To the editor:
Recently, Dominici et al very elegantly demonstrated restoration of the osteoblastic hematopoietic stem cell (HSC) niche after lethal marrow radioablation in mice.1 Based on their data, the authors propose a model in which radiation induced an increase in stromal cell–derived factor 1α (SDF-1α), causing the attraction of CXCR4-positive megakaryocytes that survived radiation. A concomitant increase in platelet-derived growth factor-β (PDGF-β) and basic fibroblast growth factor (bFGF) levels, known to promote osteoblast proliferation, leads to restoration of the HSC niche as demonstrated by local engraftment of transplanted HSCs. The effect of radiation on bone marrow (BM) SDF-1α levels in human subjects is unknown. Moreover, data on SDF-1α levels following nonmyeloablative regimens, which are increasingly used in clinical practice, are lacking.
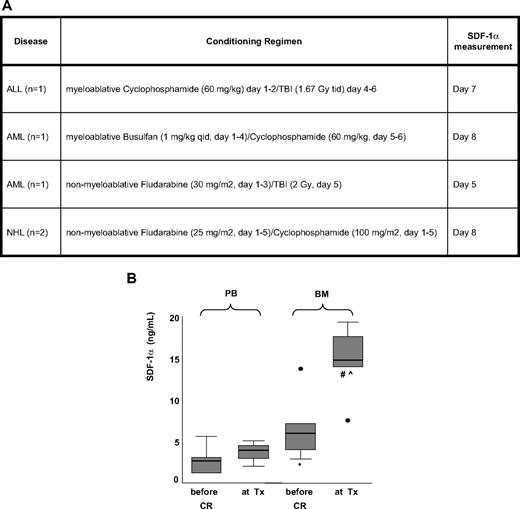
We determined SDF-1α levels in BM and peripheral blood (PB) of 5 patients who underwent an allogeneic stem cell transplantation. Samples were taken on the first day of the conditioning regimen and on the day of the stem cell infusion (Figure 1A). Indeed, we were able to show a significant 2.8-fold increase (± 1.8 SD, P = .04, Figure 1B) in BM SDF-1α levels in patients with hematologic malignancies, being treated with nonmyeloablative or myeloablative conditioning regimens. A similar although less pronounced increase in PB SDF-1α levels was observed (1.7 ± 1.1, Figure 1B). In agreement with the findings of Dominici et al in animals, the increase in protein levels was found to be the result of induction of SDF-1α gene expression, as BM SDF-1α mRNA copies increased a mean of approximately 40-fold, correlating with the increase in SDF-1 BM protein levels (r = 0.92; P = .03).
Increase in SDF-1α levels in peripheral blood and bone marrow of patients being differently conditioned. (A) Conditioning regimens in 5 patients undergoing allogeneic stem cell transplantation. Patients gave informed consent according to the institutional guidelines in accordance with the Declaration of Helsinki. (B) Effect of myeloablative and nonmyeloablative regimens on SDF-1 protein levels in peripheral blood and bone marrow. SDF-1 levels (in ng/mL) were determined in both PB plasma and BM plasma before the conditioning regimen (before CR) and at the day of stem cell transplantation (at Tx). *P = .043 BM before conditioning regimen vs PB before conditioning regimen. #P = .043 BM at Tx vs BM before conditioning regimen. ⋀P = .043 BM at Tx vs PB at Tx. ● indicates outlier (before CR; conditioned with myeloablative cyclophosphamide/TBI, at Tx; conditioned with mild nonmyeloablative fludarabine/low-dose TBI). Gray boxes indicate interquartile range (between 75th and 25th percentiles). Horizontal black line through box indicates median. Error bars indicate range.
Increase in SDF-1α levels in peripheral blood and bone marrow of patients being differently conditioned. (A) Conditioning regimens in 5 patients undergoing allogeneic stem cell transplantation. Patients gave informed consent according to the institutional guidelines in accordance with the Declaration of Helsinki. (B) Effect of myeloablative and nonmyeloablative regimens on SDF-1 protein levels in peripheral blood and bone marrow. SDF-1 levels (in ng/mL) were determined in both PB plasma and BM plasma before the conditioning regimen (before CR) and at the day of stem cell transplantation (at Tx). *P = .043 BM before conditioning regimen vs PB before conditioning regimen. #P = .043 BM at Tx vs BM before conditioning regimen. ⋀P = .043 BM at Tx vs PB at Tx. ● indicates outlier (before CR; conditioned with myeloablative cyclophosphamide/TBI, at Tx; conditioned with mild nonmyeloablative fludarabine/low-dose TBI). Gray boxes indicate interquartile range (between 75th and 25th percentiles). Horizontal black line through box indicates median. Error bars indicate range.
Interestingly, there were indications that the increase in SDF-1α was dependent on the type of conditioning regimen. In the sole patient being mildly conditioned with fludarabine/low-dose total body irradiation (TBI) no increase in bone marrow SDF-1α mRNA was found, corresponding with only a minor increase in BM SDF-1 protein levels (from 6.0 to 7.5 ng/mL, see outlier at Tx, Figure 1B). In this single patient, platelets did not recover more than 100 × 109/L in 100 days. In contrast, the other 4 patients, showing a more pronounced increase in BM SDF-1α mRNA (2.0- to 104.0-fold increase) and protein levels (1.4- to 5.2-fold increase), recovered completely within 20 days. This observation supports the hypothesis of Dominici et al that bone marrow SDF-1α levels improve stem cell engraftment. To substantiate the observation that conditioning with fludarabine/low dose TBI has only limited effects, we studied PB SDF-1α levels in an additional group of 7 patients and confirmed that there was no increase PB SDF-1α protein levels (n = 7, P = .24). In contrast, the more dose-intense nonmyeloablative regimen with fludarabine/cyclophosphamide did increase PB SDF-1α protein levels (1.7- ± 0.6-fold increase; n = 7, P = .02), as did myeloablative regimens (1.6- ± 0.6-fold increase, n = 8, P = .05).
Our data indicate that the key regulatory role of SDF-1α in restoration of the murine stem cell niche as found by Dominici et al can be translated to both the myeloablative and nonmyeloablative clinical transplantation setting in humans. Whether the type of nonmyeloablative conditioning matters warrants further research. However, our data suggest that reassessment of the agents used in conditioning regimens might improve stem cell engraftment efficiency. This may be especially relevant when limited numbers of stem cells are available, as in cord blood transplantations.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Current address of J.v.d.B.: Department of Nephrology, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands
Correspondence: Sonja Zweegman, Department of Hematology, VU University Medical Center, de Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: s.zweegman@vumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal