Abstract
The use of plasma-derived factor VIII (pdFVIII) concentrates in hemophilia A has been reported to result in reduced anti-FVIII antibody formation. In this study, we have investigated whether the cytokine microenvironment induced by pdFVIII has an influence on reducing anti-FVIII antibody titers in hemophilic mice. Microarray and confirmatory quantitative reverse transcription polymerase chain reaction (RT-PCR) experiments show that pdFVIII infusion causes a different transcriptional profile in dendritic cells than recombinant FVIII (rFVIII). Both treatments caused up-regulation of proinflammatory gene expression, but rFVIII and pdFVIII treatments promote expression of genes that induce Th1 and Th2 responses, respectively. Moreover, administration of rFVIII or pdFVIII concentrates resulted in distinct T-cell splenic cytokine microenvironments. rFVIII induced the release of Th1 cytokines and IL-10, whereas pdFVIII induced the release of Th2 cytokines and transforming growth factor-β. We have also observed high titers of anti–human von Willebrand factor (VWF) antibodies in the pdFVIII-treated mice and propose that this results from antigenic competition. We further investigated the role of this phenomenon using infusions of FVIII and increasing concentrations of recombinant human factor IX (FIX). These studies show an inverse relationship between increasing concentrations of FIX and the production of anti-FVIII antibodies. In summary, these studies report new mechanisms that contribute to reduced anti-FVIII antibody development in hemophilia A after pdFVIII infusions.
Introduction
Hemophilia A is the most common severe inherited bleeding disorder. Approximately 25% of severe hemophilia A patients undergoing factor VIII (FVIII) replacement therapy develop antibodies that inhibit the activity of the infused FVIII (FVIII inhibitors). The formation of FVIII antibodies is currently the most significant treatment-related complication in the clinical care of hemophilic patients. The available treatments for hemophilia A patients who develop FVIII antibodies include the use of hemostatic bypassing agents and the induction of immune tolerance to FVIII using various FVIII dosing schedules. These approaches are all costly and not always successful. The prevention of immunologic responses toward FVIII during FVIII replacement therapy would represent a significant therapeutic advance.
The immune system in severe hemophilia A patients is activated after FVIII treatment, in part, because no circulating normal FVIII is present in these patients; thus, the infused FVIII may be viewed as a foreign protein. Infused FVIII will be internalized by professional antigen-presenting cells (APCs) such as dendritic cells (DCs) through mechanisms that include receptor-mediated endocytosis1 or by antigen nonspecific internalization pathways including pinocytosis.2 DCs have the ability to process and present FVIII to CD4+ T cells (FVIII-DCs). FVIII-presenting DCs interact with T cells through signal 1 (major histocompatibility complex class II [MHC-II] and TCR interaction), signal 2 (costimulatory molecules and their appropriate receptors on T cells), and signal 3 (cytokines that regulate the differentiation of CD4+ T cells into either a Th1 or Th2 profile3 ). Th1 T cells release proinflammatory cytokines such as interleukin-2 (IL-2), IL-12, and interferon-γ (IFN-γ) that trigger an inflammatory immune response and activation of the T-cell cytotoxic pathway.4 In contrast, Th2 cells release anti-inflammatory cytokines such as IL-4 and IL-10 that inhibit Th1 cells and induce B-cell activation.4
Because DCs play a key role in directing the differentiation of CD4+ T-helper cells, we expect these cells to act as regulators for the formation of FVIII antibodies. We and others have shown that FVIII under in vitro sterile conditions does not induce the maturation of immature DCs.5,6 In addition, in T cell– and FVIII-immature DC cocultures, FVIII does not induce the release of proinflammatory cytokines.6 However, repetitive infusions of FVIII into hemophilic mice result in the formation of FVIII antibodies. These findings suggest that the behavior of FVIII in vivo is different to its behavior in vitro. Thus, FVIII in vivo is expected to induce a “dangerous” inflammatory microenvironment that results in the maturation of DCs and the subsequent activation of the immune system.
In this paper, we have studied the mechanisms associated with the differences in anti-FVIII antibody development in hemophilia A mice after recombinant FVIII (rFVIII) or plasma-derived factor VIII (pdFVIII) treatment. This was achieved by investigating the effects of rFVIII and pdFVIII administration on the profile of immune gene expression by DCs, DC maturation, the T-cell cytokine splenic microenvironment, and anti-FVIII antibody titers in mice after repetitive FVIII treatments.
Methods
Animals
Male and female Balb/c E16 hemophilia A mice, between 6 and 10 weeks old were used in all experiments. Mouse genotype was assessed for the interruption of FVIII exon 16 by polymerase chain reaction (PCR) using genomic DNA isolated from tail clips as described by Connelly et al.7 All mouse experiments were performed in accordance with the Canadian Council for Animal Care, and the Queen's University Animal Care Committee approved all animal protocols.
FVIII and von Willebrand factor concentrates
The following human FVIII and von Willebrand factor (VWF) concentrates were used in these studies: recombinant human FVIII (Kogenate-FS; Bayer), plasma-derived FVIII (Wilate; Octapharma AG), and a highly purified plasma-derived human VWF concentrate (Batch no. A568016; Biotest AG). The certificate of analysis for the Biotest plasma-derived VWF (pdVWF) concentrate details the following characteristics: VWF antigen (VWF:Ag) 110.8 IU/mL, FVIII less than 0.1 IU/mL, immunoglobulin G (IgG) less than 9.3 μg/mL.
Design of the immune gene microarray chip
The chip contained 1095 genes relevant to the immune response, 12 housekeeping genes, 150 negative controls, 150 spike controls, and positive controls. This chip was designed using Agilent eArray services, and the gene probes were printed by the manufacturer (Agilent Technologies). Three replicates of each gene probe were printed on each array (8 arrays per slide).
RNA isolation from FVIII in vivo–pulsed DCs
Naive hemophilia A mice between 6 and 10 weeks of age were infused intravenously with 3 different materials: 200 μL of Hepes-buffered saline solution (HBSS), 2 IU (80 IU/kg) of human recombinant FVIII Kogenate FS (rFVIII) or 2 IU (80 IU/kg) plasma-derived human FVIII Wilate (pdFVIII). Twenty-four hours later, the spleens were isolated. For each infusate (HBSS, rFVIII, and pdFVIII), 6 mouse spleens were pooled to generate 1 experimental biologic replicate. For each infusate, we had 4 experimental biologic replicates (ie, 4 × 6 pooled splenocyte samples) as shown in Figure 1. The CD11c+ DCs were isolated from each splenocyte pool using magnetic beads coated with anti-CD11c (Miltenyi Biotec) according to the manufacturer's guidelines. The CD11c cellular pellets were resuspended in Trizol (Invitrogen). RNA isolation and purification steps were performed according to the manufacturer's guidelines. RNA quality was evaluated by Nanodrop assessment, and there was no evidence of RNA degradation.
Details of the experimental protocols for the microarray and quantitative RT-PCR studies. There were 4 biologic replicates for each infusate. Each biologic replicate was tested on 2 different arrays, and the quantitative RT-PCR studies were performed in quadruplicate.
Details of the experimental protocols for the microarray and quantitative RT-PCR studies. There were 4 biologic replicates for each infusate. Each biologic replicate was tested on 2 different arrays, and the quantitative RT-PCR studies were performed in quadruplicate.
RNA labeling and hybridization
The isolated RNA was labeled with Cyanine 3-CTP and hybridized to the microarray chip according to the manufacturer's guidelines (Agilent Technologies). Each biologic replicate was hybridized on 2 separate arrays to generate 2 technical repeats. The arrays were then washed and scanned using an Agilent scanner according to the manufacturer's guidelines (Agilent Technologies).
Microarray data analysis
For microarray hybridization analysis, the difference of the median intensity subtracted from the median background was divided by the standard deviation of the background. The data were normalized, and the replicates were filtered as previously described by Stekel and Quackenbush.8,9 The DC gene expression in the HBSS-treated mice was compared with the DC gene expression from either the rFVIII- or pdFVIII-treated mice. Altered gene expression was considered significant if it had a fold-induction of greater than or equal to 2 or less than or equal to 0.5 in all of the 4 biologic replicates and their corresponding technical repeats. The microarray raw data can be found at http://clinlabs.path.queensu.ca/queens/labs/lillicrap/. The data have also been deposited into the Gene Expression Omnibus (GEO) public database under accession number GSE15809.
Quantitative real-time PCR
Expression of the differentially induced genes as determined by microarray evaluation was validated by quantitative reverse transcription PCR (RT-PCR). The RNA was isolated as described earlier. The quantitative RT-PCR experiments were conducted by SuperArray Bioscience Corporation. For each infusate, each gene was run in quadruplicate. Each sample was normalized to the (ACTB) housekeeping gene. The HBSS samples were used as the control samples for analysis. The fold-change in gene expression was calculated based on the 2ΔΔCt method.9
Treatment of hemophilia A mice with FVIII and VWF
Naive hemophilic mice between 6 and 10 weeks of age received 4 weekly intravenous infusions of 2 IU of human recombinant FVIII (416 ng based on the specific activity of this Kogenate-FS lot: equivalent to 80 IU/kg), Kogenate-FS (rFVIII), or plasma-derived human FVIII Wilate (pdFVIII) (480 ng FVIII protein based upon a FVIII:Ag to FVIII:C ratio of 1.2 to 1 for this Wilate lot). Each FVIII concentrate was diluted with HBSS into a final volume of 200 μL. The negative control mice for this experiment received 4 intravenous injections of 200 μL HBSS.
In a separate group of studies, naive hemophilia A mice between 6 and 10 weeks of age received 4 weekly intravenous treatments of 2 IU human rFVIII mixed at 22°C for 30 minutes with 2 IU highly purified human pdVWF (Biotest AG), at 100 molar excess, similar to the Wilate infusions. All treatments were diluted with HBSS in a final volume of 200 μL. The negative control mice for this experiment received 4 intravenous treatments of 200 μL 2 IU pdVWF alone, while the positive control mice received 4 intravenous treatments of 200 μL 2 IU rFVIII.
Blood sampling from the hemophilia A mice
Mice were anesthetized with 0.2 mL hypnorm/water/midazolam (1:2:1), and blood samples were obtained using uncoated microhematocrit capillary tubes (Fisher Scientific) via the retro-orbital plexus. Blood was mixed with one-tenth volume of 3.2% sodium citrate. Plasma samples were isolated by centrifugation of blood samples at 9000g for 3 minutes at 4°C, frozen on dry ice, and stored at −70°C until tested.
Measurement of FVIII antibodies in mouse blood samples
Total anti-FVIII antibody titers were quantified by enzyme-linked immunosorbent assay (ELISA). In brief, microtiter plate wells were coated overnight at 4°C with 100 μL human recombinant FVIII (Kogenate-FS) at a concentration of 10 IU/mL. Wells were blocked using 10% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Plasma samples were diluted in HBS-BSA buffer. The anti-FVIII antibody isotypes (IgG1, IgG2A, IgG2B, IgG2C, and IgG3) were studied using the Clonotype kit (Southern Biotech). An anti-FVIII standard curve was generated using an anti-human FVIII antibody of known concentation (Abcam). The standard curve was used as a reference to quantify the FVIII antibody titers in the test samples.
Plasma samples were analyzed for FVIII inhibitory antibodies using a 1-stage FVIII clotting assay. In brief, plasma samples were diluted in 2% BSA in HBS, mixed with human pool plasma in a 1:1 ratio, and incubated at 37°C for 2 hours. Samples were then assayed for residual FVIII activity using activated partial thromboplastin time (APTT) reagent (Organon Teknika) in an automated coagulometer (Coag-A-Mate; General Diagnostics) following the manufacturer's protocol. Inhibitor titers (Bethesda units) were calculated as detailed in the standard Bethesda protocol.10
Phenotypic characterization of in vivo pulsed DCs by flow cytometry
Naive hemophilia A mice between 6 and 10 weeks of age were treated with a single infusion of HBSS, 2 IU rFVIII, or 2 IU pdFVIII. Twenty-four hours later, spleens were isolated and digested in collagenase D for 30 minutes. CD11c+ DCs were isolated using magnetic beads coated with anti-CD11c antibodies (Miltenyi Biotec) according to the manufacturer's guidelines. All isolated DC populations were greater than 90% phycoerythrin (PE)-Cy5-CD11c+ by flow cytometric assessment. Costimulatory molecules CD80 and CD86 (BD Biosciences) were fluorescently labeled with PE and fluorescein isothiocyanate (FITC) to identify the maturation stage of the DCs. The cells were fixed with Cytofix/Cytoperm kit (BD Biosciences), and samples were analyzed by flow cytometry within 12 hours.
In vivo assessment of the splenic T-cell microenvironment induced by FVIII infusions
Hemophilia A mice that had previously been treated with either rFVIII or pdFVIII were used in this experiment. Mice were infused with 2 IU of either rFVIII or pdFVIII 4 months after their last FVIII treatment. Twenty-four hours later, CD4+ T cells were isolated from the spleens via the use of a CD4 negative selection isolation kit (Miltenyi Biotec) following the manufacturer's protocol. The CD4+ cells were stained with specific antibodies against CD4, CD25, and Foxp3, and the intracellular cytokines IL-2, IL-4, IL-5, IL-10, IFN-γ, and transforming growth factor-β (TGF-β) as described by the manufacturer (BD Biosciences). The cells were fixed with the Cytofix/Cytoperm kit (BD Biosciences), and samples were analyzed by flow cytometry within 12 hours. The negative control mice for this experiment initially received 4 intravenous injections of 200 μL HBSS and then, 4 months later, were treated again with 20 μL HBSS.
In vivo studies of antigenic competition between FVIII and FIX
Naive hemophilia A mice between 6 and 10 weeks of age received 4 weekly intravenous treatments of either 2 IU human rFVIII or 2 IU human rFVIII mixed with 3 μg (0.6 IU: 100 molar excess), 10 μg (2 IU: 330 molar excess), or 20 μg (4 IU: 660 molar excess) of recombinant human factor IX (FIX), BeneFix (rFIX). Large amounts of FIX protein were used in this experiment to match and exceed the quantity of VWF found in pdFVIII (ie, 1 IU VWF = 10 μg of protein; 100 molar excess over FVIII). All treatments were diluted with HBSS in a final volume of 200 μL. The negative control mice for this experiment received 4 intravenous treatments of 200 μL 20 μg of FIX alone.
Statistical analysis
All data are presented as mean plus or minus the standard error of the mean (SEM). Statistical comparisons of experimental groups were evaluated with a Student t test; all P values are shown in the figures.
Results
Treatment of hemophilia A mice with pdFVIII results in reduced anti-FVIII antibody generation
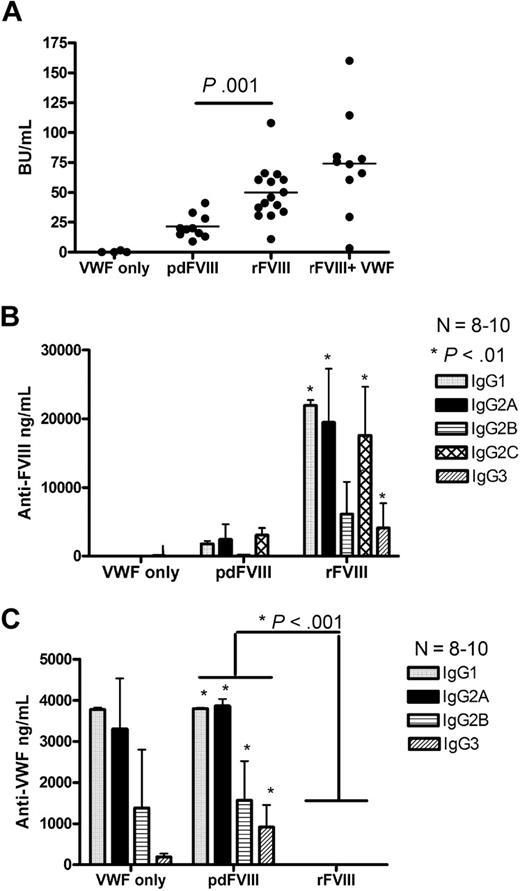
Hemophilia A mice were treated with 4 weekly infusions of either 2 IU rFVIII (416 ng FVIII:Ag) or 2 IU pdFVIII (480 ng FVIII:Ag). Our results show that the mice that received rFVIII had 2-fold higher anti-FVIII inhibitor titers compared with the pdFVIII-treated mice (Figure 2A). Anti-FVIII antibodies were not seen in the HBSS-infused mice. The anti-FVIII ELISA studies on the rFVIII-treated mice showed that the antibodies were predominantly of the IgG1 subclass (Th2-dependent), IgG2A (Th1-dependent), and also the Th1-dependent IgG2B and IgG2C subclasses (Figure 2B). Moreover, the anti-FVIII isotyping studies on the rFVIII-treated mice showed approximately 8-fold increase in the formation of anti-FVIII IgG1, IgG2A, and IgG2B subclasses in compression to pdFVIII-treated mice. The mice that were treated with pdFVIII developed high titers of anti-human VWF antibodies. These anti-VWF antibodies were predominantly IgG1 and IgG2A subclasses (Figure 2C). Thus, treating hemophilia A mice with pdFVIII results in low anti-FVIII and high anti-human VWF antibody titers.
Infusion of pdFVIII concentrate results in reduced anti-FVIII titers and the formation of anti-human VWF antibodies. Comparison of (A) FVIII inhibitors, (B) anti-FVIII IgG subclasses, and (C) anti-human VWF IgG subclasses in Balb/c mice after 4 infusions of either VWF alone, pdFVIII, rFVIII, or in vitro mixed rFVIII and highly purified pdVWF. Naive hemophilic Balb/c mice were treated with 4 weekly infusions of 2 IU VWF, 2 IU pdFVIII, 2 IU rFVIII, or 2 IU rFVIII mixed in vitro with a 50-molar excess of pdVWF. Mice were sampled 1 week after the fourth treatment. The horizontal lines and error bars represent the mean and SEM for 8 to 10 mice.
Infusion of pdFVIII concentrate results in reduced anti-FVIII titers and the formation of anti-human VWF antibodies. Comparison of (A) FVIII inhibitors, (B) anti-FVIII IgG subclasses, and (C) anti-human VWF IgG subclasses in Balb/c mice after 4 infusions of either VWF alone, pdFVIII, rFVIII, or in vitro mixed rFVIII and highly purified pdVWF. Naive hemophilic Balb/c mice were treated with 4 weekly infusions of 2 IU VWF, 2 IU pdFVIII, 2 IU rFVIII, or 2 IU rFVIII mixed in vitro with a 50-molar excess of pdVWF. Mice were sampled 1 week after the fourth treatment. The horizontal lines and error bars represent the mean and SEM for 8 to 10 mice.
In vitro mixing of rFVIII and pdVWF does not influence the tendency for anti-FVIII antibody generation
Studies involving the infusion of rFVIII that had been mixed in vitro with a highly purified pdVWF preparation at a similar molar ratio to that found in Wilate did not show a significant difference in the incidence or magnitude of FVIII inhibitor development. This result is in agreement with a previous report showing that in vitro mixing with VWF reduced the immunogenicity of just 1 of 2 rFVIII concentrates tested.11
Recombinant and plasma-derived FVIII concentrates induce different patterns of immune gene expression in splenic CD11c+ DCs
DCs play a central role in directing the immune system toward either tolerance or immunity. Therefore, to investigate the influence of rFVIII and pdFVIII concentrates on anti-FVIII antibody generation, transcriptional profiling microarray studies were performed. The immune system transcript profile of splenic CD11c+ DCs was investigated 24 hours after infusing hemophilic mice with either pdFVIII or rFVIII. The gene expression profile in DCs after administration of each concentrate (pdFVIII or rFVIII) was compared with that of DCs that were isolated from naive hemophilic mice treated with HBSS. Our results show that the pdFVIII infusion induced a different immune gene expression profile in DCs compared with the rFVIII treatment; these results are summarized in Tables 1 through 4.
Microarray-characterized gene expression in DCs isolated 24 hours after FVIII treatment: ratio of the gene expression in DCs isolated from rFVIII-treated mice to the gene expression of DCs isolated from pdFVIII-treated mice
| Gene name . | rFVIII/pdFVIII ratio . |
|---|---|
| Ccl2 | 0.25 |
| Cxcl1 | 0.39 |
| Cxcl2 | 0.41 |
| Hspb1 | 0.44 |
| Hspa1a | 0.45 |
| Il6 | 0.46 |
| Jun | 0.47 |
| Gdf15 | 0.47 |
| Egr1 | 0.47 |
| Plk2 | 0.48 |
| Egr2 | 0.49 |
| Itga2b | 2.01 |
| Cxcl4 | 2.10 |
| Ltf | 2.17 |
| Ppbp | 2.41 |
| Camp | 2.72 |
| Gene name . | rFVIII/pdFVIII ratio . |
|---|---|
| Ccl2 | 0.25 |
| Cxcl1 | 0.39 |
| Cxcl2 | 0.41 |
| Hspb1 | 0.44 |
| Hspa1a | 0.45 |
| Il6 | 0.46 |
| Jun | 0.47 |
| Gdf15 | 0.47 |
| Egr1 | 0.47 |
| Plk2 | 0.48 |
| Egr2 | 0.49 |
| Itga2b | 2.01 |
| Cxcl4 | 2.10 |
| Ltf | 2.17 |
| Ppbp | 2.41 |
| Camp | 2.72 |
Ratios of expression from genes showing at least a 2-fold change after rFVIII treatment compared with the same genes after pdFVIII treatment.
Biologic function and list of immune system genes that had a consistent 2-fold difference in expression after rFVIII treatment relative to the HBSS-treated mice
| Functional grouping: gene function . | Gene name . | Gene symbol . | Intensity ratio (rFVIII/HBSS) . |
|---|---|---|---|
| Cell-matrix adhesion: increases cell survival12 | Connective tissue growth factor | Ctgf | 0.44 |
| Host immune defense to bacteria: induces Th1 responses13 | Cathelicidin antimicrobial peptide | Camp | 3.22 |
| Surface marker: proinflammatory cytokines increasing phagocytoses14 | S100 calcium binding protein A8 | S100a8 | 2.5 |
| Host defense to bacteria: inhibits actions of TNF-α15 | Proteoglycan 2 | Prg2 | 0.36 |
| Oxidative stress: prevents inflammation16 | Glutathione peroxidase 2 | Gpx2 | 2.44 |
| Host defense to bacteria: Th1-polarized Ag-specific immune responses17 | Lactotransferrin | Ltf | 2.27 |
| Functional grouping: gene function . | Gene name . | Gene symbol . | Intensity ratio (rFVIII/HBSS) . |
|---|---|---|---|
| Cell-matrix adhesion: increases cell survival12 | Connective tissue growth factor | Ctgf | 0.44 |
| Host immune defense to bacteria: induces Th1 responses13 | Cathelicidin antimicrobial peptide | Camp | 3.22 |
| Surface marker: proinflammatory cytokines increasing phagocytoses14 | S100 calcium binding protein A8 | S100a8 | 2.5 |
| Host defense to bacteria: inhibits actions of TNF-α15 | Proteoglycan 2 | Prg2 | 0.36 |
| Oxidative stress: prevents inflammation16 | Glutathione peroxidase 2 | Gpx2 | 2.44 |
| Host defense to bacteria: Th1-polarized Ag-specific immune responses17 | Lactotransferrin | Ltf | 2.27 |
Genes that had at least a 2-fold change in expression after rFVIII or pdFVIII infusion, respectively; all gene expression ratios are relative to HBSS-infused mice.
Biologic function and list of genes that had a 2-fold difference in expression after pdFVIII treatment relative to the HBSS-treated mice
| Functional grouping: gene function . | Gene name . | Gene symbol . | Intensity ratio (pdFVIII/HBSS) . |
|---|---|---|---|
| Cytokine-cytokine receptor interaction: binds IL-8 receptor and recruits DCs and neutrophils18 | Chemokine (C-X-C motif) ligand 2 | Cxcl2 | 3.13 |
| Cytokine-cytokine receptor interaction: induce type 2 T helper cell polarization and recruits natural killer (NK) cells; see Reference19 | Chemokine (C-C motif) ligand 2 | Ccl2 | 2.08 |
| Host defense to bacteria: inhibits actions of TNF-α18 | Proteoglycan 2 | Prg2 | 2.34 |
| Involved in septic shock: enhances inflammatory responses to microbial products20 | Triggering receptor expressed on myeloid cells 1 | Trem1 | 2.94 |
| Extracellular matrix protein: inhibits DC migration to lymph nodes21 | Secreted acidic cysteine-rich glycoprotein | Sparc | 0.47 |
| Heat shock protein (toll-like receptor [TLR] interacting protein): induce CD86 expression and release of TNF-α, IL-1β, and IL-622 | Heat shock protein 1A | Hspa1a | 3.03 |
| Heat shock protein: induce the infiltration of NK cells and DCs19 | Heat shock protein 1 | Hspb1 | 2.13 |
| Functional grouping: gene function . | Gene name . | Gene symbol . | Intensity ratio (pdFVIII/HBSS) . |
|---|---|---|---|
| Cytokine-cytokine receptor interaction: binds IL-8 receptor and recruits DCs and neutrophils18 | Chemokine (C-X-C motif) ligand 2 | Cxcl2 | 3.13 |
| Cytokine-cytokine receptor interaction: induce type 2 T helper cell polarization and recruits natural killer (NK) cells; see Reference19 | Chemokine (C-C motif) ligand 2 | Ccl2 | 2.08 |
| Host defense to bacteria: inhibits actions of TNF-α18 | Proteoglycan 2 | Prg2 | 2.34 |
| Involved in septic shock: enhances inflammatory responses to microbial products20 | Triggering receptor expressed on myeloid cells 1 | Trem1 | 2.94 |
| Extracellular matrix protein: inhibits DC migration to lymph nodes21 | Secreted acidic cysteine-rich glycoprotein | Sparc | 0.47 |
| Heat shock protein (toll-like receptor [TLR] interacting protein): induce CD86 expression and release of TNF-α, IL-1β, and IL-622 | Heat shock protein 1A | Hspa1a | 3.03 |
| Heat shock protein: induce the infiltration of NK cells and DCs19 | Heat shock protein 1 | Hspb1 | 2.13 |
Genes that had at least a 2-fold change in expression after rFVIII or pdFVIII infusion, respectively; all gene expression ratios are relative to HBSS-infused mice.
Comparison of the genes and their expression ratios for mice treated with rFVIII or pdFVIII
| Gene name . | rFVIII/HBSS . | pdFVIII/HBSS . |
|---|---|---|
| Camp | 3.27 | 1.27 |
| S100a8 | 2.53 | 1.96 |
| Gpx2 | 2.47 | 1.70 |
| Ltf | 2.27 | 0.91 |
| Ctgf | 0.44 | 0.56 |
| Prg2 | 0.36 | 0.43 |
| Cxcl2 | 1.27 | 3.11 |
| Hspa1a | 1.60 | 3.01 |
| Trem1 | 1.66 | 2.92 |
| Hspb1 | 0.93 | 2.11 |
| Ccl2 | 0.57 | 2.09 |
| Sparc | 0.58 | 0.47 |
| Prg2 | 0.36 | 0.43 |
| Gene name . | rFVIII/HBSS . | pdFVIII/HBSS . |
|---|---|---|
| Camp | 3.27 | 1.27 |
| S100a8 | 2.53 | 1.96 |
| Gpx2 | 2.47 | 1.70 |
| Ltf | 2.27 | 0.91 |
| Ctgf | 0.44 | 0.56 |
| Prg2 | 0.36 | 0.43 |
| Cxcl2 | 1.27 | 3.11 |
| Hspa1a | 1.60 | 3.01 |
| Trem1 | 1.66 | 2.92 |
| Hspb1 | 0.93 | 2.11 |
| Ccl2 | 0.57 | 2.09 |
| Sparc | 0.58 | 0.47 |
| Prg2 | 0.36 | 0.43 |
Comparison of the genes that were differentially expressed in the rFVIII-treated mice relative to the same genes in the pdFVIII-treated mice; all gene expression ratios are relative to HBSS-infused mice.
There were 50 transcripts that showed a 1.5-fold differential expression between rFVIII and HBSS-treated mice, and 47 transcripts were differentially expressed between pdFVIII and HBSS-treated animals. Fifteen of these differentially expressed transcripts were documented in both the rFVIII and pdFVIII-treated animals (Table 1). All but 1 of these 15 in-common differentially expressed transcripts showed the same polarity of expression with both FVIII concentrates. Interestingly, expression of the chemokine ligand Ccl2 was suppressed in rFVIII-treated mice (0.57-fold) and enhanced in pd-FVIII treated animals (2.09-fold).
At a discriminatory value of greater than 2-fold expression variance and with consistent biologic (4 replicates, pools of 6 spleens) and technical (assayed on 2 separate arrays) reproducibility, we documented 6 and 7 differentially expressed transcripts with rFVIII and pdFVIII, respectively (Tables 2–3). This includes the proteoglycan 2 transcript (involved in TNF-α inhibition) that was up-regulated by both types of FVIII concentrate.
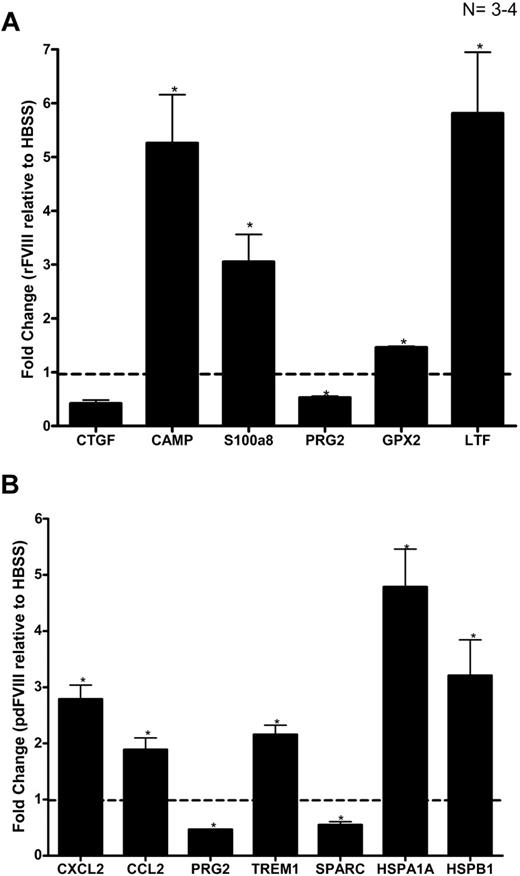
The transcripts that showed a consistent greater than 2-fold differential expression in the microrarray studies were further evaluated by quantitative RT-PCR analysis, with each biologic replicate RNA sample (N = 4) undergoing quadruplicate testing. The results of the quantitative RT-PCR studies confirm the polarity of differential expression and, in most instances, show a similar magnitude of difference to that documented in the microarray studies (Figure 3A-B)
The pdFVIII and rFVIII treatments induce the expression of different genes in DCs. Gene expression in DCs 24 hours after the (A) rFVIII or (B) pdFVIII treatments was analyzed via quantitative RT-PCR. The HBSS-treated mice were used as a control. The fold change for each gene was calculated as explained in “Quantitative real-time PCR.” Fold inductions were considered statistically significant if the P value was less than .05 in at least 3 of the 4 biologic repeats. The dashed line represents the gene expression of the control samples. The error bars represent the SEM. N = 4
The pdFVIII and rFVIII treatments induce the expression of different genes in DCs. Gene expression in DCs 24 hours after the (A) rFVIII or (B) pdFVIII treatments was analyzed via quantitative RT-PCR. The HBSS-treated mice were used as a control. The fold change for each gene was calculated as explained in “Quantitative real-time PCR.” Fold inductions were considered statistically significant if the P value was less than .05 in at least 3 of the 4 biologic repeats. The dashed line represents the gene expression of the control samples. The error bars represent the SEM. N = 4
In vivo administration of rFVIII and pdFVIII concentrates induces the maturation of splenic DCs
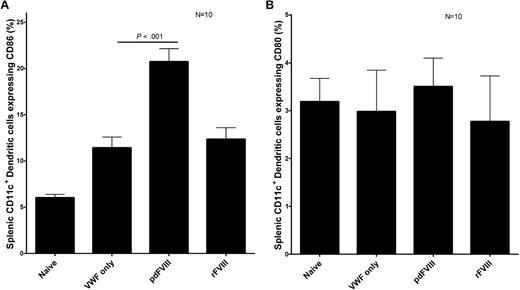
To activate the immune system toward FVIII, the FVIII protein must be sampled, processed, and presented to T cells by APCs such as DCs. We studied the in vivo maturation of DCs after FVIII infusion. Our results show that in vivo, rFVIII and pdFVIII concentrates both induce the maturation of DCs by increasing the expression of CD86 on DCs in comparison to the HBSS control DCs (Figure 4). Interestingly, the pdFVIII-DCs had a higher CD86 expression than rFVIII-DCs. DC CD86 expression was also increased after infusion of the human pdVWF concentrate. We propose that the presence of VWF and other copurified proteins found in the pdFVIII concentrate are responsible for the increased CD86 expression documented after exposure to this product.
pdFVIII and rFVIII infusions cause the maturation of DCs. Assessment of DC maturation after treating naive hemophilic Balb/c mice with either HBSS, 2 IU VWF, 2 IU pdFVIII, or 2 IU rFVIII. Mice were killed 24 hours after the infusions, and CD11c+ DCs were purified from the spleens. The expression of (A) CD86 or (B) CD80 maturation markers was quantified on DCs via flow cytometry. Error bars represent the SEM for 10 mice.
pdFVIII and rFVIII infusions cause the maturation of DCs. Assessment of DC maturation after treating naive hemophilic Balb/c mice with either HBSS, 2 IU VWF, 2 IU pdFVIII, or 2 IU rFVIII. Mice were killed 24 hours after the infusions, and CD11c+ DCs were purified from the spleens. The expression of (A) CD86 or (B) CD80 maturation markers was quantified on DCs via flow cytometry. Error bars represent the SEM for 10 mice.
In vivo, Th1 and Th2 adaptive immune responses were induced after the rFVIII and pdFVIII infusions, respectively
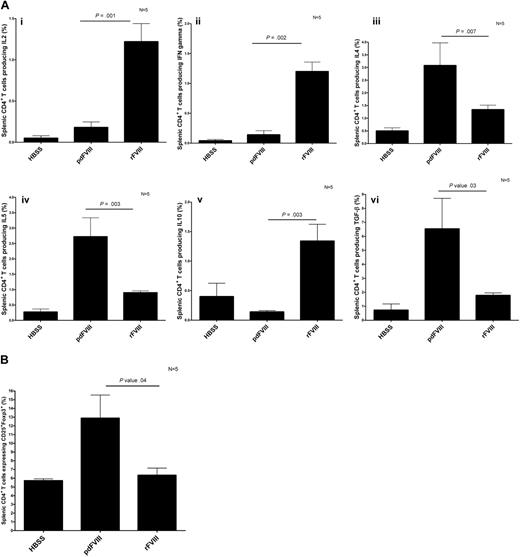
To learn more about the in vivo cytokine release induced by CD4+ T cells after infusion of rFVIII and pdFVIII concentrates, we assessed the intracellular cytokine production in CD4+ T cells isolated from rFVIII- and pdFVIII-treated mice that were rechallenged with the respective FVIII concentrates after an interval. This experimental design was chosen to ensure sufficient expansion of FVIII-specific CD4+ T cells to enable robust cytokine analysis. Our results show that the mice that were challenged with rFVIII had more T cells producing IL-2, IL-10, and IFN-γ, and fewer T cells producing IL-4, IL-5, and TGF-β (Figure 5A). A contrasting cytokine profile was documented in the mice that were challenged with pdFVIII. With pdFVIII infusion, we observed more T cells producing IL-4, IL-5, and TGF-β, and fewer T cells producing IL-2, IL-10, and IFN-γ (Figure 5A). We also evaluated the CD4+CD25+Foxp3+ regulatory T-cell population in the spleen after rFVIII or pdFVIII challenges. Our results show that the mice that received pdFVIII treatment had more splenic CD4+CD25+Foxp3+ regulatory cells (12.5% vs 6%) than mice that received rFVIII (Figure 5B).
pdFVIII concentrate induces the development of T regulatory cells and a Th2 cytokine profile, whereas rFVIII induces a Th1 cytokine profile. Detection of (A) intracellular Th1 and Th2 cytokines produced by CD4+ T cells and (B) CD4+CD25+Foxp3+ T regulatory cells isolated from HBSS-, rFVIII-, or pdFVIII-treated mice. Mice were treated with 4 infusions of HBSS, 2 IU pdFVIII, or 2 IU rFVIII; 4 months later, mice were challenged with HBSS, 2 IU rFVIII, or 2 IU pdFVIII. Twenty-four hours later, CD4+ T cells were purified from the spleens. The Th1 cytokines IL2, IFN (3Ai-ii), Th2 cytokines IL-4, IL-5, IL-10, and TGF-β (3Biii-vi), and CD4+CD25+Foxp3+ expression was quantified by flow cytometry. Error bars represent the SEM for 5 mice.
pdFVIII concentrate induces the development of T regulatory cells and a Th2 cytokine profile, whereas rFVIII induces a Th1 cytokine profile. Detection of (A) intracellular Th1 and Th2 cytokines produced by CD4+ T cells and (B) CD4+CD25+Foxp3+ T regulatory cells isolated from HBSS-, rFVIII-, or pdFVIII-treated mice. Mice were treated with 4 infusions of HBSS, 2 IU pdFVIII, or 2 IU rFVIII; 4 months later, mice were challenged with HBSS, 2 IU rFVIII, or 2 IU pdFVIII. Twenty-four hours later, CD4+ T cells were purified from the spleens. The Th1 cytokines IL2, IFN (3Ai-ii), Th2 cytokines IL-4, IL-5, IL-10, and TGF-β (3Biii-vi), and CD4+CD25+Foxp3+ expression was quantified by flow cytometry. Error bars represent the SEM for 5 mice.
Treatment of hemophilia A mice with rFVIII mixed with rFIX results in reduced levels of FVIII antibodies
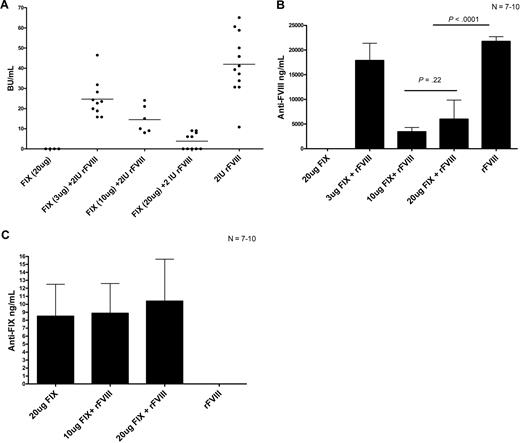
Reipert and colleagues previously suggested that VWF found in pdFVIII can compete with FVIII for immunogenic recognition.10 Therefore, to assess the possibility of antigenic competition between FVIII and other proteins, we performed the following experiment. Hemophilia A mice were treated with 4 weekly infusions of 2 IU rFVIII alone, 2 IU rFVIII plus 3 μg (0.6 IU) human rFIX, 2 IU rFVIII plus 10 μg (2 IU) human rFIX, or 2 IU rFVIII plus 20 μg (4 IU) human rFIX. Our results show that the mice that received the higher concentrations of rFIX mixed with rFVIII had reduced FVIII inhibitor titers compared with the mice that were treated with rFVIII alone (Figure 6A). Of particular note, 5 of the mice that were treated with 2 IU rFVIII plus 20 μg rFIX did not develop FVIII inhibitors. Our experiments also show that the rFVIII/rFIX-treated mice had lower total anti-FVIII antibody titers as measured by ELISA than the rFVIII-treated mice (Figure 6B). We also evaluated the presence of anti-FIX antibodies in the mice after rFVIII plus rFIX treatment. Our results show that the mice that were treated with human rFIX developed negligible levels of anti-FIX antibodies (Figure 6C). Therefore, treating hemophilia A mice with rFVIII plus rFIX results in reduced anti-FVIII titers, which might be due to antigenic competition between FIX and FVIII for immunogenic presentation by APCs and subsequent activation of effector T cells.
The presence of high human rFIX protein concentrations during FVIII treatment results in reduced anti-FVIII antibodies. Comparison of (A) FVIII inhibitors, (B) anti-FVIII antibodies, and (C) anti-human FIX antibodies in hemophilic mice after 4 treatments of either 20 μg human rFIX, 3 μg human rFIX plus 2 IU rFVIII, 10 μg human rFIX plus 2 IU rFVIII, 20 μg human rFIX plus 2 IU rFVIII, or 2 IU rFVIII. Mice were sampled 1 week after the fourth treatment. Horizontal and error bars represent the mean and SEM, respectively, for 7 to 10 mice.
The presence of high human rFIX protein concentrations during FVIII treatment results in reduced anti-FVIII antibodies. Comparison of (A) FVIII inhibitors, (B) anti-FVIII antibodies, and (C) anti-human FIX antibodies in hemophilic mice after 4 treatments of either 20 μg human rFIX, 3 μg human rFIX plus 2 IU rFVIII, 10 μg human rFIX plus 2 IU rFVIII, 20 μg human rFIX plus 2 IU rFVIII, or 2 IU rFVIII. Mice were sampled 1 week after the fourth treatment. Horizontal and error bars represent the mean and SEM, respectively, for 7 to 10 mice.
Discussion
We have shown that treating hemophilia A mice with rFVIII results in higher levels of anti-FVIII antibodies compared with pdFVIII-infused mice. We have subsequently investigated the key players in the initiation of the adaptive immune response, DCs and T cells. Our results suggest that the differences in immune responsiveness between the rFVIII- and pdFVIII-treated mice may be related to the different splenic cytokine milieu induced by each type of concentrate. The cytokine context of antigen presentation is an important determinant of immunogenicity, and recent studies have documented an association between FVIII antibody generation and polymorphisms in the promoter regions of the IL-10 and TNF-α genes.23,24 Moreover, Sasgary et al reported an association between rFVIII treatment and proinflammatory IFN-γ production by T cells isolated from hemophilic mice.26
DCs regulate the immune response by differentially expressing cell surface proteins and by releasing cytokines and chemokines that influence the interaction with T cells. The immune gene expression profile of DCs isolated from pdFVIII- or rFVIII-treated mice in this study was distinct. The rFVIII and pdFVIII treatments resulted in a consistent greater than 2-fold change of expression of 6 and 7 genes involved in the immune response, respectively. Analysis of the differentially expressed genes after rFVIII treatment shows enhanced expression of genes that induce inflammation, promote Th1 responses, enhance phagocytosis, and prevent oxidative metabolic stress. The pdFVIII treatment induced the expression of genes that recruit inflammatory cells, promote inflammation, and stimulate the production of TNF-α, IL-1β, and IL-6 inflammatory cytokines. These studies indicate that the rFVIII and pdFVIII treatments induce expression of 2 different sets of immune system genes in DCs. The Ltf and CAMP genes that were up-regulated after rFVIII administration are involved in inducing Th1 cytokines. In contrast, the Ccl2 gene, which is involved in inducing Th2 T-cell cytokine polarization, was up-regulated after pdFVIII treatment. Because DCs regulate the differentiation of T cells at least in part via signal 3 (cytokine secretion), we expect that the expression of these genes has an influence on the development of Th1 and Th2 T cells after rFVIII and pdFVIII treatment, respectively.
Levels of the costimulatory molecule, CD86, were found to be higher in pdFVIII-treated mice in this study. This can be explained, at least in part, by the fact that the pdFVIII mice overexpressed the heat shock protein A1, which is known to increase CD86 expression. We have also shown that DCs overexpress CD86 after the infusion of VWF alone. Interestingly, the expression of CD80 was not increased after either rFVIII or pdFVIII treatment. This observation agrees with the findings of Qian et al, who showed that CD86, but not CD80, is involved in the primary anti-FVIII immune response in hemophilic mice.27
Our intracellular cytokine studies have shown major differences in the secondary immune response after rFVIII and pdFVIII infusions. We have shown that rFVIII and pdFVIII treatments induce the generation of a predominantly Th1 and Th2 secondary immune response, respectively. Reding et al have suggested that Th1 cytokines have an essential role in maintaining the anti-FVIII immune response (secondary immune response25 ), and Sasgary et al reported that FVIII-specific T cells are dependent on the production of IL-2, IL-10, and IFN-γ.26 We observed high levels of these cytokines produced by T cells isolated from the rFVIII-treated mice and reduced levels expressed by T cells obtained from pdFVIII-treated mice. Our findings support the results obtained by Hu et al and Sasgary et al and indicate that rFVIII induces the production of IL-2, IL-10, and IFN-γ by CD4+ T cells.26,28,29 We further investigated the importance of IFN-γ on the formation of FVIII inhibitors by pretreating hemophilic mice with neutralizing anti–IFN-γ antibodies before and after the 4 weekly 2-IU rFVIII treatments. The anti–IFN-γ–treated mice showed a 30% reduction in FVIII inhibitor formation in comparison to isotype control-treated mice (data not shown). This provides additional evidence for the importance of IFN-γ on the formation of FVIII antibodies. In contrast to the results of rFVIII infusion, after pdFVIII administration, IL-4, IL-5, IL-6, and TGF-β were expressed by CD4+ T cells. Notably, TGF-β has been reported previously to suppress immune reactivity in hemophilic mice resulting in a reduced anti-FVIII titer.30,31
In this study, we also observed higher levels of splenic CD4+CD25+Foxp3+ T regulatory cells in mice that were treated with pdFVIII. There are likely to be several factors that contribute to this finding, including the release of the immunosuppressive cytokine TGF-β by the CD4+ T cells in pdFVIII-treated mice. The increased numbers of T regulatory cells may play a role in reducing immune reactivity toward FVIII, but additional experiments must be conducted to confirm this hypothesis. The involvement of T regulatory cells in reducing the formation of anti-FVIII antibodies in pdFVIII-treated mice has previously been reported by Kallas et al.31
A reduction in FVIII inhibitor development after pdFVIII infusion into hemophilia A mice has been observed previously.11,31 However, the mechanisms responsible for this phenomenon remain unresolved. Reipert et al have suggested that antigenic competition between FVIII and other proteins in the pdFVIII concentrates, including VWF, may account for a reduction in presentation of FVIII by DCs.10 Our results show that pdFVIII induces DC maturation, the release of Th2 cytokines, and the formation of high titer anti-human VWF antibodies. Thus, in the hemophilia A mouse model, human VWF appears to compete very effectively for immunologic recognition. In contrast, in human hemophiliacs, who are tolerant to VWF, VWF (and other proteins in the pdFVIII concentrates) may still compete for antigen presentation but should not result in a productive immune response.
Of note, when we mixed a highly purified human pdVWF protein with rFVIII in vitro, before IV infusion, we observed no reduction in FVIII inhibitor levels in the hemophilic mice. This observation replicates a previous report with this same rFVIII concentrate.11 Nevertheless, in the earlier study, preincubation of VWF with another rFVIII concentrate did reduce its immunogenicity. This finding suggests that there may be differences between rFVIII concentrates in the manner in which they interact with VWF.
In this study, we have further explored the possibility of antigenic competition between FVIII and another unrelated protein, FIX. Our results agree with previous investigations of antigenic competition.32,33 These studies suggest that the presence of high concentrations of other infused proteins during the FVIII treatment (in these studies, an approximately 6-fold molar excess of FIX molecules) will result in reduced titers of anti-FVIII antibodies. By increasing the concentrations of FIX, we expect that we are increasing the competition between FIX and FVIII for antigenic sites on APCs. Thus, high FIX concentrations during FVIII treatment might result in fewer APCs presenting FVIII, and thus a reduced anti-FVIII antibody titer. Significantly, we did not observe high anti-human FIX antibodies in these mice. We propose that this is likely due to at least 2 factors: the expression of normal murine FIX protein in hemophilia A mice, and the inherent reduced immunogenicity of FIX due to its homology with other vitamin K–dependent plasma proteins.
As a result of these studies, we propose that the following mechanisms contribute to the observed reduction of FVIII inhibitor formation in the pdFVIII-treated mice. First, proteins found in pdFVIII concentrates will be internalized by splenic DCs and will compete for antigenic presentation. Second, we have shown that pdFVIII concentrates elicit a different profile of immune gene expression in splenic DCs compared with rFVIII. This difference in gene expression will influence the maturation state and cytokine production of DCs. In the hemophilia A mice, more DCs will present VWF to T cells resulting in the activation of more VWF-specific T cells. With the subsequent generation of a Th2 cytokine response, and the activation of VWF-specific B cells, a potent anti-human VWF antibody response develops. Thus, through mechanisms involving antigenic competition and differential immune gene expression, the anti-FVIII immune response will be substantially reduced. It is also noteworthy that in clinical practice, FVIII is administered based upon FVIII activity units (IU/kg). In rFVIII concentrates, the specific activity of the protein can vary by 2- to 3-fold and thus significant differences in FVIII antigen loads can be experienced depending upon the specific lot of rFVIII being administered.
It is important to place the results of these studies in hemophilic mice in the context of the FVIII immune response in human hemophiliacs. Whereas all hemophilia A mice develop anti-FVIII antibodies when infused repeatedly with human FVIII, this only occurs in approximately 25% of human patients. However, although the incidence of this response is different between man and mouse, there are many similarities between the underlying immunologic mechanisms in these 2 species. As such, the differences in the immune reactivity that we have documented after exposure to the 2 FVIII concentrates in this study may well be indicative of similar phenomena in human hemophiliacs. Not surprisingly, these differences are complex and involve influences ranging from competition for initial antigen presentation and differences in DC immune gene expression to altered patterns of cytokine expression during secondary immune responses. Overall, these results indicate that it is very likely that the immune response to rFVIII and pdFVIII products in human hemophiliacs would be expected to be different.
In summary, we have shown that treating hemophilia A mice with pdFVIII results in the reduction of FVIII antibody formation. We propose that this differential effect occurs as a result of several factors including competition for antigen presentation, a different immune gene expression profile in splenic DCs, and the production of Th2 instead of Th1 cytokines.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded by an operating grant from the Canadian Institutes for Health Research (MOP-10 912). M.Q. was supported by an Ontario Graduate Scholarship. D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis.
Authorship
Contribution: M.Q. designed and performed research, analyzed data, and wrote the manuscript; B.W. and R.C. helped in designing the research; M.Q., E.B., S.B., C.H., M.O., and D.L. contributed in performing the research; M.Q., M.O., and D.L. participated in writing the paper; and D.L. was involved in the management of the research activities.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Richardson Laboratory, Queen's University, Kingston, ON, Canada K7L 3N6; e-mail: lillicrap@cliff.path.queensu.ca.