Guideline . | Medication . | Administration . |
|---|---|---|
| 1 | Paracetamol | 500-1000 mg orally, ≤ 6×/d is the initial medication of choice; if not effective, go to Guideline 2. |
| 2 | Celecoxib (COX2 inhibitor) | 100-200 mg orally, 1-2×/d; if not effective, go to Guideline 3. |
| 3 | Paracetamol + codeine orParacetamol + tramadol | 10-20 mg orally, no more than 6×/d, or50-100 mg orally, 3-4×/d; if not effective, go to Guideline 4. |
| 4 | Morphine | Use a slow-release product, starting with 20 mg 2×/d, with an escape to a rapid-release product 10 mg 4×/d. Increase the slow-release product if the rapid-release product is used more than 4×/d. |
Chronic blood-borne infections
The long-term consequences of HCV infection are looming large on those elderly PWHs, particularly with genotype 1 and HIV coinfection, who failed to achieve a sustained viral response with pegylated interferon and ribavirin.8,9 A significant proportion of them develop liver cirrhosis and carry a high risk of hepatocellular carcinoma (HCC).8,15,17 The curative option of liver transplantation is made difficult by various factors: advanced age, frequent HIV coinfection, and the often multifocal presentation of HCC. Periodic ultrasound screening is the only recommendation that can be made to detect HCC earlier in PWHs with cirrhosis. It is also hoped that newer and more efficacious antiviral agents, such as protease inhibitors,40 may soon become safe and effective enough to eradicate HCV in a higher proportion of PWHs unresponsive to pegylated interferon/ribavirin.9
Because of their coagulation defect, PWHs with cirrhosis and portal hypertension probably bleed from the upper gastrointestinal tract more frequently and severely than cirrhosis patients without hemophilia. Therefore, preventive band ligation should be carried out as early as possible in the presence of gastroesophageal varices at high risk of bleeding.
In HIV-infected PWHs, combined antiretroviral therapy (cART) has dramatically reduced the high mortality rate seen before the advent of this treatment in the middle 1990s. cART has also reduced the previously frequent occurrence of non-Hodgkin lymphomas.41,42 On the other hand, cART increases the risk of the metabolic syndrome, diabetes, renal insufficiency, and atherothrombotic cardiovascular disease in nonhemophilia patients.43 Although there are few data on the long-term clinical course of HIV infection in elderly PWHs, it is suspected that cART will induce the same long-term alterations. Monitoring serum lipid, glucose, and creatinine at regular intervals is recommended.
Bleeding pattern
The bleeding pattern typical of younger PWHs may change with aging. Acute hemarthrosis, which accounts for 70% to 80% of hemorrhages in the young, usually becomes less frequent because of a less physically active pattern of life. In some PWHs with severe arthropathy, acute painful bleedings may occur that are unresponsive to CFC. These are more likely to be arterial instead of synovial bleedings, which may require angiographic embolization for adequate control.44
Age-related diseases in elderly persons with hemophilia
Cancer
With the exception of HCC in HCV-infected patients and non-Hodgkin lymphomas in HIV-infected patients, it is unclear whether or not in elderly PWHs the incidence of cancer differs from that in their age-group peers without hemophilia. Experimental data indicate that hemophilic mice form less metastasis from experimental melanomas, perhaps because of less thrombin formation.45 It is uncertain whether or not this is also true in humans.46 Two population studies found a lower incidence of cancer, particularly in patients with severe hemophilia,4,7 but the prevalence of tumors other than HCC and non-Hodgkin lymphoma was 4-fold higher than in the age-matched general population in a small study of elderly PWHs.47
Literature data on the treatment of cancer in elderly PWHs are limited to a few case reports, so that there is no evidence-based recommendation for the optimal management of these cases. Cancer that develops in the elderly PWH should be handled as in any other person with the same type of malignancy, but this logical approach is not without problems. The risk of bleeding inherent in the congenital coagulation defect is increased by several factors, such as the use of invasive diagnostic and therapeutic procedures and chemotherapy- and radiotherapy-induced thrombocytopenia. Replacement therapy should be administered not only on an episodic basis at the time of diagnostic or therapeutic invasive procedures, but also as continuous prophylaxis when chemotherapy or radiotherapy is accompanied by severe thrombocytopenia. It is unknown which platelet count is safe in a PWH with cancer and, hence, does not entail the need of prophylaxis. In patients who develop cancer and are not already on continuous prophylaxis, there is no need to start it if thrombocytopenia is moderate (platelet count > 30 000/μL). On the other hand, continuous prophylaxis with CFC according to the schedule recommended in Table 1 is advised when platelet counts are lower. In patients already receiving continuous prophylaxis, this regimen should continue throughout the period of chemotherapy and/or radiotherapy. In some cases, transfusion with platelets may be necessary. Thrombocytopenia is not itself antithrombotic, so that antithrombotic prophylaxis should be prescribed when cancer is associated with a high risk of thrombosis. After oncologic surgery, low molecular weight heparins should be preferably administered starting postoperatively, with dosages and treatment intervals similar or higher than those recommended for major orthopedic surgery (Table 2).
Cardiovascular disease
Several data indicate that in PWHs mortality from coronary artery disease is lower than in the general male population. Tuinenburg et al48 showed that the standard mortality ratio (calculated by dividing the number of observed deaths by those expected in the general population) ranged from 0.2 to 0.6 in PWHs in the time period spanning from 1989 to 2006.48 On the other hand, coronary artery and other atherothrombotic diseases do indeed occur in PWHs, as it stems from a review on the causes of death in PWHs,49 from a review article based on 42 cases50 and the experiences gained in our large tertiary-care centers. This is not unexpected because if, on one hand, PWHs may be protected from thrombus formation by their hypocoagulability, on the other hand, they are exposed at least as much as normal males to such risk factors for atherosclerosis as aging, smoking, and overweight.51 Other risk factors, such as hypertension, physical inactivity, and chronic renal disease,52 may be even more frequent in PWHs than in the general aging population. Finally, HIV infection and cART may per se increase the risk of the metabolic syndrome, diabetes, renal insufficiency, and hence of atherothrombotic diseases.53
Evidence-based guidelines for the acute treatment and secondary prophylaxis of cardiovascular diseases in PWHs are currently lacking, nor is it known how to handle the increased risk of bleeding associated with invasive procedures and the short- and long-term use of antithrombotic drugs. Our general principle is to treat PWHs like their age-group peers without hemophilia, provided replacement therapy is adapted to the baseline plasma factor deficiency and the added risk of bleeding carried by therapeutic procedures and antithrombotic drugs. With this as background, we elected to provide more details on the management of 2 cardiovascular diseases, acute coronary syndromes and nonvalvular atrial fibrillation (AF), which probably are the most frequent cardiovascular diseases in elderly PWHs, as they are in the population at large.54
Table 4 shows how replacement therapy is planned in PWHs when they develop acute coronary syndromes. Peak factor levels should reach at least 80 U/dL at the time of coronary angiography and percutaneous coronary intervention (PCI) with stenting, maintaining these factor levels for as long as therapeutic dosages of heparin are given. Because most PCI-related bleeding complications occur at the arterial access site, a radial access should be preferred over the femoral access.55 Moreover, for as long as dual antiplatelet therapy is indicated after PCI and stenting, trough plasma levels of 30 U/dL are pursued. The use of bare metal stents instead of drug-eluting stents is preferable, to limit to 1 month the period of dual antiplatelet therapy with aspirin and P2Y12 inhibitors (such as clopidogrel or prasugrel). The choice of bare metal stents, however, must be balanced against the advantage of less restenosis with drug-eluting stents. However, the latter warrant up to 12 months of dual antiplatelet therapy, and hence more prolonged continuous prophylaxis, as indicated in Table 4.
Schedules of replacement therapy with FVIII or FIX concentrates to reach target factor levels in elderly persons with severe hemophilia during cardiac procedures
| Procedure . | Replacement regimen . | |
|---|---|---|
| Dosage . | Target . | |
| Diagnostic coronary angiography | Bolus infusion of 40 U/kg FVIII (80 U/kg FIX), followed by 20 U/kg FVIII (30 U/kg FIX) after 12 h | Peak factor level ≥ 80 U/dL |
| PCI with bare metal stents | ||
| Before the procedure | Bolus infusion of 40 U/kg FVIII (80 U/kg FIX), followed by 20 U/kg FVIII (30 U/kg FIX) after 12 h | Peak factor level ≥ 80 U/dL |
| During dual antiplatelet therapy with aspirin and clopidogrel | Infusions of 50 U/kg FVIII every other day (60-70 U/kg FIX) for 1 mo | Trough factor levels ≥ 30 U/dL |
| During single antiplatelet therapy with aspirin | Infusions of 25-40 U/kg FVIII every other day or 25-50 U/kg FIX 2-3×/wk for 1 y or indefinitely | Trough factor levels ≥ 5 U/dL |
| Fibrinolysis | Bolus infusion of 40 U/kg FVIII (80 U/kg FIX), followed by continuous infusion (3 U/kg/h FVIII or FIX) or 20 U/kg FVIII (30 U/kg FIX) every 12 h for 48 h | Peak factor levels ≥ 80 U/dL, followed by trough levels ≥ 50 U/dL |
| Procedure . | Replacement regimen . | |
|---|---|---|
| Dosage . | Target . | |
| Diagnostic coronary angiography | Bolus infusion of 40 U/kg FVIII (80 U/kg FIX), followed by 20 U/kg FVIII (30 U/kg FIX) after 12 h | Peak factor level ≥ 80 U/dL |
| PCI with bare metal stents | ||
| Before the procedure | Bolus infusion of 40 U/kg FVIII (80 U/kg FIX), followed by 20 U/kg FVIII (30 U/kg FIX) after 12 h | Peak factor level ≥ 80 U/dL |
| During dual antiplatelet therapy with aspirin and clopidogrel | Infusions of 50 U/kg FVIII every other day (60-70 U/kg FIX) for 1 mo | Trough factor levels ≥ 30 U/dL |
| During single antiplatelet therapy with aspirin | Infusions of 25-40 U/kg FVIII every other day or 25-50 U/kg FIX 2-3×/wk for 1 y or indefinitely | Trough factor levels ≥ 5 U/dL |
| Fibrinolysis | Bolus infusion of 40 U/kg FVIII (80 U/kg FIX), followed by continuous infusion (3 U/kg/h FVIII or FIX) or 20 U/kg FVIII (30 U/kg FIX) every 12 h for 48 h | Peak factor levels ≥ 80 U/dL, followed by trough levels ≥ 50 U/dL |
For secondary antithrombotic prophylaxis in patients who recovered from an acute coronary syndrome, we recommend low-dose aspirin (100 mg) under protection with low-dose CFC prophylaxis (Table 4, recommended dosages). Until now, continuous prophylaxis with bypassing agents was never implemented by us in patients with inhibitors who developed an atherothrombotic event because the issue of thrombosis as a potential complication of the continuous prophylactic use of bypassing agents remains a concern.
Fibrinolytic therapy with a tissue-type plasminogen activator is still the most frequently adopted reperfusion strategy for patients who develop ST-elevation myocardial infarction and cannot reach within the recommended timelines an interventional cardiology unit with expertise in PCI. Coagulation factors should be kept at normal levels (80-100 U/dL), preferably by continuous intravenous infusion, for at least 24 to 48 hours during and after fibrinolysis. In the presence of 3-vessel coronary artery disease or stenosis of the left main coronary artery, cardiopulmonary bypass surgery should be chosen and carried out as in nonhemophilic patients, with clotting factor levels kept within normal ranges (80-100 U/dL). Continuous infusion of CFC before, during, and after surgery until wound healing is sufficiently advanced should be preferred over bolus infusion.56,57
Despite the use of these strategies of replacement therapy, the aforementioned revascularization procedures and the associated antithrombotic regimens sometimes cause excessive bleeding, which may be difficult to control and increase mortality. The hemostatic agents that are currently used in persons without hemophilia at the time of bleeding complications should be considered also in those with hemophilia, such as rFVIIa.58
Another cardiovascular disease likely to occur with increasing frequency in the elderly PWH is nonvalvular AF. In the absence of evidence indicating that PWHs are protected from cardiac embolism by their underlying coagulation defect, the occurrence of AF in PWHs confronts us with the issue of how to handle antithrombotic treatment, during and after cardioversion as well as for the long-term prevention of embolic complications.49
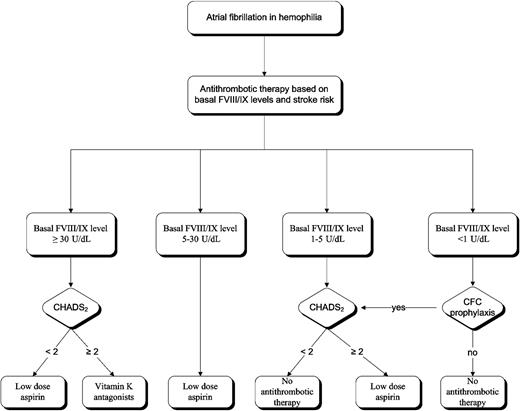
Our strategy for the long-term thromboprophylaxis in PWHs with chronic AF is outlined in Figure 1. In nonhemophilia patients with AF, the risk of cardioembolic stroke is stratified according to several risk factors included in the CHADS2 score59 (Table 5). In principle, oral anticoagulation with vitamin K antagonists should be chosen in PWHs at high risk of embolization and stroke (CHADS2 score of ≥ 2).59-61 In practice, vitamin K antagonists are chosen only when baseline FVIII levels are at least 30 U/dL because in severe and moderate hemophilia these drugs would require the use of continuous prophylaxis during the whole period of treatment, entailing a huge consumption of CFC and exorbitant costs. Hence, low-dose aspirin (100 mg daily) is usually preferred because this antiplatelet agent, although less efficacious than vitamin K antagonists in persons without hemophilia,60 warrants much lower trough levels of coagulation factors (5 U/dL) to avoid significant bleeding in PWHs (Figure 1). A huge problem is how to handle antithrombotic prophylaxis in patients with FVIII inhibitors who develop AF. We would not recommend routine use of aspirin because the risk of bleeding appears too high to us.
Recommendations for antithrombotic and replacement therapy with coagulation factor concentrates in hemophilia A patients with nonvalvular atrial fibrillation.
Recommendations for antithrombotic and replacement therapy with coagulation factor concentrates in hemophilia A patients with nonvalvular atrial fibrillation.
CHADS2 score: risk for stroke in nonhemophilia patients
| CHADS2 score . | Adjusted stroke rate per 100 patient-years . |
|---|---|
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
| CHADS2 score . | Adjusted stroke rate per 100 patient-years . |
|---|---|
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
CHADS2 score is calculated by adding 1 point for congestive heart failure, hypertension, age ≥ 75 years, or diabetes mellitus, and 2 points for prior stroke or transient ischemic attacks. Low risk = 0; moderate risk = 1; high risk ≥ 2.
Pertaining to the choice of rhythm versus rate control in AF, no significant differences were seen in cardiovascular mortality between the 2 approaches,61 although a recent study found better quality of life and left ventricular function if rhythm control were chosen.62 In patients without hemophilia, no differences were found in the occurrence of stroke between high-risk patients in the rate control or sinus rhythm control arms,63 indicating the need for antithrombotic prophylaxis even after successful cardioversion in patients at high risk. It is essential that the selection of PWHs with a high chance of successful cardioversion involves a cardiologist in the team of the specialized hemophilia treatment center.
A recommended strategy for cardioversion in PWHs is outlined in Figure 2. Patients with AF lasting more than 48 hours eligible for cardioversion require transesophageal echocardiography to rule out atrial thrombi and at least 4 weeks of anticoagulation with vitamin K antagonists (international normalized ratio target 2.5; range, 2.0-3.0), whichever method of cardioversion is chosen. The use of transesophageal echocardiography avoids the need for a 4-week period of anticoagulation before cardioversion. This strategy was suitable and safe in our hands, provided adequate CFC prophylaxis was given, with the goal to maintain trough FVIII levels of at least 30 IU/dL. After cardioversion, long-term anticoagulant therapy is needed, as suggested in Figure 1.
Recommendations for antithrombotic and replacement therapy in hemophilia A patients with nonvalvular AF. TEE denotes transesophageal echocardiography.
Recommendations for antithrombotic and replacement therapy in hemophilia A patients with nonvalvular AF. TEE denotes transesophageal echocardiography.
In hemophilia B, particular problems are caused by FIX replacement, which may interfere with vitamin K antagonists and spuriously affect the international normalized ratio. Moreover, one must be aware that oral anticoagulant therapy converts mild hemophilia B into a more severe form, so that replacement therapy with CFC should be implemented in the majority of patients aiming at trough levels of 30 U/dL when vitamin K antagonists are needed.
When cardiac valve replacement is indicated, a bioprosthetic valve should be the first choice to avoid the need of indefinite anticoagulation; but, if necessary, mechanical artificial valves can be used, provided trough factor levels are kept more than 30 U/dL by long-term continuous prophylaxis. We have never been challenged with the formidable problem of PWHs with inhibitors who needed a cardiac valve replacement or operations of cardiac surgery.
These general principles of replacement therapy can also be applied to other cardiovascular diseases. Our preferred antithrombotic therapies are those recommended by the American College of Chest of Physicians,64,65 individually adapted to the peculiar condition of patients with a bleeding tendency that must be corrected by short- or long-term prophylactic replacement therapy, particularly when invasive procedures and antithrombotic drugs are used. It is obvious that all the aforementioned schedules of prophylaxis do dramatically increase the costs of therapy.
Other morbidities in elderly persons with hemophilia
Chronic renal disease probably develops with increasing frequency in the aging PWH because of multiple concomitant risk factors (HIV infection, cART, hematuria, structural renal damage, use of antifibrinolytic amino acids).52 Hence, there may be an increasing need to resort to dialysis. In theory, peritoneal dialysis is preferable to hemodialysis because it does not require the placement of an arterovenous fistula or the administration of heparin to prevent clot formation in the dialyzer, so that coverage with replacement therapy is needed only at the time of catheter insertion. However, this procedure entails a high risk of peritoneal infections, particularly in HCV- and HIV-infected patients. Hence we chose more often hemodialysis, using both heparin and a single dose of CFC replacement aiming at factor levels of 80 U/dL, before and after each procedure.
According to the results of a small cohort study,17 cognitive impairment is no more frequent in PWHs than in their age-group peers, but it must be pointed out that 78 years was the oldest age.17 In cases with severe depression, serotonin reuptake inhibitors are frequently used. However, interference of these drugs with primary hemostasis was reported,66 and their use might increase the risk of upper gastrointestinal bleeding.67 To bypass these uncertainties, we usually use drugs other than serotonin reuptake inhibitors to treat depression.
An important aspect of aging is erectile dysfunction resulting not only from the normal aging process with the related biosocial problems but also comorbidity as painful arthropathy affecting sexual desire and conditions that affect erectile function, such as arteriosclerosis and hypertension.68 The use of multiple drugs to treat the latter may also compromise sexual function. On the whole, living with hemophilia often strains the relationship with the sex partner, and approximately half of the PWH reported their limitations during sexual intercourse, especially when they had painful joints and contractures in hips and knees.68 The oral phosphodiesterase-5 inhibitors (sildenafil and tadalafil) slightly inhibit platelet aggregation in vitro.69,70 Yet they are being used satisfactorily by some of our elderly PWHs without an apparent increase in the incidence of bleeding, despite no prophylactic CFC therapy. As seen in normal persons, a few PWHs develop previously unnoticed epistaxis during the intake of these drugs, because they cause some degree of nasal congestion.71
Prostatic hypertrophy is a common problem in elderly PWHs, and α-reductase inhibitors, such as finasteride, or α-blockers, such as tamsulosin, are commonly used. There are no reports of clinically relevant hemostatic changes while using these drugs, so they can be used safely in PWHs. Genitourinary diseases and prostatic hypertrophy would facilitate the onset of hematuria, and upper gastrointestinal bleeding may become more frequent because of concomitant cirrhosis or malignancies in the gastrointestinal tract. The use of multiple drugs for the treatment of multimorbidity is another potential reason for an increased bleeding tendency.
Cataract surgery needs replacement therapy with CFC. However, we give only a single injection 1 hour before cataract extraction, more with the goal to cover the risk of bleeding associated with local anesthesia than to avoid blood loss because of cataract extraction.
Conclusions
Because of a life expectancy approaching that of the general population, PWHs develop more and more age-related clinical conditions never experienced before, so that treatment centers must enlist the expertise of specialists rarely needed before, such as cardiologists and oncologists. Integrated expertise can usually be offered only by large centers specialized in the comprehensive management of PWHs because they can provide, at the same time, proficiency in coagulation laboratory, hemophilia care, and other surgical and medical subspecialties that are probably not available to the general hematologist.
In general, our recommended approach is to treat the diseases occurring in the elderly PWH as they would be treated in age-group peers without a bleeding disorder. Hence, in this article, we mainly suggested when and how replacement therapy should be changed and adapted when the baseline risk of bleeding is heightened by the use of invasive procedures and/or drugs that, as antithrombotics and chemotherapeutics, cause a bleeding tendency per se. We are aware of the limits of these general recommendations because of the presence of many interacting clinical variables: the strength of the indication for antithrombotic therapy, the magnitude of the risk of thrombosis if antithrombotic therapy is withheld but, on the other hand, the risk of bleeding if therapy is continued. Hence, very often clinical decisions are ultimately made on a case-by-case basis.
What can be done to improve this situation of lack of evidence-based recommendations? Because of the current relative rarity of elderly PWHs, the aforementioned clinical problems cannot readily be answered by adequately powered and controlled clinical trials. Perhaps the best approach is to establish international registries on age-related diseases in elderly PWHs, to collect data at regular intervals from large populations on the events of interest (eg, cardiovascular disease and cancer), on how they are handled, and on the corresponding outcomes. This endeavor is being tackled by European Haemophilia Safety Surveillance (EUHASS; http://euhass.org), a prospective system of reporting adverse events in PWHs in Europe. The 46 hemophilia treatment centers involved in the EUHASS network represent 26 countries and care for more than 14 500 persons with hemophilia, severe von Willebrand disease, and other rare bleeding disorders. Every 3 months, each participating center is asked to report whether or not there were new adverse events, including cardiovascular disease and malignancies. More details on treatment and outcome are requested when events develop. Among other purposes, this registry is meant to facilitate a more evidence-based approach to the management of comorbidities and age-related diseases in the aging PWH and to establish improved guidelines. These are particularly needed because the intensive replacement therapy that we recommend in many of the clinical situations depicted in this article do dramatically increase the usage of CFC and will create in the elderly PWH a second period of intensive consumption, which adds to the early peak of consumption for prophylaxis in children and adolescents with severe hemophilia. It cannot be ruled out that this second peak in the elderly PWH might produce an increase in inhibitor incidence, particularly in patients with mild disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Milan and Utrecht Hemophilia Centres received financial support for this study in the frame of EUHASS. The EUHASS project is supported by the European Union in the frame of the Public Health Program.
Authorship
Contribution: P.M.M. wrote the first draft and the revision of the manuscript; and R.E.G.S., E.S., and E.P.M.-B. reviewed the first draft and revision and made significant additions and contributions to the final version.
Conflict-of-interest disclosure: All authors have received speaker fees and honoraria for consulting by most pharmaceutical companies manufacturing coagulation factors for hemophilia treatment. The authors declare no other competing financial interests.
Correspondence: Pier M. Mannucci, A. Bianchi Bonomi Hemophilia and Thrombosis Center, Department of Medicine and Medical Specialties, Via Pace 9, 20122 Milano, Italy; e-mail: pmmannucci@libero.it.