Abstract
The urokinase receptor (uPAR) plays an important role in regulation of fibronolysis, cell migration, and adhesion. In this study, we examined whether uPAR plays a role in modulating efferocytosis of neutrophils. Macrophages from uPAR−/− mice demonstrated enhanced ability to engulf viable wild-type (WT) neutrophils in vitro and in vivo in the lungs. The increased phagocytic activity of uPAR−/− macrophages was abrogated by incubation with soluble uPAR (suPAR), arginine-glycine-aspartic acid (RGD)–containing peptides, or anti-integrin antibodies. There was increased uptake of viable uPAR−/− neutrophils by WT macrophages. Incubation of uPAR−/− neutrophils with suPAR or anti-integrin antibodies diminished uptake by WT macrophages to baseline. Uptake of uPAR−/− neutrophils by uPAR−/− macrophages was not enhanced. However, incubation of uPAR−/− neutrophils or uPAR−/− macrophages, but not both, with suPAR enhanced the uptake of viable uPAR−/− neutrophils by uPAR−/− macrophages. The adhesion of WT neutrophils to uPAR−/− macrophages was higher than to WT macrophages. uPAR−/− neutrophils demonstrated increased adhesion to suPAR, which was abrogated by blocking of low-density lipoprotein related protein and integrins. Expression of uPAR on the surface of apoptotic neutrophils was reduced compared with levels on viable neutrophils. These results demonstrate a novel role for uPAR in modulating recognition and clearance of neutrophils.
Introduction
Clearance of apoptotic cells by phagocytes, also referred to as efferocytosis, plays an essential role in maintaining normal tissue homeostasis and in the regulation of immune responses.1,2 Phagocytosis of dying cells prevents leakage of cytotoxic or antigenic intracellular contents into the extracellular milieu with the resultant initiation of inflammatory processes and tissue injury.2 Ingestion of apoptotic cells by macrophages results in the release of anti-inflammatory cytokines, such as IL-10, that suppress the production of proinflammatory mediators.3,4 Defects or delays in apoptotic corpse elimination are associated with autoimmune and inflammatory diseases, including systemic lupus erythematosus and acute lung injury.5-7
Several molecules on the apoptotic cell surface have been demonstrated to be involved in efferocytosis. Phosphatidylserine (PtdSer) and calreticulin (CRT) play an important role in modulating uptake of apoptotic cells by phagocytes, functioning as “eat me” signals.1,8 PtdSer is located on the inner side of the cell membrane of viable cells and is externalized during apoptosis.9,10 PtdSer is recognized and bound indirectly by PtdSer receptors, such as integrins and Mer kinase through opsonins, including milk-fat globule EGF-factor 811,12 and growth arrest-specific gene 6.13 However, recent evidence indicates that PtdSer is also directly recognized by membrane proteins on phagocytes, including TIM4 and stabilin-2.14,15 CRT mediates efferocytosis through binding to the low-density lipoprotein related protein (LRP) on phagocytes.8,16 The expression of CRT is increased on the apoptotic cell surface, and such enhanced expression contributes to apoptotic cell engulfment.8 However, despite the ubiquitous presence of CRT on the surface of viable cells and exposure of PtdSer on localized regions of stimulated, viable cells, the concomitant expression of “don't eat me” molecules, such as CD47, CD31, and PAI-1, prevents the uptake of viable cells by adjacent phagocytes.7,8,17
The urokinase-type plasminogen activator receptor (uPAR) consists of 3 globule-like domains (D1, D2, and D3) but lacks trans-membrane and cytoplasmic structures.18-20 Instead, uPAR is anchored on the cell membrane through a glycosylphosphatidylinositol (GPI) moiety.18-20 uPAR can be released from the plasma membrane by GPI-specific phospholipase C or D to form soluble uPAR (suPAR).18 uPAR itself does not transduce extracellular signals. However, association between uPAR and other membrane proteins enables uPAR to participate in the activation of downstream signaling events.18,19 For example, uPAR laterally binds to integrins, such as αM, αV, β1, β2, and β3, and regulates cell adhesion and migration mediated by these integrins.18,19,21-26 suPAR also interacts with the same integrins as does membrane-linked uPAR.18
uPAR expression is increased in cytokine or bacteria-activated macrophages, monocytes, and other cell populations and contributes to the infiltration of inflammatory cells into infected tissues or organs, including the lungs.27-31 Given the association between uPAR and integrins, as well as the involvement of integrins in efferocytosis and the importance of neutrophil clearance in the resolution of inflammation, we investigated the potential role that uPAR may occupy in the regulation of neutrophil efferocytosis. The present studies show that uPAR is an active participant in efferocytosis under both in vitro and in vivo conditions.
Methods
Materials
Custom cocktail antibodies and negative selection columns for neutrophil isolation were purchased from StemCell Technologies. Brewer thioglycollate was from Sigma-Aldrich. Annexin V–FITC and propidium iodide were from Calbiochem. Mouse anti-integrin αM, αV, β1, β2, β3, or β4 antibodies, and mouse suPAR were from R&D Systems. Rabbit anti-uPAR, rabbit anti–PAI-1, and mouse anti-CD47 antibodies were from Santa Cruz Biotechnology. Mouse anti-LRP antibody was from American Diagnostica. Alexa 488–conjugated goat anti–rabbit antibodies and 2-μm carboxylate-modified beads were from Invitrogen. RGD- and RAD (Arg-Ala-Asp)–containing peptides were from BIOMOL Research Laboratories. LRP-specific blocking peptide RAP was a gift from Dr Dudley Strickland (University of Maryland, Baltimore, MD).
Mice
uPAR knockout mice that have been backcrossed with C57/BL6 mice for at least 9 generations and age- and sex-matched control C57/BL6 mice were purchased from The Jackson Laboratory. Eight- to 10-week-old animals were used for experiments. Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama (Birmingham, AL).
Isolation and culture of bone marrow neutrophils and peritoneal macrophages
Isolation of bone marrow neutrophils was performed as previously described.32
Induction and assessment of neutrophil apoptosis
Apoptosis of neutrophils was induced as previously described.32
In vitro efferocytosis assay
Assays for in vitro efferocytosis of neutrophils were performed as previously described.7 To determine the phagocytosis of carboxylate-modified beads, 2-μm carboxylate-modified beads were presented to wild-type (WT) or uPAR−/− macrophages for 1 hour. The percentage of macrophages that ingested beads was taken as phagocytic index.
In vivo efferocytosis assay
In vivo efferocytosis assay was performed as previously described.7
Flow cytometric analysis
The expression of uPAR on the surface of WT or uPAR−/− macrophages and neutrophils was assessed by flow cytometry. Macrophages or neutrophils were incubated with anti-uPAR antibodies (1 μg/mL) for 1 hour on ice. The cells were then fixed in phosphate-buffered saline (PBS) containing 4% paraformaldehyde and 3% sucrose for 20 minutes. After 3 washes with PBS, the macrophages or neutrophils were incubated with Alexa 488–conjugated goat anti–rabbit antibody for 60 minutes on ice. The fluorescent intensity was then determined by flow cytometry analysis. To determine the effects of anti-integrin antibodies on the binding of suPAR to uPAR−/− macrophages, the cells were incubated with anti-integrin αV, β3, β4 antibodies (1 μg/mL) or isotype IgG control for 30 minutes followed by addition of Chromeo 488 (Active Motif)–labeled mouse suPAR (1 μg/mL) for 30 minutes and then washed with PBS 5 times.
Adhesion assay
To determine the adhesion of viable WT and uPAR−/− neutrophils to suPAR, 96-well culture plates were precoated with 1 μg/mL suPAR or bovine serum albumin (BSA) for 3 hours at room temperature and washed 3 times with PBS. Fluorescent-labeled WT and uPAR−/− neutrophils were added to the wells, incubated 30 minutes at 37°C, and then the wells washed 5 times with PBS. Fluorescence intensity was determined by a plate reader at excitation wavelength 488 nM. The ratio of the residual fluorescence intensity to the initial fluorescence intensity of each well was calculated. To determine the heterogenic adhesion of WT neutrophils to WT and uPAR−/− macrophages, WT and uPAR−/− macrophages were plated on 96-well plates. Fluorescent-labeled WT neutrophils were added to the macrophages for the indicated time, and the wells were washed 7 times with PBS. The ratio of the residual fluorescence intensity to the initial fluorescence intensity of each well was calculated.
Immunofluorescent confocal microscopy
Viable neutrophils or macrophages were incubated with Chromeo 488–labeled mouse suPAR (1 μg/mL) or mouse albumin for 30 minutes. The cells were then fixed and stained without or with anti-integrin αV or β3 antibodies overnight and then incubated with secondary antibodies conjugated with Alexor 594 for 1 hour and confocal images obtained. To examine the expression of uPAR on the surface of viable or apoptotic neutrophils, the cells were stained with anti-uPAR antibodies. Confocal images were taken for viable and apoptotic cells under the same settings.
Statistical analysis
For each experiment, macrophages or neutrophils were isolated and pooled from groups of mice (n = 3 or 4). One-way analysis of variance (for multiple groups) or Student t test (for comparisons between 2 groups) was used. A P value less than .05 was considered to be statistically significant.
Results
uPAR−/− macrophages demonstrate enhanced phagocytosis of viable neutrophils
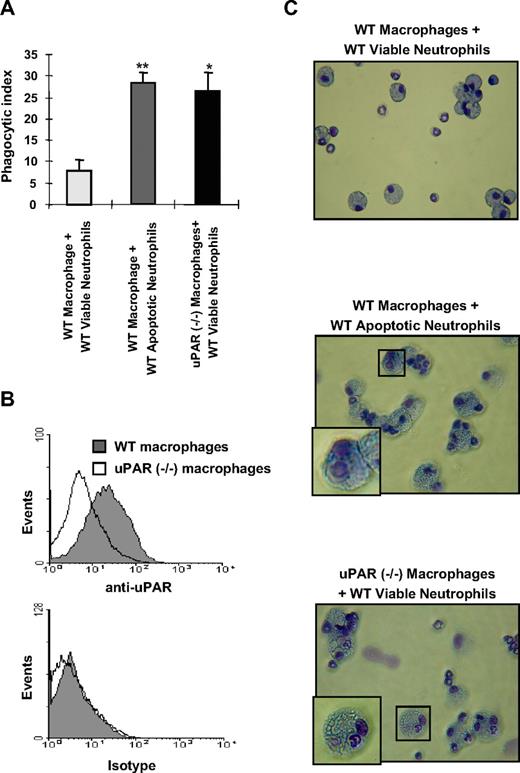
To investigate whether uPAR regulates the phagocytic activities of macrophages, we isolated peritoneal macrophages from WT and uPAR−/− mice and determined their ability to ingest viable neutrophils. uPAR−/− macrophages demonstrated significantly higher phagocytosis of WT viable neutrophils than did WT macrophages (Figure 1A). To confirm that uPAR is expressed on the surface of WT macrophages, we performed flow cytometry using anti-uPAR antibodies and found that there is much higher uPAR expression on WT compared with uPAR−/− macrophages, which only showed low levels of nonspecific staining (Figure 1B).
uPAR−/− macrophages demonstrate enhanced phagocytosis of viable neutrophils. (A) The phagocytosis of viable WT neutrophils by uPAR−/− macrophages is significantly increased compared with phagocytic indices when WT neutrophils are incubated with WT macrophages, and is similar to that present when WT macrophages are exposed to apoptotic WT neutrophils. A total of 106 viable or apoptotic WT neutrophils were added into each well of a 96-well plate containing adherent WT or uPAR−/− macrophage monolayers, and efferocytosis assays performed as described in “In vitro efferocytosis assay.” Phagocytic indices are expressed as the percentage of macrophages containing at least 1 ingested neutrophil. *P < .05, **P < .01, compared with “WT macrophages + viable WT neutrophils.” (B) uPAR is expressed on the surface of WT, but not uPAR−/− macrophages. WT and uPAR−/− macrophages were incubated with anti-uPAR antibodies for 1 hour. The cells were then incubated with FITC-conjugated secondary antibodies and flow cytometry performed. (C) uPAR−/− macrophages actively phagocytose viable WT neutrophils. Viable or apoptotic WT neutrophils were added to WT or uPAR−/− macrophages. After 60 minutes, cytospin slides were prepared for Wright-Giemsa staining. Slides were viewed with a Labor LUX 12 microscope (Leitz) using a Phaco 2 lens at 40×/0.65. Images were acquired using a Sony cybershot camera model DSC-H2 and were processed with Adobe Photoshop version 7.0 software (Adobe Systems). As shown in the representative figures, the WT viable neutrophils ingested by uPAR−/− macrophages showed normal nuclear and chromatin patterns, consistent with being viable and not apoptotic.
uPAR−/− macrophages demonstrate enhanced phagocytosis of viable neutrophils. (A) The phagocytosis of viable WT neutrophils by uPAR−/− macrophages is significantly increased compared with phagocytic indices when WT neutrophils are incubated with WT macrophages, and is similar to that present when WT macrophages are exposed to apoptotic WT neutrophils. A total of 106 viable or apoptotic WT neutrophils were added into each well of a 96-well plate containing adherent WT or uPAR−/− macrophage monolayers, and efferocytosis assays performed as described in “In vitro efferocytosis assay.” Phagocytic indices are expressed as the percentage of macrophages containing at least 1 ingested neutrophil. *P < .05, **P < .01, compared with “WT macrophages + viable WT neutrophils.” (B) uPAR is expressed on the surface of WT, but not uPAR−/− macrophages. WT and uPAR−/− macrophages were incubated with anti-uPAR antibodies for 1 hour. The cells were then incubated with FITC-conjugated secondary antibodies and flow cytometry performed. (C) uPAR−/− macrophages actively phagocytose viable WT neutrophils. Viable or apoptotic WT neutrophils were added to WT or uPAR−/− macrophages. After 60 minutes, cytospin slides were prepared for Wright-Giemsa staining. Slides were viewed with a Labor LUX 12 microscope (Leitz) using a Phaco 2 lens at 40×/0.65. Images were acquired using a Sony cybershot camera model DSC-H2 and were processed with Adobe Photoshop version 7.0 software (Adobe Systems). As shown in the representative figures, the WT viable neutrophils ingested by uPAR−/− macrophages showed normal nuclear and chromatin patterns, consistent with being viable and not apoptotic.
As shown in Figure 1C (top panel), there was only minimal uptake of viable WT neutrophils by WT macrophages. However, coincubation of uPAR−/− macrophages with viable WT neutrophils clearly demonstrated enhanced phagocytosis of viable neutrophils, which did not display the condensed nuclei present with apoptotic neutrophils (compare Figure 1C middle panel with Figure 1C bottom panel). These findings confirm that uPAR−/− macrophages do indeed have significantly greater ability to phagocytose viable WT neutrophils than do WT macrophages.
Modulation of uPAR on the surface of macrophages regulates phagocytosis of viable neutrophils
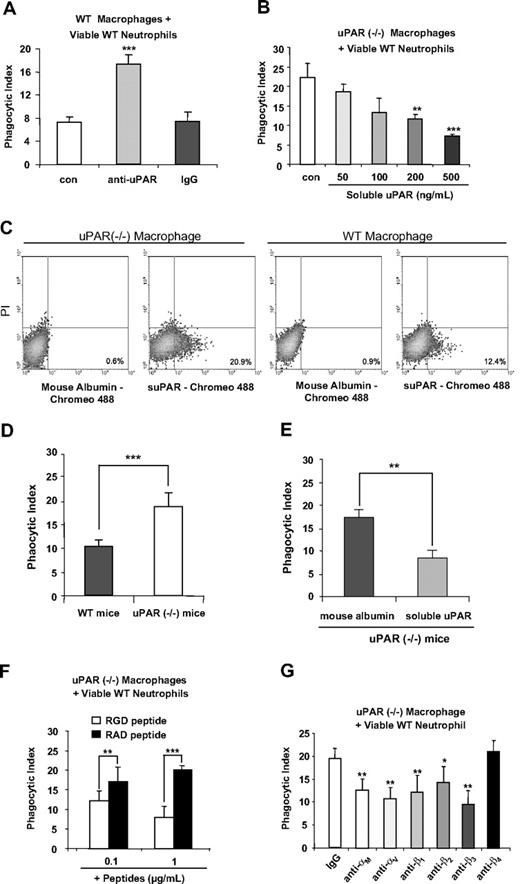
Although we found that uPAR−/− macrophages demonstrate enhanced phagocytosis of viable neutrophils, it was unknown whether these effects are a direct result of the absence of uPAR from the cell surface or of secondary events resulting from deletion of uPAR. To examine this issue, we pretreated WT macrophages with anti-uPAR antibodies before the phagocytosis assays. As was seen with uPAR−/− macrophages, WT macrophages treated with anti-uPAR antibodies demonstrated significantly higher phagocytosis of viable WT neutrophils than did WT macrophages treated with control antibodies (Figure 2A), suggesting that uPAR is directly involved in the phagocytosis of viable neutrophils.
Modulation of uPAR on the surface of macrophages regulates the phagocytosis of viable neutrophils in vitro and in vivo, and is dependent on macrophage-associated integrins. (A) Blockade of uPAR enhances the ability of WT macrophages to phagocytose viable WT neutrophils. WT macrophages were preincubated without (con) or with 1 μg/mL anti-uPAR antibodies or rabbit IgG for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes and the phagocytic index determined. ***P < .001 compared with the control group. (B) suPAR dose-dependently decreases the ability of uPAR−/− macrophages to phagocytose viable WT neutrophils. uPAR−/− macrophages were preincubated without (con) or with suPAR at the indicated concentrations for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes and the phagocytic index determined. **P < .01, ***P < .001 compared with the control group. (C) suPAR binds to the surface of uPAR−/− and WT macrophages. The cells were incubated with Chromeo 488–labeled mouse suPAR (1 μg/mL) or mouse albumin for 30 minutes and then washed with PBS 5 times. Fluorescent intensity was determined by flow cytometry. (D) uPAR−/− alveolar macrophages demonstrate enhanced ability to phagocytose viable WT neutrophils in vivo. A total of 10 × 106 viable WT neutrophils were injected intratracheally into WT or uPAR−/− mice (n = 5 in each group). After 90 minutes, the mice were killed and bronchoalveolar lavage performed with 3 mL PBS. Cytospin slides were prepared using 250 μL bronchoalveolar lavage fluids. Phagocytic indices were determined as described in “In vivo efferocytosis assay.” (E) Exposure to suPAR in vivo abrogates the enhanced ability of uPAR−/− alveolar macrophages to phagocytose viable WT neutrophils in vivo. A total of 10 μg suPAR or mouse albumin was intratracheally instilled 1 hour before intratracheal administration of 10 × 106 viable WT neutrophils into uPAR−/− mice (n = 5 in each group). Bronchoalveolar lavage was performed 90 minutes after injection of neutrophils and phagocytic indices determined. n = 5 in each group. **P < .01. ***P < .001. (F) RGD-, but not RAD-containing, peptides abrogate the enhanced activity of uPAR−/− macrophages to phagocytose viable WT neutrophils. uPAR−/− macrophages were preincubated with 0.1 or 1 μg/mL RGD or RAD peptides for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes, after which the phagocytic index was determined. **P < .01. ***P < .001. (G) Integrins participate in the enhanced ability of uPAR−/− macrophages to phagocytose viable WT neutrophils. uPAR−/− macrophages were preincubated with 1 μg/mL mouse IgG, or antibodies to the integrins αM, αV, β1, β2, β3, or β4 for 30 minutes. The medium was then changed to RPMI plus 5% FBS. and viable WT neutrophils were added for 60 minutes, after which the phagocytic index was determined. *P < .05, **P < .01, compared with the control group treated with IgG.
Modulation of uPAR on the surface of macrophages regulates the phagocytosis of viable neutrophils in vitro and in vivo, and is dependent on macrophage-associated integrins. (A) Blockade of uPAR enhances the ability of WT macrophages to phagocytose viable WT neutrophils. WT macrophages were preincubated without (con) or with 1 μg/mL anti-uPAR antibodies or rabbit IgG for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes and the phagocytic index determined. ***P < .001 compared with the control group. (B) suPAR dose-dependently decreases the ability of uPAR−/− macrophages to phagocytose viable WT neutrophils. uPAR−/− macrophages were preincubated without (con) or with suPAR at the indicated concentrations for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes and the phagocytic index determined. **P < .01, ***P < .001 compared with the control group. (C) suPAR binds to the surface of uPAR−/− and WT macrophages. The cells were incubated with Chromeo 488–labeled mouse suPAR (1 μg/mL) or mouse albumin for 30 minutes and then washed with PBS 5 times. Fluorescent intensity was determined by flow cytometry. (D) uPAR−/− alveolar macrophages demonstrate enhanced ability to phagocytose viable WT neutrophils in vivo. A total of 10 × 106 viable WT neutrophils were injected intratracheally into WT or uPAR−/− mice (n = 5 in each group). After 90 minutes, the mice were killed and bronchoalveolar lavage performed with 3 mL PBS. Cytospin slides were prepared using 250 μL bronchoalveolar lavage fluids. Phagocytic indices were determined as described in “In vivo efferocytosis assay.” (E) Exposure to suPAR in vivo abrogates the enhanced ability of uPAR−/− alveolar macrophages to phagocytose viable WT neutrophils in vivo. A total of 10 μg suPAR or mouse albumin was intratracheally instilled 1 hour before intratracheal administration of 10 × 106 viable WT neutrophils into uPAR−/− mice (n = 5 in each group). Bronchoalveolar lavage was performed 90 minutes after injection of neutrophils and phagocytic indices determined. n = 5 in each group. **P < .01. ***P < .001. (F) RGD-, but not RAD-containing, peptides abrogate the enhanced activity of uPAR−/− macrophages to phagocytose viable WT neutrophils. uPAR−/− macrophages were preincubated with 0.1 or 1 μg/mL RGD or RAD peptides for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes, after which the phagocytic index was determined. **P < .01. ***P < .001. (G) Integrins participate in the enhanced ability of uPAR−/− macrophages to phagocytose viable WT neutrophils. uPAR−/− macrophages were preincubated with 1 μg/mL mouse IgG, or antibodies to the integrins αM, αV, β1, β2, β3, or β4 for 30 minutes. The medium was then changed to RPMI plus 5% FBS. and viable WT neutrophils were added for 60 minutes, after which the phagocytic index was determined. *P < .05, **P < .01, compared with the control group treated with IgG.
We next determined whether incubation of uPAR−/− macrophages with suPAR abrogates the enhanced phagocytosis of neutrophils. As shown in Figure 2B, exposure of uPAR−/− macrophages to suPAR dose-dependently decreased phagocytosis of viable neutrophils. To determine whether suPAR binds to uPAR−/− macrophages, the cells were incubated with Chromeo 488–labeled suPAR and flow cytometry analysis performed. As shown in Figure 2C, suPAR, but no mouse albumin, bound to uPAR−/− macrophages. Of note, suPAR also bound to WT macrophages, but to a lesser extent than was found with uPAR−/− macrophages, suggesting that the receptors for suPAR may be occupied by endogenous uPAR on the WT cell surface.
uPAR−/− alveolar macrophages demonstrate enhanced phagocytic activity in vivo
To determine whether the enhanced ability of uPAR−/− macrophages to engulf viable WT neutrophils also occurs under in vivo conditions, viable WT neutrophils were intratracheally injected into WT and uPAR−/− mice and the phagocytic indices of alveolar macrophages determined. As shown in Figure 2D, there was significantly enhanced phagocytosis of viable WT neutrophils by uPAR−/− alveolar macrophages in vivo. In addition, we found that the enhanced phagocytosis of viable WT neutrophils by uPAR−/− alveolar macrophages was efficiently abrogated when suPAR was administered intratracheally before injection of viable WT neutrophils (Figure 2E).
Integrins contribute to the enhanced phagocytosis of neutrophils by uPAR−/− macrophages
uPAR is a GPI-anchored protein and does not directly transduce extracellular signals.18 Thus, it is doubtful that the enhanced ability of uPAR−/− macrophages to engulf viable neutrophils is simply the result of loss of uPAR acting as a “don't eat” signal on the cell surface. Rather, because uPAR has interactions with multiple membrane proteins, including integrins,18,33,34 a possible mechanism for the ability of uPAR to modulate phagocytosis of neutrophils is through regulating the exposure of uPAR-associated membrane proteins. To explore this hypothesis, we examined whether integrins are involved in the enhanced phagocytic activities of uPAR−/− macrophages. In initial experiments, uPAR−/− macrophages were incubated with integrin-blocking RGD-containing peptides before the phagocytosis assays. As shown in Figure 2F, exposure of uPAR−/− macrophages to RGD-containing peptides efficiently abrogated the enhanced phagocytosis of viable WT neutrophils by these macrophages. In contrast, addition of RAD-containing peptides, which do not block integrin binding to extracellular matrix ligands, did not affect phagocytosis of neutrophils. These data suggest that integrins are necessary for the enhanced phagocytosis of viable WT neutrophils by uPAR−/− macrophages and also that uPAR negatively regulates such activities.
RGD-containing peptides block the ligand-binding activities of multiple integrins.35 To elucidate which integrins are involved in the enhanced phagocytic activities of uPAR−/− macrophages, the macrophages were incubated with antibodies to the integrins αM, αV, β1, β2, β3, or β4 before being included in phagocytosis assays. As shown in Figure 2G, antibodies to αM, αV, β1, β2, and β3, but not to β4, blocked the enhanced phagocytosis of viable WT neutrophils by uPAR−/− macrophages.
Previous studies found that uPAR is associated with vitronectin (Vn).36,37 To demonstrate whether Vn is involved in modulating the uptake of viable neutrophils by macrophages, we performed the phagocytosis experiments in serum-free medium with or without supplementation with Vn. As shown in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), uPAR−/− macrophages ingested similar numbers of viable neutrophils in serum-free medium as when medium containing 5% FBS was used. Addition of Vn to the neutrophil/macrophage cultures had no effect on neutrophil phagocytosis by uPAR−/− macrophages, demonstrating that Vn did not contribute to the enhanced phagocytic index associated with absence of uPAR from the macrophage surface.
LRP mediates the enhanced phagocytosis of neutrophils by uPAR−/− macrophages
Integrins, particularly αVβ3, are essential to the phagocytosis of apoptotic cells.1 Although we found that integrins also contribute to the enhanced phagocytic activities of uPAR−/− macrophages, there are no previous data showing that integrins directly participate in the phagocytosis of viable cells. However, it has been demonstrated that the LRP mediates viable cell phagocytosis by binding to CRT present on the surface of viable cells.8
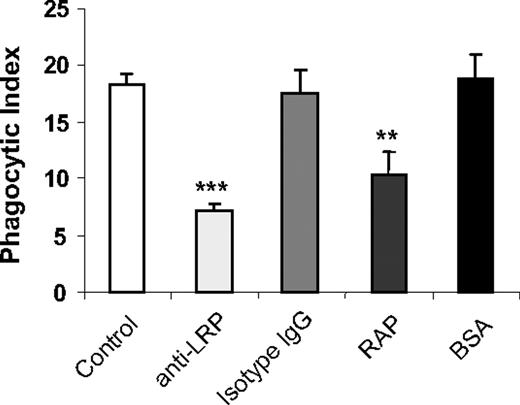
To determine whether LRP participates in the enhanced phagocytosis of viable WT neutrophils by uPAR−/− macrophages, we incubated uPAR−/− macrophages with anti-LRP antibodies or the LRP specific blocking peptide, RAP, before inclusion in phagocytosis assays. As shown in Figure 3, anti-LRP antibodies or RAP diminished the enhanced phagocytic activities of uPAR−/− macrophages. As a control, preincubation of uPAR−/− macrophages with isotype-matched IgG or BSA had no effect on the phagocytic index.
LRP participates in the enhanced phagocytosis of viable WT neutrophils by uPAR−/− macrophages. uPAR−/− macrophages were preincubated without or with 1 μg/mL mouse IgG, anti-LRP antibodies, BSA, or RAP for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes, after which the phagocytic index was determined. **P < .01, compared with the group treated with isotype IgG. ***P < .001, compared with the group treated with BSA.
LRP participates in the enhanced phagocytosis of viable WT neutrophils by uPAR−/− macrophages. uPAR−/− macrophages were preincubated without or with 1 μg/mL mouse IgG, anti-LRP antibodies, BSA, or RAP for 30 minutes. The medium was then changed to RPMI plus 5% FBS, and viable WT neutrophils were added for 60 minutes, after which the phagocytic index was determined. **P < .01, compared with the group treated with isotype IgG. ***P < .001, compared with the group treated with BSA.
uPAR−/− neutrophils demonstrate increased phagocytosis when incubated with WT macrophages
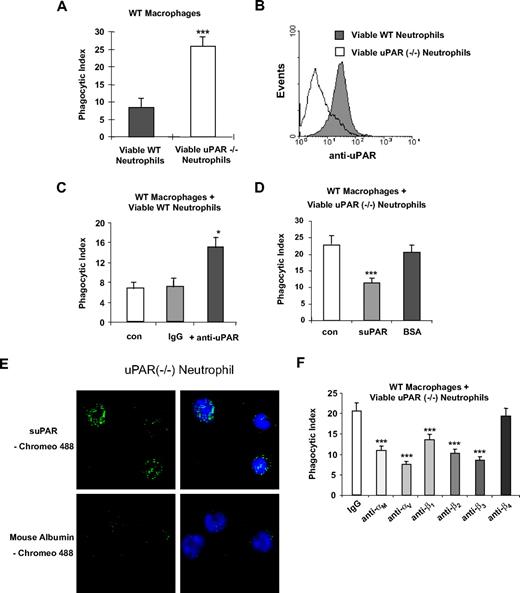
The enhanced phagocytic activities of uPAR−/− macrophages prompted us to ask whether uPAR deficiency on the surface of neutrophils would affect their engulfment by macrophages. To address this issue, we isolated neutrophils from WT and uPAR−/− mice and then performed phagocytosis assays with WT macrophages. As shown in Figure 4A, phagocytosis of viable uPAR−/− neutrophils by WT macrophages was significantly greater than that found when WT neutrophils were incubated with WT macrophages. Figure 4B confirms that the uPAR−/− neutrophils had no staining with anti-uPAR antibodies compared with WT neutrophils.
Phagocytosis of viable uPAR−/− neutrophils by WT macrophages is increased. (A) Increased phagocytosis of uPAR−/− neutrophils by WT macrophages. A total of 106 viable WT or uPAR−/− neutrophils were added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. ***P < .001, compared with the phagocytosis with viable WT neutrophils. (B) uPAR is expressed on the surface of WT, but not uPAR−/− neutrophils. Flow cytometry assays were performed with anti-uPAR antibodies as in Figure 1B. (C) Blockade of uPAR enhances the phagocytosis of viable WT neutrophils by WT macrophages. WT neutrophils were preincubated without (con) or with 1 μg/mL anti-uPAR antibodies or rabbit IgG for 30 minutes. The cells were then resuspended in RPMI plus 5% FBS and added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. *P < .05, compared with the group treated with IgG. (D) suPAR abrogates the enhanced phagocytosis of viable WT neutrophils by WT macrophages. Viable uPAR−/− neutrophils were preincubated without (con) or with 1 μg/mL suPAR or BSA for 30 minutes. The cells was then resuspended in RPMI plus 5% FBS and added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. ***P < .001, compared with the group treated with BSA. (E) suPAR binds to the surface of viable neutrophils. The cells were incubated with Chromeo 488-labeled mouse suPAR (1 μg/mL) or mouse albumin for 30 minutes and washed 5 times with PBS. Representative confocal images are shown. (F) Integrins participate in the enhanced phagocytosis of viable uPAR−/− neutrophils by WT macrophages. uPAR−/− neutrophils were preincubated with 1 μg/mL mouse IgG or antibodies to the integrins αM, αV, β1, β2, β3, or β4 for 30 minutes. The cells were then resuspended in RPMI plus 5% FBS and added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. **P < .01, ***P < .001, compared with the control group treated with IgG.
Phagocytosis of viable uPAR−/− neutrophils by WT macrophages is increased. (A) Increased phagocytosis of uPAR−/− neutrophils by WT macrophages. A total of 106 viable WT or uPAR−/− neutrophils were added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. ***P < .001, compared with the phagocytosis with viable WT neutrophils. (B) uPAR is expressed on the surface of WT, but not uPAR−/− neutrophils. Flow cytometry assays were performed with anti-uPAR antibodies as in Figure 1B. (C) Blockade of uPAR enhances the phagocytosis of viable WT neutrophils by WT macrophages. WT neutrophils were preincubated without (con) or with 1 μg/mL anti-uPAR antibodies or rabbit IgG for 30 minutes. The cells were then resuspended in RPMI plus 5% FBS and added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. *P < .05, compared with the group treated with IgG. (D) suPAR abrogates the enhanced phagocytosis of viable WT neutrophils by WT macrophages. Viable uPAR−/− neutrophils were preincubated without (con) or with 1 μg/mL suPAR or BSA for 30 minutes. The cells was then resuspended in RPMI plus 5% FBS and added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. ***P < .001, compared with the group treated with BSA. (E) suPAR binds to the surface of viable neutrophils. The cells were incubated with Chromeo 488-labeled mouse suPAR (1 μg/mL) or mouse albumin for 30 minutes and washed 5 times with PBS. Representative confocal images are shown. (F) Integrins participate in the enhanced phagocytosis of viable uPAR−/− neutrophils by WT macrophages. uPAR−/− neutrophils were preincubated with 1 μg/mL mouse IgG or antibodies to the integrins αM, αV, β1, β2, β3, or β4 for 30 minutes. The cells were then resuspended in RPMI plus 5% FBS and added to WT macrophages. After 60 minutes of incubation, the phagocytic index was determined. **P < .01, ***P < .001, compared with the control group treated with IgG.
Modulation of uPAR on the surface of neutrophils regulates their phagocytosis by macrophages
To explore more completely the role that uPAR occupies in modulating the phagocytosis of neutrophils by macrophages, we incubated WT neutrophils with anti-uPAR antibodies. Similar to findings with viable uPAR−/− neutrophils, the phagocytosis of WT neutrophils preincubated with anti-uPAR antibodies by WT macrophages was increased compared with that found with WT neutrophils incubated with isotype-matched IgG (Figure 4C). We next determined whether incubation of uPAR−/− neutrophils with suPAR affects their phagocytosis by WT macrophages. uPAR−/− neutrophils were preincubated with suPAR before inclusion in phagocytosis assays. As shown in Figure 4D, exposure of viable uPAR−/− neutrophils to suPAR abrogated the increased phagocytosis found when uPAR−/− neutrophils were cultured with WT macrophages. Of note, incubation of uPAR−/− neutrophils with suPAR resulted in binding of suPAR to the neutrophil surface (Figure 4E).
Integrins contribute to the enhanced phagocytosis of uPAR−/− neutrophils
In the aforementioned experiments (Figure 2), we found that integrins are involved in the enhanced phagocytic activities of uPAR−/− macrophages. To determine whether this is also true with uPAR−/− neutrophils, antibodies to the integrins αM, αV, β1, β2, β3, or β4 were incubated with viable uPAR−/− neutrophils before culture with WT macrophages. As shown in Figure 4F, and similar to the results with uPAR−/− macrophages, antibodies to αM, αV, β1, β2, and β3, but not β4 integrins abrogated the enhanced phagocytosis of viable uPAR−/− neutrophils by WT macrophages. These data suggest that the enhanced ability of uPAR−/− macrophages to phagocytose WT viable neutrophils and the increased phagocytosis of viable uPAR−/− neutrophils by WT macrophages may involve similar integrin-based mechanisms.
Although we found that integrins contribute to the enhanced phagocytosis of uPAR−/− neutrophils, there is still a possibility that the expression of “eat me” and/or “don't eat me” signals, such as CRT, CD47, and PtdSer, are altered on the surface of uPAR−/− neutrophils, thus rendering the cells more susceptible to ingestion. To test this hypothesis, we examined the levels of CRT, CD47, and PtdSer on the surface of uPAR−/− neutrophils by flow cytometry. As shown in supplemental Figure 2A through C, there were no noticeable changes in the expression of these molecules on the surface of uPAR−/− neutrophils. suPAR-treated WT or uPAR−/− neutrophils also demonstrated no changes in the expression of these molecules (supplemental Figure 2D). These data suggest that the enhanced uptake or the abrogation of the enhanced uptake by suPAR of uPAR−/− neutrophils is not caused by alterations in the levels of alternate “eat me” or “don't eat me” molecules on the neutrophil surface.
To compare the activity of uPAR with other “don't eat me” molecules, such as CD47 and PAI-1, in reducing ingestion of viable neutrophils, WT and uPAR−/− neutrophils were preincubated with anti-CD47 or anti–PAI-1 antibodies before being cultured with macrophages. As expected, blocking CD47 or PAI-1 promoted the uptake of WT neutrophils. However, blocking CD47 or PAI-1 did not further increase the uptake of viable uPAR−/− neutrophils by WT macrophages (supplemental Figure 3).
The presence of uPAR on the surface of either macrophages or neutrophils is required for the phagocytosis of viable neutrophils by macrophages
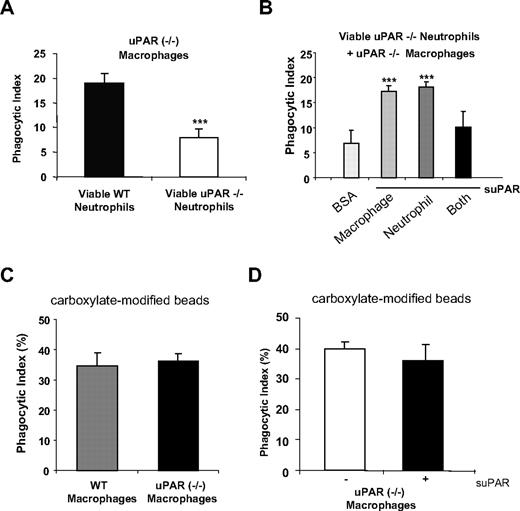
As shown in the aforementioned experiments, increased phagocytic indices are found when viable WT neutrophils are incubated with uPAR−/− macrophages or when viable uPAR−/− neutrophils are cultured with WT macrophages. We next asked whether uPAR−/− macrophages also demonstrate increased ability to engulf viable uPAR−/− neutrophils. As shown in Figure 5A, the phagocytic index found when uPAR−/− macrophages were incubated with viable uPAR−/− neutrophils was low, and similar to that found when WT macrophages and WT neutrophils were mixed. These data suggest that uPAR on the surface of either macrophages or neutrophil is necessary to enhance the phagocytosis of viable cells. To further explore this issue, we incubated uPAR−/− macrophages and/or neutrophils with suPAR before inclusion in phagocytosis assays. We found that incubation of uPAR−/− macrophages with suPAR enhanced their ability to engulf uPAR−/− neutrophils (Figure 5B). Similarly, incubation of uPAR−/− neutrophils with suPAR also increased their engulfment by uPAR−/− macrophages (Figure 5B). However, uPAR−/− macrophages incubated with suPAR did not demonstrate enhanced phagocytosis of uPAR−/− neutrophils that also had been exposed to suPAR before inclusion in the phagocytosis assays (Figure 5B). These data therefore suggest that the presence or absence of uPAR on both macrophages and neutrophils prevents phagocytosis of viable neutrophils. However, the presence of uPAR on either of neutrophils or macrophages is required to enhance phagocytosis of viable neutrophils. To examine whether the enhanced phagocytic activity of uPAR−/− macrophages is a specific phenomenon requiring the presence of uPAR on the surface of target cells, such as neutrophils, or simply reflects increased phagocytic activity of uPAR−/− macrophages, we examined the ability of WT and uPAR−/− macrophages to take up carboxylate-modified beads that do not express uPAR. As shown in Figure 5C, WT and uPAR−/− macrophages demonstrated comparable phagocytosis of the carboxylate-modified beads. Furthermore, suPAR did not enhance the uptake of the beads by uPAR−/− macrophages (Figure 5D).
The presence of uPAR on the surface of either macrophages or neutrophils is required for enhanced phagocytosis of viable neutrophils by macrophages. (A) uPAR−/− macrophages do not demonstrate enhanced phagocytosis when combined with viable uPAR−/− neutrophils. A total of 106 viable WT or uPAR−/− neutrophils were added to uPAR−/− macrophages and phagocytosis assays were performed. ***P < .001, compared with phagocytosis with viable WT neutrophils. (B) Incubation of uPAR−/− neutrophils or macrophages, but not both, with suPAR restores the ability of uPAR−/− macrophages to phagocytose viable uPAR−/− neutrophils. Viable uPAR−/− neutrophils alone (neutrophil), uPAR−/− macrophages alone (macrophage), or both (Both) were preincubated with 1 μg/mL suPAR for 30 minutes. The cells were then resuspended in RPMI plus 5% FBS and added to WT macrophages for phagocytosis assays. Phagocytosis of viable uPAR−/− neutrophils preincubated with 1 μg/mL BSA by uPAR−/− macrophages preincubated BSA were used as a control. **P < .01, ***P < .001, compared with the control group treated with BSA. (C) WT and uPAR−/− macrophages demonstrate similar ability in ingesting carboxylate-modified beads. Carboxylate-modified beads (2 μm) were incubated WT or uPAR−/− macrophages for 1 hour. The phagocytic index was calculated as the percentage of macrophages that ingested beads. (D) suPAR did not enhance the uptake of beads by uPAR−/− macrophages. uPAR−/− macrophages were preincubated without or with 1 μg/mL suPAR, and phagocytosis performed as in panel C.
The presence of uPAR on the surface of either macrophages or neutrophils is required for enhanced phagocytosis of viable neutrophils by macrophages. (A) uPAR−/− macrophages do not demonstrate enhanced phagocytosis when combined with viable uPAR−/− neutrophils. A total of 106 viable WT or uPAR−/− neutrophils were added to uPAR−/− macrophages and phagocytosis assays were performed. ***P < .001, compared with phagocytosis with viable WT neutrophils. (B) Incubation of uPAR−/− neutrophils or macrophages, but not both, with suPAR restores the ability of uPAR−/− macrophages to phagocytose viable uPAR−/− neutrophils. Viable uPAR−/− neutrophils alone (neutrophil), uPAR−/− macrophages alone (macrophage), or both (Both) were preincubated with 1 μg/mL suPAR for 30 minutes. The cells were then resuspended in RPMI plus 5% FBS and added to WT macrophages for phagocytosis assays. Phagocytosis of viable uPAR−/− neutrophils preincubated with 1 μg/mL BSA by uPAR−/− macrophages preincubated BSA were used as a control. **P < .01, ***P < .001, compared with the control group treated with BSA. (C) WT and uPAR−/− macrophages demonstrate similar ability in ingesting carboxylate-modified beads. Carboxylate-modified beads (2 μm) were incubated WT or uPAR−/− macrophages for 1 hour. The phagocytic index was calculated as the percentage of macrophages that ingested beads. (D) suPAR did not enhance the uptake of beads by uPAR−/− macrophages. uPAR−/− macrophages were preincubated without or with 1 μg/mL suPAR, and phagocytosis performed as in panel C.
uPAR−/− neutrophils demonstrate enhanced adhesion to uPAR
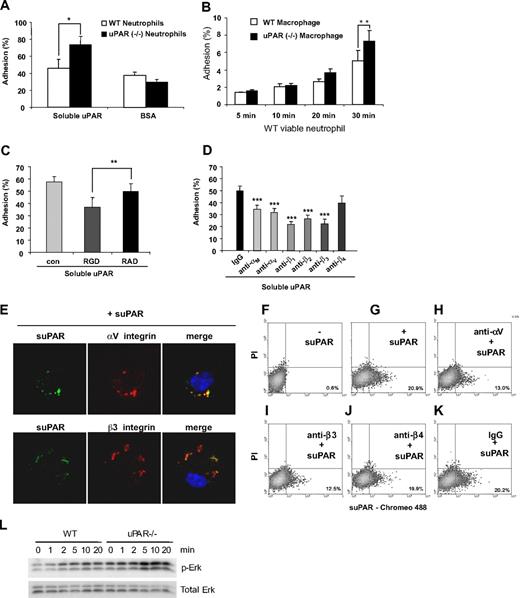
Previous studies showed that integrins, including αM, αV, β1, β2, and β3, but not β4, can associate with uPAR either in trans (lateral) or in cis.18,19,21-24,38 Because recognition and binding of target cells by macrophages are the initial step in efferocytosis and because the absence of uPAR on either macrophages or neutrophils increases phagocytosis of neutrophils, we hypothesized that the absence of uPAR on either macrophages or neutrophils may lead to an increase in the adhesion of the uPAR−/− cells to uPAR. To examine this issue, we first determined adhesion of viable WT and uPAR−/− neutrophils to suPAR coated on tissue culture plates. As shown in Figure 6A, viable WT neutrophils have slightly higher adhesion to suPAR than to BSA. Viable uPAR−/− neutrophils showed a similar adhesion to BSA compared with WT cells. However, the adhesion of uPAR−/− neutrophils to suPAR was significantly higher than that found with WT neutrophils. Furthermore, the heterotypic adhesion of WT neutrophils to uPAR−/− macrophages was significantly higher than to WT macrophages (Figure 6B). These data support the hypothesis that uPAR on the surface of neutrophils or macrophages prevents their adhesion to uPAR on opposing cells.
uPAR−/− neutrophils demonstrate enhanced adhesion to uPAR. (A) Adhesion of uPAR−/− neutrophils to uPAR is increased. A total of 106 viable WT and uPAR−/− neutrophils were added into each well of a 96-well plate precoated with 1 μg/mL BSA or suPAR for 1 hour, and adhesion assays performed as described in “Adhesion assay.” *P < .05. (B) Adhesion of WT neutrophils to uPAR−/− macrophages is increased. A total of 106 viable WT neutrophils were added into each well of a 96-well plate plated with WT and uPAR−/− macrophages, and adhesion assays performed as described in “Adhesion assay.” (C) RGD, but not RAD, peptides, abrogate the enhanced adhesion of uPAR−/− neutrophils to suPAR. uPAR−/− neutrophils were preincubated with 1 μg/mL BSA (con) or 1 μg/mL RGD or RAD peptides for 30 minutes and adhesion assays performed as in panel A. **P < .01. (D) Antibodies to integrins diminish the enhanced adhesion of viable uPAR−/− neutrophils to suPAR. uPAR−/− neutrophils were preincubated with 1 μg/mL mouse IgG or antibodies to the integrins αM, αV, β1, β2, β3, or β4 for 30 minutes. The cells were then resuspended in RPMI 1640 medium and added to suPAR-coated plates for adhesion assays. **P < .01, ***P < .001, compared with the control group treated with control IgG. (E) suPAR and integins are colocalized on the surface of uPAR−/− macrophages. uPAR−/− macrophages were incubated with Chromeo 488–labeled mouse suPAR (1 μg/mL) for 30 minutes. The cells were fixed and then stained overnight without or with antibodies to the integrins αV or β3. Representative confocal images are shown. (F-K) Anti-integrin antibodies prevent the binding of suPAR to the surface of uPAR−/− macrophages. Cells were preincubated without (F,G) or with antibodies (1 μg/mL) to the integrins αV (H), β3 (I), β4 (J), or isotype IgG control (K) for 30 minutes. The cells were then incubated with Chromeo 488–labeled mouse suPAR (1 μg/mL) for 1 hour (G-K) and washed 5 times with PBS. Fluorescent intensity was determined by flow cytometry. (L) uPAR−/− macrophages demonstrate greater Erk phosphorylation after exposure to viable WT neutrophils than do WT macrophages. A total of 4 × 106 viable WT neutrophils were added for the indicated time into each well of a 12-well plate plated with WT or uPAR−/− macrophages. The cells were then collected and the levels of phosphorylated Erk and total Erk determined by Western blot analysis.
uPAR−/− neutrophils demonstrate enhanced adhesion to uPAR. (A) Adhesion of uPAR−/− neutrophils to uPAR is increased. A total of 106 viable WT and uPAR−/− neutrophils were added into each well of a 96-well plate precoated with 1 μg/mL BSA or suPAR for 1 hour, and adhesion assays performed as described in “Adhesion assay.” *P < .05. (B) Adhesion of WT neutrophils to uPAR−/− macrophages is increased. A total of 106 viable WT neutrophils were added into each well of a 96-well plate plated with WT and uPAR−/− macrophages, and adhesion assays performed as described in “Adhesion assay.” (C) RGD, but not RAD, peptides, abrogate the enhanced adhesion of uPAR−/− neutrophils to suPAR. uPAR−/− neutrophils were preincubated with 1 μg/mL BSA (con) or 1 μg/mL RGD or RAD peptides for 30 minutes and adhesion assays performed as in panel A. **P < .01. (D) Antibodies to integrins diminish the enhanced adhesion of viable uPAR−/− neutrophils to suPAR. uPAR−/− neutrophils were preincubated with 1 μg/mL mouse IgG or antibodies to the integrins αM, αV, β1, β2, β3, or β4 for 30 minutes. The cells were then resuspended in RPMI 1640 medium and added to suPAR-coated plates for adhesion assays. **P < .01, ***P < .001, compared with the control group treated with control IgG. (E) suPAR and integins are colocalized on the surface of uPAR−/− macrophages. uPAR−/− macrophages were incubated with Chromeo 488–labeled mouse suPAR (1 μg/mL) for 30 minutes. The cells were fixed and then stained overnight without or with antibodies to the integrins αV or β3. Representative confocal images are shown. (F-K) Anti-integrin antibodies prevent the binding of suPAR to the surface of uPAR−/− macrophages. Cells were preincubated without (F,G) or with antibodies (1 μg/mL) to the integrins αV (H), β3 (I), β4 (J), or isotype IgG control (K) for 30 minutes. The cells were then incubated with Chromeo 488–labeled mouse suPAR (1 μg/mL) for 1 hour (G-K) and washed 5 times with PBS. Fluorescent intensity was determined by flow cytometry. (L) uPAR−/− macrophages demonstrate greater Erk phosphorylation after exposure to viable WT neutrophils than do WT macrophages. A total of 4 × 106 viable WT neutrophils were added for the indicated time into each well of a 12-well plate plated with WT or uPAR−/− macrophages. The cells were then collected and the levels of phosphorylated Erk and total Erk determined by Western blot analysis.
To determine whether integrins participate in the adhesion of uPAR−/− neutrophils to suPAR, uPAR−/− neutrophils were preincubated with integrin-blocking RGD or control RAD peptides before being added to suPAR-coated plates. As shown in Figure 6C, RGD-, but not RAD-containing peptides efficiently abrogated the increased adhesion of uPAR−/− neutrophils to suPAR. Το delineate which integrin subunits mediate the increased adhesion of uPAR−/− neutrophils to suPAR, viable uPAR−/− neutrophils were incubated with antibodies to the integrins αM, αV, β1, β2, β3, and β4 before being added to suPAR-coated plates. We found that antibodies to αM, αV, β1, β2, and β3, but not to β4 integrins abrogated the enhanced adhesion of uPAR−/− neutrophils to suPAR (Figure 6D).
suPAR binds to integrins
As shown in the aforementioned experiments, anti-integrin antibodies attenuated the enhanced phagocytic activity of uPAR−/− macrophages and the elevated uptake of uPAR−/− neutrophils by WT macrophages. Anti-integrin antibodies also diminished the adhesion of uPAR−/− neutrophils to suPAR. These data suggest that uPAR modulates the ingestion of viable cells through interactions with integrins. To explore this possibility more completely, we examined the localization of integrins and suPAR after uPAR−/− macrophages had been incubated with suPAR. As shown in Figure 6E, suPAR was found to be colocalized with αV and β3 integrins on the surface of uPAR−/− macrophages. To determine whether anti-integrin antibodies block the binding of suPAR to the cell surface, uPAR−/− macrophages were preincubated with antibodies to αV, β3, β4, or control IgG before exposure to suPAR. Anti-αV and β3 antibodies, but not IgG or antibodies to β4 attenuated suPAR binding to uPAR−/− macrophages (Figure 6F-K). These data suggest that anti-integrin antibodies may attenuate the enhanced phagocytic activity of uPAR−/− macrophages and the elevated uptake of uPAR−/− neutrophils through preventing trans binding of integrins and uPAR.
Integrin-associated signaling events are increased in uPAR−/− macrophages when exposed to viable neutrophils
The enhanced adhesion of neutrophils to uPAR−/− macrophages and the trans binding of uPAR to integrins led us to speculate that integrin-associated signaling events, such as Erk phosphorylation, may be differentially activated in WT and uPAR−/− macrophages after exposure to WT neutrophils. As shown in Figure 6L, Erk was phosphorylated in a time-dependent manner in both WT and uPAR−/− macrophages exposed to viable WT neutrophils. However, Erk phosphorylation was markedly higher in uPAR−/− macrophages than in WT macrophages at later time points (10 and 20 minutes after neutrophil addition). These data suggest that trans binding of uPAR and integrins promotes cellular activation through integrin-mediated pathways.
uPAR inhibits phagocytosis of apoptotic cells
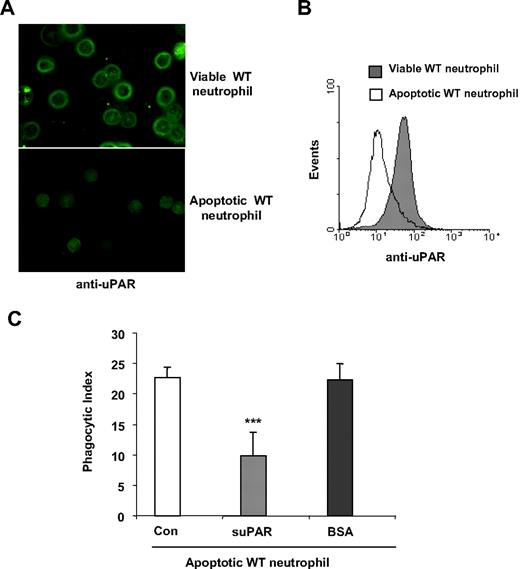
As demonstrated in the aforementioned experiments, the presence of uPAR on either neutrophils or macrophages is involved in uptake of viable cells. To explore whether uPAR also can participate in the phagocytosis of apoptotic cells, we first examined whether apoptosis was associated with alterations in the expression of uPAR on neutrophils. As shown in Figure 7A and B, uPAR levels were significantly decreased when neutrophils became apoptotic. These data suggest that loss of uPAR expression may contribute to the enhanced clearance of apoptotic cells, a hypothesis that was confirmed by reduction in phagocytosis of apoptotic neutrophils after incubation with suPAR (Figure 7C).
uPAR inhibits the phagocytosis of apoptotic cells. (A,B) Expression of uPAR is decreased in apoptotic neutrophils. Viable or apoptotic neutrophils were stained with anti-uPAR antibodies and expression of uPAR determined by confocal microscopy (A) or flow cytometry (B). The images were acquired using double bidirectional scans of viable and apoptotic neutrophils with a Leica DMIRBE inverted epifluorescence/Nomarski microscope outfitted with Leica TCS NT laser confocal optics (Leica Inc). The levels of fluorescence were averaged using SimplePCI software (Compix). Images were processed using IPLab Spectrum and Adobe Photoshop (Adobe Systems) software. (C) suPAR inhibits phagocytosis of apoptotic cells. A total of 1 μg/mL suPAR or BSA was added to cultures of apoptotic WT neutrophils, and macrophages and phagocytosis assays were performed.
uPAR inhibits the phagocytosis of apoptotic cells. (A,B) Expression of uPAR is decreased in apoptotic neutrophils. Viable or apoptotic neutrophils were stained with anti-uPAR antibodies and expression of uPAR determined by confocal microscopy (A) or flow cytometry (B). The images were acquired using double bidirectional scans of viable and apoptotic neutrophils with a Leica DMIRBE inverted epifluorescence/Nomarski microscope outfitted with Leica TCS NT laser confocal optics (Leica Inc). The levels of fluorescence were averaged using SimplePCI software (Compix). Images were processed using IPLab Spectrum and Adobe Photoshop (Adobe Systems) software. (C) suPAR inhibits phagocytosis of apoptotic cells. A total of 1 μg/mL suPAR or BSA was added to cultures of apoptotic WT neutrophils, and macrophages and phagocytosis assays were performed.
Discussion
uPAR is a GPI-anchored protein and a primary receptor for uPA.18,39 Through its association with uPA, uPAR focuses the proteolytic activities of uPA, thereby promoting degradation of the extracellular matrix and facilitating migration of cells.18,39 In addition, uPAR also regulates cell adhesion and migration through its association with integrins by mechanisms that are independent of the interactions between uPAR and uPA that are involved in fibrinolytic processes.31,40-42 In this study, we demonstrated a previously unrecognized role of uPAR in modulating the phagocytosis of viable and apoptotic neutrophils. In particular, uPAR deficiency on either neutrophils or macrophages, but not both cellular populations, promoted the ability of macrophages to phagocytose neutrophils through integrin-dependent mechanisms.
It has been proposed that efferocytosis is a default process.43 The resistance of viable cells to phagocytosis is the result of the presence of “don't eat me” signals, such as CD47, CD31, and PAI-1, on the surface of the target cells.7,8,17,43 Disruption of “don't eat me” signals or enhanced expression of “eat me” signals can trigger the engulfment of target cells, whether viable or apoptotic.7,8,43 In the present experiments, we found increased phagocytosis of viable neutrophils by uPAR−/− macrophages, suggesting that uPAR on the macrophage surface plays an inhibitory role in the phagocytosis of viable cells. uPAR has no transmembrane domain; thus, its inhibitory activity should be mediated by proteins associated with uPAR on the surface of macrophages. uPAR deficiency on the surface of viable neutrophils also promoted their uptake by macrophages, suggesting that uPAR can act as a “don't eat me” signal. However, the inhibitory role of uPAR on neutrophils is not identical to that of other “don't eat me” signals because there is no enhanced phagocytosis of uPAR−/− neutrophils by uPAR−/− macrophages. These data therefore suggest that uPAR on the surface of neutrophils does not act as a classic “don't eat me” signal, such as CD47,8 which prevents efferocytosis under all conditions, and indicate that the inhibitory activity of uPAR on phagocytosis is mediated by associated proteins on the surface of neutrophils. In addition, the requirement for the presence of uPAR on either macrophages or neutrophils to enhance phagocytosis of viable neutrophils indicates that cis association of uPAR with membrane proteins, in this case integrins, inhibits, whereas trans association of uPAR with the same integrins, facilitates phagocytosis of viable neutrophils, which is probably mediated by constitutive interaction of CRT and its receptor, LRP (supplemental Figure 4).
Previous studies have shown that uPAR can be associated with the integrins αMβ2, αVβ3, α3β1, and α5β1 on the cell surface.18,19,21-24,44,45 The integrin binding sites are exclusively located within D2 and/or D3 domains of uPAR.18,38,44,46 The uPAR binding sites on integrins vary but are primarily located in regions that are part of or adjacent to the ligand-binding sites.18,38,47,48 Through such associations with integrins, uPAR regulates integrin-mediated adhesion to the extracellular matrix proteins Vn and fibronectin.18,38,42 Our present data indicate that integrins also directly participate in modulating the effects of uPAR on efferocytosis. In particular, preincubation of uPAR−/− macrophages or neutrophils with antibodies to the integrins αM, αV, β1, β2, and β3 abrogated the enhanced phagocytosis associated with absence of uPAR from the cell surface. However, antibodies to the β4 integrin, which does not associate with uPAR, did not modify the effects of uPAR on phagocytosis. Collectively, these data indicate that deficiency of uPAR promotes the ability of integrins on the cell surface to mediate phagocytosis of viable cells through binding to uPAR on the opposing cell surface.
The involvement of integrins in enhancing trans binding to uPAR on the opposing cell surface is corroborated by our findings that uPAR−/− neutrophils have significantly higher binding to suPAR than do uPAR+/+ cells. Furthermore, we demonstrated that the binding of uPAR−/− cells to suPAR is mediated by integrins because antibodies to αM, αV, β1, β2, and β3, but not to β4 integrins, blocked the enhanced binding of uPAR−/− neutrophils to suPAR. These data are consistent with the participation of multiple integrins, previously known to associate with uPAR, in mediating the enhancing effects of uPAR absence on the uptake of viable cells.18,19,21-24,38
We found that the enhanced activity of uPAR−/− macrophages to phagocytose viable neutrophils is abrogated by incubation with anti-LRP antibodies or by RAP, an LRP specific blocking peptide.49 Binding between LRP and CRT has previously been shown to increase phagocytosis of viable cells when the “don't eat me” signal CD47 is disrupted.7,8 The present results therefore suggest that the absence of uPAR on either macrophages or neutrophils results in the loss of cis binding of uPAR to integrins as well as increased trans interactions between integrins and uPAR, thereby tilting the balance toward phagocytosis, even when the targets are viable cells. The phagocytosis of the viable neutrophils is mediated by the constitutive force generated by the binding of LRP to its ligand, CRT. However, how trans binding of integrins to uPAR specifically triggers LRP-CRT-dependent phagocytosis of viable neutrophils requires further studies.
It was previously shown that uPAR expression is up-regulated on cytokine-activated inflammatory cells and also that circulating levels of suPAR are increased in inflammatory and infectious processes, including endotoxemia, HIV infection, and rheumatoid arthritis.28-30,50-52 Phagocytosis and clearance of apoptotic cells are critical to the resolution of inflammation.1,2 Integrins, especially the integrin αVβ3, are major receptors for PtdSer and play a major role in the clearance of apoptotic cells.1,2 Although we found that the presence of uPAR inhibits the trans binding of integrins to uPAR, it is also probable that cis interactions between integrins and uPAR repress the binding of integrins to PtdSer, thereby inhibiting phagocytosis of apoptotic cells. Increased local and circulating levels of uPAR and suPAR may therefore lead to impaired phagocytosis of apoptotic neutrophils and other cellular populations, providing a new mechanism through which uPAR modulates inflammation and tissue injury in chronic and acute autoimmune and infectious disorders.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the National Institutes of Health (NIH, Bethesda, MD; grant R01HL076206).
National Institutes of Health
Authorship
Contribution: Y.-J.P. performed research and analyzed data; G.L. designed and performed research, analyzed data, and wrote the paper; Y.T. and E.L. performed research; and E.A. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward Abraham, Department of Medicine, University of Alabama at Birmingham, 420 Boshell Bldg, 1808 7th Ave South, Birmingham, AL 35294; e-mail: eabraham@uab.edu.
References
Author notes
*Y.-J.P. and G.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal