Abstract
Angiogenesis and lymphangiogenesis are essential for organogenesis but also play important roles in tissue regeneration, chronic inflammation, and tumor progression. Here we applied in vivo forward chemical genetics to identify novel compounds and biologic mechanisms involved in (lymph)angiogenesis in Xenopus tadpoles. A novel 2-step screening strategy involving a simple phenotypic read-out (edema formation or larval lethality) followed by semiautomated in situ hybridization was devised and used to screen an annotated chemical library of 1280 bioactive compounds. We identified 32 active compounds interfering with blood vascular and/or lymphatic development in Xenopus. Selected compounds were also tested for activities in a variety of endothelial in vitro assays. Finally, in a proof-of-principle study, the adenosine A1 receptor antagonist 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine, an inhibitor of blood vascular and lymphatic development in Xenopus, was shown to act also as a potent antagonist of VEGFA-induced adult neovascularization in mice. Taken together, the present chemical library screening strategy in Xenopus tadpoles represents a rapid and highly efficient approach to identify novel pathways involved in (lymph)angiogenesis. In addition, the recovered compounds represent a rich resource for in-depth analysis, and their drug-like features will facilitate further evaluation in preclinical models of inflammation and cancer metastasis.
Introduction
Lymphatic vessels play a major role in tissue pressure homeostasis, immune responses, and the uptake of dietary fat and fat-soluble vitamins, as well as in inflammation and cancer progression.1 Recent studies indicate that both lymphatic and blood vessels are involved in chronic inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis.1–3 But the formation and activation of both types of endothelium have also important roles in the progression and metastasis of the majority of human cancers.2,3 Tumors need to induce the growth of new blood vessels (angiogenesis) to secure the sufficient supply of oxygen and nutrients. The growth of new lymphatic vessels (lymphangiogenesis) has been shown to promote cancer metastasis to sentinel lymph nodes and beyond,4–8 a phenomenon that is also found in human neoplasm.9,10 Indeed, studies have revealed that tumor-induced lymphangiogenesis around the primary neoplasm is the most significant prognostic indicator to predict the occurrence of regional lymph node metastasis in human malignant melanomas of the skin.9 More recently, it has been found that tumors can induce lymphangiogenesis in their draining lymph nodes, even before they metastasize, and that induction of lymph node lymphangiogenesis promotes the further metastatic cancer spread to distant sites.7,8 Thus, tumor-induced lymphatic growth and activation represent a promising target for treating or preventing advanced cancer.
A strong correlation between the expression levels of the lymphangiogenic factor vascular endothelial growth factor-C (VEGFC), tumor lymphangiogenesis, and lymph node metastasis has been found in human and in experimental tumors.11 VEGFC promotes lymphangiogenesis by activating VEGF receptor-2 (VEGFR2) and VEGFR3 on lymphatic endothelial cells.12 VEGF-C-deficient mice fail to develop a functional lymphatic system,13 and transgenic expression of a soluble VEGFR3 results in pronounced lymphedema.12 However, blockade of the VEGFC/VEGFR3 axis only partially inhibits lymphatic metastasis, indicating that additional pathways are involved in mediating the formation and growth of lymphatic vessels. There have been previous attempts to identify lymphatic-specific receptors and pathways by transcriptional and proteomic profiling of cultured lymphatic endothelial cells (LECs).14–16 However, large-scale functional in vivo screens to identify molecular pathways or drug-like small molecule modulators of lymphatic vessel formation have been missing to date.
Amphibians offer many of the same experimental advantages that have made zebrafish a popular vertebrate model for in vivo drug screens,17 such as rapid extrauterine development, the transparency of developing tadpoles, and the permeability of the skin for small molecules. However, amphibians have a common evolutionary history with mammals that is an estimated 100 million years longer than between zebrafish and mammals.18 Being both tetrapods, amphibians and mammals share extensive synteny at the level of the genomes and have many similarities in organ development, anatomy, and physiology.19,20 These traits favor the use of amphibians for large-scale in vivo drug screens. In the past, embryos and tadpoles of the African clawed frog (Xenopus laevis) have served as a powerful animal model to study blood vascular development and angiogenesis.21–24 More recently, Xenopus embryos were shown to develop also a complex, well-defined lymphatic vascular system.25 Similar to the development of the mammalian lymphatic vascular system, LECs transdifferentiate from venous blood vascular endothelial cells and lymphangioblasts contribute in Xenopus to newly forming lymph vessels that mature to drain fluids from the peripheral tissues back to the blood circulation. Antisense-morpholino knockdown studies of the lymphangiogenic factor VEGFC in Xenopus embryos cause lymphatic vessel defects similar to the phenotype observed in VEGFC-deficient mice, including impaired LEC sprouting and migration, and the formation of lymphedema.13,25
Here, we have applied, for the first time, an unbiased forward chemical genetics approach in combination with a simple phenotypic readout and semiautomated in situ hybridization to uncover pathways involved in the development of the lymphatic and blood vascular system in Xenopus tadpoles. Our studies revealed novel compounds and pathways not previously known to mediate lymphatic and/or vascular development. This included an adenosine A1 receptor antagonist that not only inhibited lymphatic and blood vessel formation in Xenopus tadpoles but was also shown to block VEGFA-induced neovascularization in adult mice. The recovered compounds and molecular pathways will accelerate the development and testing of new drug candidates for the treatment of disorders, such as chronic inflammation and cancer.
Methods
The Xenopus and mouse studies were conducted under protocols approved by the Veterinary Office of the Canton of Zürich, Switzerland (permits 123/2005 and 199/2007). Complete information on methods is available in supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
A chemical library screen identifies a subset of compounds with pharmacologic activity in Xenopus embryos
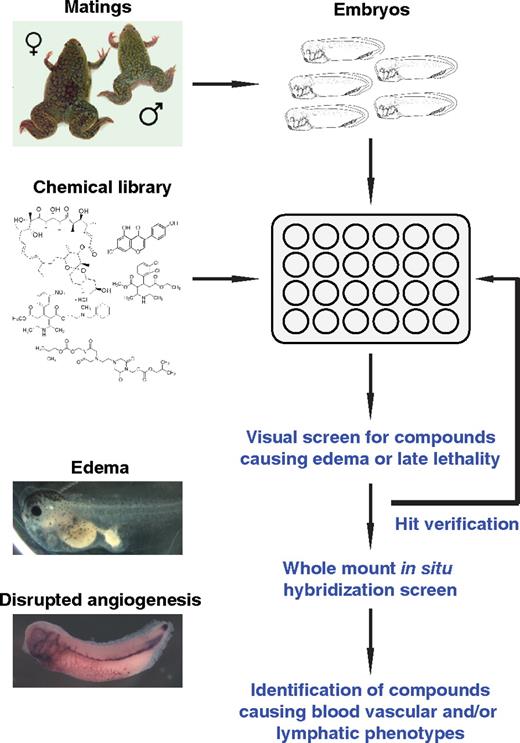
A 2-step whole organism-based chemical screening strategy was developed to rapidly identify novel small-molecule modulators of angiogenesis and lymphangiogenesis during Xenopus embryogenesis (Figure 1). The Library of Pharmacologically Active Compounds (LOPAC1280, Sigma-Aldrich), comprising 1280 bioactive compounds, was selected for the embryo-based screenings. The annotated compound library consists of marketed drugs, failed development candidates, and “gold standards” that have well-characterized activities (supplemental Table 1). The compounds represent 56 pharmacologic classes with diverse and experimentally validated biologic mechanisms, such as G-protein coupled receptors and kinases.26
A chemical library screening strategy to identify small-molecule modulators of vascular development in Xenopus embryos. Xenopus embryos were arrayed in multiwell plates, and single compounds from the LOPAC1280 chemical library were added to the water in each well. Embryos were screened visually for developmental defects (edema and/or late lethality). Positive hits are verified by repeating the phenotypic assay. Compounds interfering with vascular development are identified by whole-mount in situ hybridization of compound-treated Xenopus embryos.
A chemical library screening strategy to identify small-molecule modulators of vascular development in Xenopus embryos. Xenopus embryos were arrayed in multiwell plates, and single compounds from the LOPAC1280 chemical library were added to the water in each well. Embryos were screened visually for developmental defects (edema and/or late lethality). Positive hits are verified by repeating the phenotypic assay. Compounds interfering with vascular development are identified by whole-mount in situ hybridization of compound-treated Xenopus embryos.
Our primary screening strategy used edema formation in compound-treated tadpoles as a rapid phenotypic read-out. Edemas present as abnormal fluid-filled swellings in any organ of the body. They are caused by imbalanced fluid homeostasis either by increased secretion of fluid into the interstitium or impaired removal of this fluid. The underlying pathophysiologic causes include increased hydrostatic pressure or reduced oncotic pressure in the circulatory system, obstruction of the lymphatic system, and retention of sodium and water. Edema formation can therefore be used as a convenient indicator of impaired cardiovascular, lymphatic, and/or excretory system functions.
Xenopus late tailbud embryos at stage 31 (37 hpf) were selected for compound treatment. This embryonic stage coincides with the onset of lymphatic system formation and intersomitic vein (ISV) angiogenesis during Xenopus embryogenesis.22,25 Embryos were arrayed into 48-well dishes (5 per well) and treated with test compounds at a concentration of 20 μM in the presence of 1% dimethyl sulfoxide (DMSO). As a positive control, embryos were treated with compound E, a cell-permeable inhibitor of γ-secretase,27 which potently induces edemas (data not shown). All 48-well dishes also contained replicates of negative controls (1% DMSO). Embryos were monitored daily over a period of 4 days for edema formation. In addition, drug-induced lethality and other externally visible phenotypes were scored. On average, 320 compounds were screened per week. The phenotypes observed were highly reproducible as all embryos treated with a specific compound showed the same phenotype. Furthermore, all of the compounds generating hits were retested and yielded comparable results.
The majority (1166 compounds; 91%) of the 1280 compounds tested did not cause any discernable phenotypes in embryos and tadpoles until stage 47 (120 hpf), when treatments were terminated (Table 1). The remaining 114 (9%) compounds scored as hits. Fifty compounds representing 4% of the LOPAC1280 library caused either embryonic or larval lethality without evidence of edemas. They include 32 compounds that were severely cytotoxic, resulting in early embryonic lethality within 14 hours of compound treatment (supplemental Table 2), and 18 compounds that were lethal in tailbud embryos and/or tadpoles from at stage 37/38 (54 hpf) onwards (supplemental Table 3). Sixty-four (5%) compounds were nonlethal but caused specific, externally discernable phenotypes in embryos. Edemas were observed after treatment with 48 (4%) of the 1280 compounds tested (supplemental Table 4). In addition, 10 compounds affected skin pigmentation (supplemental Table 5), and 6 compounds caused other phenotypic changes (supplemental Table 6).
Summary of the results from the phenotypic chemical library screen of Xenopus embryos
| Phenotype . | No. of compounds . | Percentage of compounds tested . |
|---|---|---|
| Normal embryogenesis | 1166 | 91.1 |
| Abnormal embryogenesis by phenotype class | ||
| Cytotoxicity | 32 | 2.5 |
| Lethality | 18 | 1.4 |
| Edema formation | 48 | 3.7 |
| Pigmentation defects | 10 | 0.8 |
| Other defects | 6 | 0.5 |
| All abnormal embryogenesis | 114 | 8.9 |
| Total | 1280 | 100.0 |
| Phenotype . | No. of compounds . | Percentage of compounds tested . |
|---|---|---|
| Normal embryogenesis | 1166 | 91.1 |
| Abnormal embryogenesis by phenotype class | ||
| Cytotoxicity | 32 | 2.5 |
| Lethality | 18 | 1.4 |
| Edema formation | 48 | 3.7 |
| Pigmentation defects | 10 | 0.8 |
| Other defects | 6 | 0.5 |
| All abnormal embryogenesis | 114 | 8.9 |
| Total | 1280 | 100.0 |
Compounds with comparable in vivo activities target distinct pharmacologic pathways
The 1280 compounds in the LOPAC library represent 56 distinct pharmacologic classes of which 34 (61%) were active in the Xenopus screen (Table 2). Among the 114 active compounds, those affecting phosphorylation (30; 26%) were most prominently represented, followed by 9 (8%) interfering with the dopamine pathway and 8 (7%) modulating Ca+ channels. For 7 pharmacologic classes, which include phosphorylation, dopamine, and hormone signaling, a given compound was able to contribute to one of 3 distinct phenotype classes. Thirteen compound classes were contributing to at least 2 phenotype classes, whereas 14 were associated with a single phenotype class only. The latter included, for example, several representatives of the adenosine and cyclic nucleotide classes, which specifically caused edema in tadpoles. It was also evident that characteristic sets of active compound classes were associated with each phenotype class. Finally, compounds targeting the same common molecular pathway (ie, Ca2+ channels and adenosine A1 receptors) frequently manifest with the same characteristic edema phenotype (supplemental methods; supplemental Figure 1; supplemental Table 7).
Bioactive pharmacologic classes by phenotype
| Class* . | Total agents . | Screen . | Cytotoxicity . | Lethality . | Edema . | Pigmentation . | Other . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total active . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | ||
| Adenosine | 55 | 3 | — | — | — | — | 3 | 5.5 | — | — | — | — |
| Adrenoreceptor | 104 | 2 | — | — | — | — | 1 | 1.0 | 1 | 1.0 | — | — |
| Antibiotic | 28 | 3 | 1 | 3.6 | — | — | 1 | 3.6 | 1 | 3.6 | — | — |
| Apoptosis | 12 | 4 | 1 | 8.3 | — | — | 2 | 16.7 | — | — | 1 | 8.3 |
| Biochemistry | 46 | 2 | 1 | 2.2 | — | — | 1 | 2.2 | — | — | — | — |
| Ca2+ channel | 18 | 8 | — | — | 2 | 11.1 | 6 | 33.3 | — | — | — | — |
| Cannabinoid | 6 | 1 | 1 | 16.7 | — | — | — | — | — | — | — | — |
| Cell cycle | 15 | 3 | 1 | 6.7 | 1 | 6.7 | — | — | 1 | 6.7 | — | — |
| Cell stress | 19 | 3 | 1 | 5.3 | — | — | 1 | 5.3 | — | — | 1 | 5.3 |
| Cholinergic | 77 | 2 | 1 | 1.3 | 1 | 1.3 | — | — | — | — | — | — |
| Cl− channel | 3 | 1 | 1 | 33.3 | — | — | — | — | — | — | — | — |
| Cyclic nucleotides | 31 | 3 | — | — | — | — | 3 | 9.7 | — | — | — | — |
| Cytoskeleton and ECM | 10 | 3 | 1 | 10.0 | — | — | 2 | 20.0 | — | — | — | — |
| Dopamine | 114 | 9 | 1 | 0.9 | — | — | 3 | 2.6 | 5 | 4.4 | — | — |
| G protein | 4 | 1 | 1 | 25.0 | — | — | — | — | — | — | — | — |
| GABA | 42 | 1 | — | — | — | — | — | — | — | — | 1 | 2.4 |
| Gene regulation | 1 | 1 | — | — | — | — | 1 | 100.0 | — | — | — | — |
| Glutamate | 88 | 1 | — | — | — | — | 1 | 1.1 | — | — | — | — |
| Histamine | 31 | 2 | — | — | 1 | 3.2 | 1 | 3.2 | — | — | — | — |
| Hormone | 34 | 4 | — | — | 1 | 2.9 | 2 | 5.9 | — | — | 1 | 2.9 |
| Intracellular calcium | 8 | 3 | 2 | 25.0 | 1 | 12.5 | — | — | — | — | — | — |
| Ion pump | 17 | 1 | 1 | 5.9 | — | — | — | — | — | — | — | — |
| K+ channel | 19 | 1 | — | — | — | — | 1 | 5.3 | — | — | — | — |
| Leukotriene | 10 | 3 | 2 | 20.0 | 1 | 10.0 | — | — | — | — | — | — |
| Lipid | 10 | 3 | 2 | 20.0 | 1 | 10.0 | — | — | — | — | — | — |
| Multidrug resistance | 12 | 1 | — | — | — | — | — | — | — | — | 1 | 8.3 |
| Neurotransmission | 46 | 3 | 1 | 2.2 | 2 | 4.3 | — | — | — | — | — | — |
| Nitric oxide | 37 | 1 | — | — | — | — | — | — | — | — | 1 | 2.7 |
| Phosphorylation | 92 | 30 | 11 | 12.0 | 6 | 5.5 | 13 | 14.1 | — | — | — | — |
| Prostaglandin | 24 | 2 | — | — | 1 | 4.2 | 1 | 4.2 | — | — | — | — |
| Serotonin | 87 | 3 | — | — | — | — | 2 | 2.3 | 1 | 1.1 | — | — |
| Tachykinin | 5 | 1 | — | — | — | — | — | — | 1 | 20 | — | — |
| Thromboxane | 2 | 1 | 1 | 50.0 | — | — | — | — | — | — | — | — |
| Transcription | 12 | 4 | 1 | 8.3 | — | — | 3 | 25.0 | — | — | — | — |
| Entire screen | 1280 | 114 | 32 | 2.5† | 18 | 1.4† | 48 | 3.8† | 10 | 0.8† | 6 | 0.5† |
| Class* . | Total agents . | Screen . | Cytotoxicity . | Lethality . | Edema . | Pigmentation . | Other . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total active . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | Active agents . | Percentage of total . | ||
| Adenosine | 55 | 3 | — | — | — | — | 3 | 5.5 | — | — | — | — |
| Adrenoreceptor | 104 | 2 | — | — | — | — | 1 | 1.0 | 1 | 1.0 | — | — |
| Antibiotic | 28 | 3 | 1 | 3.6 | — | — | 1 | 3.6 | 1 | 3.6 | — | — |
| Apoptosis | 12 | 4 | 1 | 8.3 | — | — | 2 | 16.7 | — | — | 1 | 8.3 |
| Biochemistry | 46 | 2 | 1 | 2.2 | — | — | 1 | 2.2 | — | — | — | — |
| Ca2+ channel | 18 | 8 | — | — | 2 | 11.1 | 6 | 33.3 | — | — | — | — |
| Cannabinoid | 6 | 1 | 1 | 16.7 | — | — | — | — | — | — | — | — |
| Cell cycle | 15 | 3 | 1 | 6.7 | 1 | 6.7 | — | — | 1 | 6.7 | — | — |
| Cell stress | 19 | 3 | 1 | 5.3 | — | — | 1 | 5.3 | — | — | 1 | 5.3 |
| Cholinergic | 77 | 2 | 1 | 1.3 | 1 | 1.3 | — | — | — | — | — | — |
| Cl− channel | 3 | 1 | 1 | 33.3 | — | — | — | — | — | — | — | — |
| Cyclic nucleotides | 31 | 3 | — | — | — | — | 3 | 9.7 | — | — | — | — |
| Cytoskeleton and ECM | 10 | 3 | 1 | 10.0 | — | — | 2 | 20.0 | — | — | — | — |
| Dopamine | 114 | 9 | 1 | 0.9 | — | — | 3 | 2.6 | 5 | 4.4 | — | — |
| G protein | 4 | 1 | 1 | 25.0 | — | — | — | — | — | — | — | — |
| GABA | 42 | 1 | — | — | — | — | — | — | — | — | 1 | 2.4 |
| Gene regulation | 1 | 1 | — | — | — | — | 1 | 100.0 | — | — | — | — |
| Glutamate | 88 | 1 | — | — | — | — | 1 | 1.1 | — | — | — | — |
| Histamine | 31 | 2 | — | — | 1 | 3.2 | 1 | 3.2 | — | — | — | — |
| Hormone | 34 | 4 | — | — | 1 | 2.9 | 2 | 5.9 | — | — | 1 | 2.9 |
| Intracellular calcium | 8 | 3 | 2 | 25.0 | 1 | 12.5 | — | — | — | — | — | — |
| Ion pump | 17 | 1 | 1 | 5.9 | — | — | — | — | — | — | — | — |
| K+ channel | 19 | 1 | — | — | — | — | 1 | 5.3 | — | — | — | — |
| Leukotriene | 10 | 3 | 2 | 20.0 | 1 | 10.0 | — | — | — | — | — | — |
| Lipid | 10 | 3 | 2 | 20.0 | 1 | 10.0 | — | — | — | — | — | — |
| Multidrug resistance | 12 | 1 | — | — | — | — | — | — | — | — | 1 | 8.3 |
| Neurotransmission | 46 | 3 | 1 | 2.2 | 2 | 4.3 | — | — | — | — | — | — |
| Nitric oxide | 37 | 1 | — | — | — | — | — | — | — | — | 1 | 2.7 |
| Phosphorylation | 92 | 30 | 11 | 12.0 | 6 | 5.5 | 13 | 14.1 | — | — | — | — |
| Prostaglandin | 24 | 2 | — | — | 1 | 4.2 | 1 | 4.2 | — | — | — | — |
| Serotonin | 87 | 3 | — | — | — | — | 2 | 2.3 | 1 | 1.1 | — | — |
| Tachykinin | 5 | 1 | — | — | — | — | — | — | 1 | 20 | — | — |
| Thromboxane | 2 | 1 | 1 | 50.0 | — | — | — | — | — | — | — | — |
| Transcription | 12 | 4 | 1 | 8.3 | — | — | 3 | 25.0 | — | — | — | — |
| Entire screen | 1280 | 114 | 32 | 2.5† | 18 | 1.4† | 48 | 3.8† | 10 | 0.8† | 6 | 0.5† |
— indicates not applicable.
Includes only bioactive classes.
Frequency of whole screen.
Small-molecule compounds affecting blood vessel development and angiogenesis
We identified 48 compounds that induced edema in Xenopus tadpoles (supplemental Table 2) and 18 causing larval lethality at stage 37/38 (54 hpf) or later (supplemental Table 3). Together, the 66 compounds compose 5.1% of all compounds tested. Edema formation or late-stage lethality may result from excretory system defects and (cardio)vascular and/or lymphatic vascular pathologies. We next used semiautomated whole-mount in situ hybridization to visualize the development of blood and lymphatic vessels in Xenopus embryos treated with hits from the primary screen. apj and vegfr3 (flt4) were used as marker genes for the blood23 and lymphatic vasculature,25 respectively. Xenopus embryos were treated with compounds at stage 31 and fixed for in situ hybridization either at stage 35/36 to analyze vasculogenesis and ISV angiogenesis defects or at stage 42 to evaluate possible defects in lymph vessel development (Figures 2,Figure 3–4). A total of 32 (49%) of 65 compounds tested interfered with lymphatic and/or blood vascular development. Eighteen compounds selectively blocked blood vessel development, 6 compounds interfered with both blood and lymphatic vessel development, and 8 compounds affected lymphatic development only (Tables 3,Table 4–5).
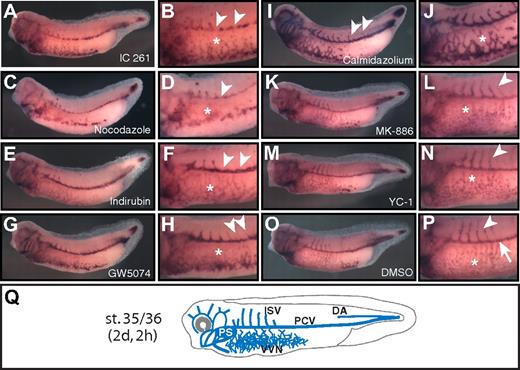
Compounds affecting distinct aspects of blood vessel development in vivo. Compound-treated (A-N) and control (DMSO-treated) Xenopus embryos (O-P) were analyzed by whole-mount in situ hybridization for expression of the vascular marker gene apj. Stage 35/36 embryos are shown in lateral views with anterior to the left. Close-up views of the trunk illustrate the morphology of the blood vessels. Compound names are indicated. (A-D) Hypoplastic VVN (*) and PCV (arrowheads). (E-F) Lack of ISVs (arrowheads). Note that assembly of PCVs and VVN (*) is unaffected. (G-H) Ectopic ISVs (arrowheads) and dysplastic VVN (*). (I-J) Ectopic ISV (arrowheads) and hyperplastic VVN (*). (K-N) Hypoplastic, dispersed VVN (*), but normal ISV angiogenesis (arrowheads). (O-P) Control embryos with normal VVN (*), PCVs (arrow), and ISVs (arrowhead). (Q) Scheme of the blood vasculature (blue) of the stage 35/36 embryo. Calmidazolium indicates calmidazolium chloride; Indirubin, indirubin-3′-oxime; and PS, pronephric sinus.
Compounds affecting distinct aspects of blood vessel development in vivo. Compound-treated (A-N) and control (DMSO-treated) Xenopus embryos (O-P) were analyzed by whole-mount in situ hybridization for expression of the vascular marker gene apj. Stage 35/36 embryos are shown in lateral views with anterior to the left. Close-up views of the trunk illustrate the morphology of the blood vessels. Compound names are indicated. (A-D) Hypoplastic VVN (*) and PCV (arrowheads). (E-F) Lack of ISVs (arrowheads). Note that assembly of PCVs and VVN (*) is unaffected. (G-H) Ectopic ISVs (arrowheads) and dysplastic VVN (*). (I-J) Ectopic ISV (arrowheads) and hyperplastic VVN (*). (K-N) Hypoplastic, dispersed VVN (*), but normal ISV angiogenesis (arrowheads). (O-P) Control embryos with normal VVN (*), PCVs (arrow), and ISVs (arrowhead). (Q) Scheme of the blood vasculature (blue) of the stage 35/36 embryo. Calmidazolium indicates calmidazolium chloride; Indirubin, indirubin-3′-oxime; and PS, pronephric sinus.
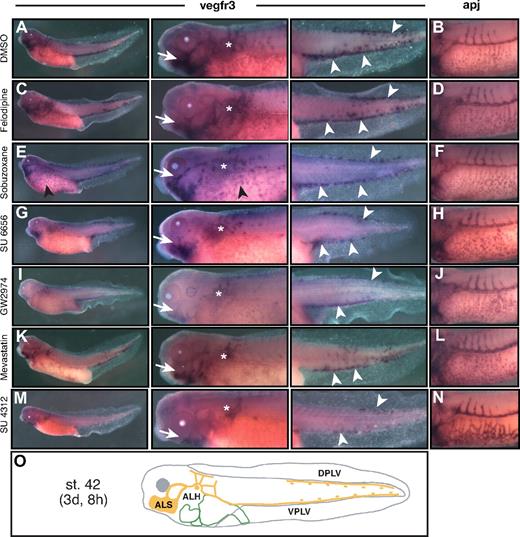
Compounds affecting blood and lymph vessel development in Xenopus tadpoles. Control DMSO- (A-B) and compound-treated (C-J) Xenopus embryos were analyzed by whole-mount in situ hybridization for expression of the blood vascular marker gene apj at stage 35/36 and the lymphatic marker gene vegfr3 at stage 42. Panels of the embryonic blood vasculature (apj) are accompanied by close-up views illustrating ISV angiogenesis and VVN development in the embryonic trunk. The panels visualizing the developing lymphatic system (vegfr3) include close-ups of the head and midtrunk region (middle panels) for the ALSs and the ALHs, and enlargements (left panels) of the tail for PLVs. (A) Normal ISVs (arrowhead) and VVN (*). (B) Normal ALSs (arrow), ALHs (*), and PLVs (arrowheads). (C) Stunted ISVs (arrowhead), normal VVN (*). (D) Hypoplastic PLV (arrowheads), impaired ALS (arrow), and ALH (*) lymphatics. (E) Stunted ISVs (arrowhead), hypoplastic VVN (*). (F) Stunted ALS lymphatics (arrow), dysplastic ALH lymphatics (*), hypoplastic PLVs (arrowheads). (G) Normal ISVs (arrowhead), hyperplastic VVN. (H) Hypoplastic PLVs (arrowheads), impaired ALS (arrow), and ALH (*) lymphatics. (I) Stunted ISVs (arrowhead), hyperplastic VVN. (J) Complete lack of ALS (arrow), ALH (*) and tail lymphatics. Note that vegfr3 expression persists in the VVN (arrowhead). (K) Scheme of the blood vasculature (blue) of the stage 35/36 embryo. (L) Scheme of the lymphatic vasculature (orange) of the stage 42 embryo. 7-Cyclo indicates 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; Naphthalimide, 4-amino-1,8-naphthalimide; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; PS; pronephric sinus; DPLV, dorsal posterior lymph vessel; and VPLV, ventral posterior lymph vessel.
Compounds affecting blood and lymph vessel development in Xenopus tadpoles. Control DMSO- (A-B) and compound-treated (C-J) Xenopus embryos were analyzed by whole-mount in situ hybridization for expression of the blood vascular marker gene apj at stage 35/36 and the lymphatic marker gene vegfr3 at stage 42. Panels of the embryonic blood vasculature (apj) are accompanied by close-up views illustrating ISV angiogenesis and VVN development in the embryonic trunk. The panels visualizing the developing lymphatic system (vegfr3) include close-ups of the head and midtrunk region (middle panels) for the ALSs and the ALHs, and enlargements (left panels) of the tail for PLVs. (A) Normal ISVs (arrowhead) and VVN (*). (B) Normal ALSs (arrow), ALHs (*), and PLVs (arrowheads). (C) Stunted ISVs (arrowhead), normal VVN (*). (D) Hypoplastic PLV (arrowheads), impaired ALS (arrow), and ALH (*) lymphatics. (E) Stunted ISVs (arrowhead), hypoplastic VVN (*). (F) Stunted ALS lymphatics (arrow), dysplastic ALH lymphatics (*), hypoplastic PLVs (arrowheads). (G) Normal ISVs (arrowhead), hyperplastic VVN. (H) Hypoplastic PLVs (arrowheads), impaired ALS (arrow), and ALH (*) lymphatics. (I) Stunted ISVs (arrowhead), hyperplastic VVN. (J) Complete lack of ALS (arrow), ALH (*) and tail lymphatics. Note that vegfr3 expression persists in the VVN (arrowhead). (K) Scheme of the blood vasculature (blue) of the stage 35/36 embryo. (L) Scheme of the lymphatic vasculature (orange) of the stage 42 embryo. 7-Cyclo indicates 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; Naphthalimide, 4-amino-1,8-naphthalimide; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; PS; pronephric sinus; DPLV, dorsal posterior lymph vessel; and VPLV, ventral posterior lymph vessel.
Compounds specifically affecting lymph vessel development in Xenopus tadpoles. Control DMSO- (A-B) and compound-treated (C-N) Xenopus embryos were analyzed by whole-mount in situ hybridization as described in the legend to Figure 3. Close-up views of apj-stained embryos illustrate normal ISV angiogenesis and VVN development in the embryonic trunk. ALSs, ALHs, and PLVs are highlighted by arrows, asterisks, and arrowheads, respectively. (A-B) Normal ALS and ALH lymphatics, and PLVs. (C-D) Dysplastic ALH lymphatics. (E-F) Dysplastic ALS and ALH lymphatics, and hypoplastic PLVs. Note persistent vegfr3 expression in the VVN (black arrowhead). (G-H) Stunted ALS lymphatics, dysplastic ALH lymphatics, and hypoplastic PLVs. (I-J) Impaired ALS lymphangiogenesis, stunted ALH lymphatics, and hypoplastic PLVs. (K-N) Impaired ALS and ALH lymphangiogenesis, and hypoplastic PLVs. (O) Scheme of the lymphatic vasculature (orange) of the stage 42 embryo. DPLV indicates dorsal posterior lymph vessel; and VPLV, ventral posterior lymph vessel.
Compounds specifically affecting lymph vessel development in Xenopus tadpoles. Control DMSO- (A-B) and compound-treated (C-N) Xenopus embryos were analyzed by whole-mount in situ hybridization as described in the legend to Figure 3. Close-up views of apj-stained embryos illustrate normal ISV angiogenesis and VVN development in the embryonic trunk. ALSs, ALHs, and PLVs are highlighted by arrows, asterisks, and arrowheads, respectively. (A-B) Normal ALS and ALH lymphatics, and PLVs. (C-D) Dysplastic ALH lymphatics. (E-F) Dysplastic ALS and ALH lymphatics, and hypoplastic PLVs. Note persistent vegfr3 expression in the VVN (black arrowhead). (G-H) Stunted ALS lymphatics, dysplastic ALH lymphatics, and hypoplastic PLVs. (I-J) Impaired ALS lymphangiogenesis, stunted ALH lymphatics, and hypoplastic PLVs. (K-N) Impaired ALS and ALH lymphangiogenesis, and hypoplastic PLVs. (O) Scheme of the lymphatic vasculature (orange) of the stage 42 embryo. DPLV indicates dorsal posterior lymph vessel; and VPLV, ventral posterior lymph vessel.
Vascular phenotypes resulting from small-molecule treatment of Xenopus embryos (compounds affecting blood vessel development only)
| Phenotype class/compound name . | Pharmacologic class . | Selectivity . | ISV phenotype . | VVN phenotype . | PCV phenotype . | Lymphatic phenotype . | Embryonic phenotype . |
|---|---|---|---|---|---|---|---|
| Defective vasculogenesis | |||||||
| Nocodazole | Cytoskeleton and ECM | Beta-tubulin | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| Podophyllotoxin | Cytoskeleton and ECM | Impaired | Hypoplastic | Hypoplastic | NA | EDE | |
| 2-Methoxyestradiol | Hormone | Estrogen | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| IC 261 | Phosphorylation | CK-1delta/epsilon | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| NSC 95397 | Phosphorylation | Cdc25 | Impaired | Hypoplastic | Hypoplastic | NA | LET |
| Tyrphostin AG 494 | Phosphorylation | EGFR | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| Meclofenamic acid sodium | Prostaglandin | COX/5-lipoxygenase | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| Defective angiogenesis | |||||||
| Retinoic acid | Apoptosis | Impaired | Hypoplastic | Normal | NA | EDE | |
| Forskolin | Cyclic nucleotides | Adenylate cyclase | Impaired | Hypoplastic | Normal | NA | EDE |
| L-162,313 | Neurotransmission | AT1 | Impaired | Hypoplastic | Normal | Normal | LET |
| Indirubin-3′-oxime | Phosphorylation | CDK | Impaired | Normal | Normal | Normal | LET |
| SU 5416 | Phosphorylation | VEGFR PTK | Impaired | Hypoplastic | Normal | NA | LET |
| Ectopic angiogenic sprouting | |||||||
| Calmidazolium chloride | Intracellular calcium | Ca2+ATPase | Ectopic ISVs | Hyperplastic | Normal | NA | LET |
| GW5074 | Phosphorylation | Raf1 kinase | Ectopic ISVs | Dysplastic | Normal | NA | EDE |
| VVN hypoplasia | |||||||
| YC-1 | Cyclic nucleotides | Guanylyl cyclase | Normal | Hypoplastic | Normal | Normal | EDE |
| Zardaverine | Cyclic nucleotides | PDE III/PDE IV | Normal | Hypoplastic | Normal | NA | EDE |
| MK-886 | Leukotriene | Normal | Hypoplastic | Normal | NA | LET | |
| Purvalanol A | Phosphorylation | CDK | Normal | Hypoplastic | Normal | Normal | LET |
| Phenotype class/compound name . | Pharmacologic class . | Selectivity . | ISV phenotype . | VVN phenotype . | PCV phenotype . | Lymphatic phenotype . | Embryonic phenotype . |
|---|---|---|---|---|---|---|---|
| Defective vasculogenesis | |||||||
| Nocodazole | Cytoskeleton and ECM | Beta-tubulin | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| Podophyllotoxin | Cytoskeleton and ECM | Impaired | Hypoplastic | Hypoplastic | NA | EDE | |
| 2-Methoxyestradiol | Hormone | Estrogen | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| IC 261 | Phosphorylation | CK-1delta/epsilon | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| NSC 95397 | Phosphorylation | Cdc25 | Impaired | Hypoplastic | Hypoplastic | NA | LET |
| Tyrphostin AG 494 | Phosphorylation | EGFR | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| Meclofenamic acid sodium | Prostaglandin | COX/5-lipoxygenase | Impaired | Hypoplastic | Hypoplastic | NA | EDE |
| Defective angiogenesis | |||||||
| Retinoic acid | Apoptosis | Impaired | Hypoplastic | Normal | NA | EDE | |
| Forskolin | Cyclic nucleotides | Adenylate cyclase | Impaired | Hypoplastic | Normal | NA | EDE |
| L-162,313 | Neurotransmission | AT1 | Impaired | Hypoplastic | Normal | Normal | LET |
| Indirubin-3′-oxime | Phosphorylation | CDK | Impaired | Normal | Normal | Normal | LET |
| SU 5416 | Phosphorylation | VEGFR PTK | Impaired | Hypoplastic | Normal | NA | LET |
| Ectopic angiogenic sprouting | |||||||
| Calmidazolium chloride | Intracellular calcium | Ca2+ATPase | Ectopic ISVs | Hyperplastic | Normal | NA | LET |
| GW5074 | Phosphorylation | Raf1 kinase | Ectopic ISVs | Dysplastic | Normal | NA | EDE |
| VVN hypoplasia | |||||||
| YC-1 | Cyclic nucleotides | Guanylyl cyclase | Normal | Hypoplastic | Normal | Normal | EDE |
| Zardaverine | Cyclic nucleotides | PDE III/PDE IV | Normal | Hypoplastic | Normal | NA | EDE |
| MK-886 | Leukotriene | Normal | Hypoplastic | Normal | NA | LET | |
| Purvalanol A | Phosphorylation | CDK | Normal | Hypoplastic | Normal | Normal | LET |
ISV indicates intersomitic vessels; VVN, vitelline vein network; PCV, posterior cardinal vein; NA, not applicable; EDE, edema phenotype; ECM, extracellular matrix; LET, lethal before stage 48; ALS, anterior lymph sac; ALH, anterior lymph heart; PLV, posterior lymph vessels; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; and 7-Cyclo, 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine.
Vascular phenotypes resulting from small-molecule treatment of Xenopus embryos (compounds affecting blood and lymph vessel formation)
| Phenotype class/compound name . | Pharmacologic class . | Selectivity . | ISV phenotype . | VVN phenotype . | PCV phenotype . | ALS phenotype . | ALH phenotype . | PLV phenotype . | Embryonic phenotype . |
|---|---|---|---|---|---|---|---|---|---|
| Defective blood and lymph angiogenesis | |||||||||
| Naphthyridine | Adenosine | A1 | Stunted | Hypoplastic | Normal | Stunted | Dysplastic | Hypoplastic | EDE |
| 7-Cyclo | Phosphorylation | lck | Stunted | Normal | Normal | Impaired | Impaired | Hypoplastic | EDE |
| SP600125 | Phosphorylation | c-JNK | Stunted | Dysplastic | Normal | Impaired | Impaired | Impaired | LET |
| VVN hyperplasia and defective lymph angiogenesis | |||||||||
| 1,3-Diethyl-8-phenylxanthine | Adenosine | A1 | Normal | Hyperplastic | Normal | Stunted | Stunted | Hypoplastic | EDE |
| 4-Amino-1,8-naphthalimide | Apoptosis | PARP | Normal | Hyperplastic | Normal | Impaired | Stunted | Hypoplastic | EDE |
| Genistein | Phosphorylation | Tyrosine kinase | Stunted | Hyperplastic | Normal | Impaired | Impaired | Impaired | EDE |
| Phenotype class/compound name . | Pharmacologic class . | Selectivity . | ISV phenotype . | VVN phenotype . | PCV phenotype . | ALS phenotype . | ALH phenotype . | PLV phenotype . | Embryonic phenotype . |
|---|---|---|---|---|---|---|---|---|---|
| Defective blood and lymph angiogenesis | |||||||||
| Naphthyridine | Adenosine | A1 | Stunted | Hypoplastic | Normal | Stunted | Dysplastic | Hypoplastic | EDE |
| 7-Cyclo | Phosphorylation | lck | Stunted | Normal | Normal | Impaired | Impaired | Hypoplastic | EDE |
| SP600125 | Phosphorylation | c-JNK | Stunted | Dysplastic | Normal | Impaired | Impaired | Impaired | LET |
| VVN hyperplasia and defective lymph angiogenesis | |||||||||
| 1,3-Diethyl-8-phenylxanthine | Adenosine | A1 | Normal | Hyperplastic | Normal | Stunted | Stunted | Hypoplastic | EDE |
| 4-Amino-1,8-naphthalimide | Apoptosis | PARP | Normal | Hyperplastic | Normal | Impaired | Stunted | Hypoplastic | EDE |
| Genistein | Phosphorylation | Tyrosine kinase | Stunted | Hyperplastic | Normal | Impaired | Impaired | Impaired | EDE |
Abbreviations are as in Table 3.
Vascular phenotypes resulting from small-molecule treatment of Xenopus embryos (compounds affecting lymph vessel formation only)
| Phenotype class/compound name . | Pharmacologic class . | Selectivity . | Blood vessel phenotype . | ALS phenotype . | ALH phenotype . | PLV phenotype . | Embryonic phenotype . |
|---|---|---|---|---|---|---|---|
| Defective lymph angiogenesis | |||||||
| Felodipine | Ca2+ channel | L-type | Normal | Normal | Dysplastic | Normal | EDE |
| Sobuzoxane | Gene regulation | Topo II | Normal | Dysplastic | Dysplastic | Hypoplastic | EDE |
| Nicardipine hydrochloride | Ca2+ channel | L-type | Normal | Dysplastic | Dysplastic | Hypoplastic | EDE |
| SU 6656 | Phosphorylation | Src family kinase | Normal | Stunted | Dysplastic | Hypoplastic | EDE |
| Dequalinium dichloride | K+ channel | Normal | Stunted | Stunted | Hypoplastic | EDE | |
| GW2974 | Phosphorylation | EGFR/ErbB2-2 | Normal | Impaired | Stunted | Hypoplastic | EDE |
| Mevastatin | Antibiotic | Ras, Rho | Normal | Impaired | Impaired | Hypoplastic | EDE |
| SU 4312 | phosphorylation | KDR | Normal | Impaired | Impaired | Hypoplastic | EDE |
| Phenotype class/compound name . | Pharmacologic class . | Selectivity . | Blood vessel phenotype . | ALS phenotype . | ALH phenotype . | PLV phenotype . | Embryonic phenotype . |
|---|---|---|---|---|---|---|---|
| Defective lymph angiogenesis | |||||||
| Felodipine | Ca2+ channel | L-type | Normal | Normal | Dysplastic | Normal | EDE |
| Sobuzoxane | Gene regulation | Topo II | Normal | Dysplastic | Dysplastic | Hypoplastic | EDE |
| Nicardipine hydrochloride | Ca2+ channel | L-type | Normal | Dysplastic | Dysplastic | Hypoplastic | EDE |
| SU 6656 | Phosphorylation | Src family kinase | Normal | Stunted | Dysplastic | Hypoplastic | EDE |
| Dequalinium dichloride | K+ channel | Normal | Stunted | Stunted | Hypoplastic | EDE | |
| GW2974 | Phosphorylation | EGFR/ErbB2-2 | Normal | Impaired | Stunted | Hypoplastic | EDE |
| Mevastatin | Antibiotic | Ras, Rho | Normal | Impaired | Impaired | Hypoplastic | EDE |
| SU 4312 | phosphorylation | KDR | Normal | Impaired | Impaired | Hypoplastic | EDE |
Abbreviations are as in Table 3.
With regard to the blood vascular defects, we identified 4 different classes: (1) inhibition of vasculogenesis, as evidenced by hypoplasia of the vitelline vein network (VVN) and posterior cardinal veins (PCVs) and absence of ISVs; (2) inhibition of angiogenesis, as reflected by defective ISV outgrowth and normal PCVs; (3) ectopic angiogenic sprouting of the ISV and VVN defects; and (4) hypoplasia of the VVN. Compounds that interfered with blood vessel vasculogenesis and PCV assembly included the casein kinase 1 inhibitor IC 261 (Figure 2A-B) and nocodazole, which disrupts cytoskeleton assembly (Figure 2C-D). Both of these compounds also impaired the development of the ISV and VVN (equivalent to the extraembryonic vasculature of the avian chorio-allantoic membrane and the mammalian yolk sac). Similar effects were observed after treatment with the tyrosine kinase inhibitor tyrphostin AG 494, 2-methoxyestradiol, the cytoskeleton inhibitor podophyllotoxin, the Cdc25 phosphatase inhibitor NSC 95397, and the dual cyclooxygenase and 5-lipoxygenase inhibitor meclofenamic acid (Table 3).
Angiogenesis inhibitors, as evaluated by the ability of compounds to block ISV outgrowth, included indirubin-3′-oxime, a cyclin-dependent kinase inhibitor (Figure 2E-F), and the VEGF receptor phosphotyrosine kinase inhibitor SU 5416 (Tables 3,Table 4–5), which is in agreement with previous studies in zebrafish.28–30 We further identified retinoic acid, the adenylate cyclase activator forskolin, and the angiotensin receptor 1 agonist L-162,313 as potent inhibitors of developmental angiogenesis (Table 3).
Two compounds induced VVN defects and promoted premature angiogenic sprouting of ISVs. After treatment with the Raf1 kinase inhibitor GW5074 (Figure 2G-H), the VVN was dysplastic and, instead of forming a regular vessel network, the blood vascular endothelial cells were dispersed throughout the ventral parts of the embryo. Moreover, thin ectopic ISV sprouting was evident in the posterior parts of the PCV compared with DMSO-treated control embryos (Figure 2G-H,O-P). This ectopic sprouting phenotype was even more pronounced after treatment with the calmodulin-dependent Ca2+ATPase inhibitor calmidazolium chloride (Figure 2I-J,O-P). In addition, the blood vessels, including the VVN, were larger and fused, representing hyperplastic vasculature. This hyperplastic endothelial phenotype is reminiscent of the phenotype observed after overexpression of vegfa in Xenopus embryos.23
A fourth group of compounds only interfered with the assembly of the VVN. The leukotriene synthesis inhibitor MK-886 led to hypoplasia of the VVN but did not interfere with PCV assembly and ISV outgrowth (Figure 2K-L). Similarly, treatments with the NO-independent guanylyl cyclase activator YC-1 (Figure 2M-N) resulted in dispersed, punctuated patterns of VVN endothelia, which might indicate a block of endothelial cell proliferation or VVN assembly.31 Other compounds of this group include the phosphodiesterase III/IV inhibitor zardaverine and the cyclin-dependent protein kinase inhibitor purvalanol A (Tables 3).
Small-molecule inhibitors of blood and lymphatic vessel development
Three primary sites of lymphatic system development can be observed in Xenopus embryos: the anterior lymph sacs (ALSs) of the head, the region of the anterior lymph hearts (ALHs) in the trunk, and the posterior lymph vessels (PLVs) in the tail (Figure 3B,L). Along with the analysis for blood vessel defects, we assessed specifically these areas in compound-treated embryos for abnormalities in early lymphatic vessel development. Based on this differential analysis, we identified 2 general compound-induced phenotype classes: compounds affecting both blood vessel and lymph vessel formation and compounds disrupting selectively lymph vessel development only (Table 4).
The former phenotype class was composed of 6 compounds. The src family tyrosine kinase inhibitor 7-cyclopentyl-5-(-4-phenoxy)phenyl-7H-pyrrolo(2,3-d)pyrimidin-4-ylamin (7-Cyclo) interfered with angiogenic ISV sprouting but not with VVN assembly (Figure 3C). Formation of ALS and ALH rudiments occurred, but lymphangiogenesis was strongly suppressed and the lymph vessel assembly in the tadpole tails was disrupted (Figure 3D). The A1 adenosine receptor antagonist 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine (naphthyridine) interfered not only with ISV angiogenesis but also with VVN assembly (Figure 3E). At later stages, tadpoles displayed stunted, disorganized lymphatic vessels arising from the ALSs and ALHs, and only a few LECs, but no lymph vessels, were detected in the tail (Figure 3F). Similar vascular phenotypes were observed after treatment with the JNK inhibitor SP600125 (Table 4). A subgroup of 3 compounds manifested with lymphangiogenesis defects accompanied by VVN hyperplasia. Treatment with the adenosine A1 receptor antagonist 1,3-diethyl-8-phenylxanthine or the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide caused hyperplasia of the VVN, whereas ISV angiogenesis occurred unaffected (Figure 3G and data not shown). Interestingly, lymphangiogenesis was largely suppressed (Figure 3H and data not shown). Treatment with the tyrosine kinase inhibitor genistein induced a severe blood vessel phenotype with stunted ISVs and hyperplastic VVN (Figure 3I). At later stages, blood vessels were readily detected, whereas lymphatic vessels and LECs were absent (Figure 3J). This suggests that genistein may interfere with the specification of lymphatic cell lineages.
Small-molecule inhibitors of lymphatic vessel development
We recovered 8 compounds that specifically inhibited lymphatic vessel development without affecting the blood vasculature from the chemical library screen (Table 5; Figure 4). Each compound induced highly characteristic lymphatic defects, ranging from subtle regional lymph vessel dysplasia (Figure 4C) to severe, widespread disruption of lymph vessel development (Figure 4I,K,M). The L-type calcium channel blocker felodipine caused abnormal, dysplastic lymphatic vessel sprouting without affecting other areas of lymphangiogenesis (Figure 4C). Treatment with the DNA topoisomerase II inhibitor sobuzoxane and the Ca2+ channel blocker nicardipine resulted in the formation of discontinuous, dysplastic lymphatic vessels (Figure 4E and data not shown). In addition, vegfr3 expression persisted in the VVN of subuzoxane-treated embryos (Figure 4E). The src family kinase inhibitor SU 6656 and the K+ channel blocker dequalinium dichloride resulted in embryos with poorly developed, stunted lymphatics emerging from the ALSs, ALHs, and hypoplastic PLVs (Figure 4G and data not shown). The most severe defects in lymphatic vessel development were observed with the protein prenylation inhibitor mevastatin and the tyrosine kinase inhibitors GW2974 and SU 4312 (Figure 4I-M). After compound treatment, the 3 primary sites of lymphatic vessel development (ALSs, ALHs, and PLVs) were detectable, but lymphangiogenesis was largely suppressed and the assembly of lymphatics in the tail was disrupted. This phenotype was most pronounced after treatment with SU 4312, which is known as a VEGF receptor-1/-2 (VEGFR) inhibitor (Figure 4M). Interestingly, blood vascular development is critically dependent on VEGFR1/VEGFR2 signaling but appears to be unaffected in SU 4312-treated embryos as demonstrated by normal angiogenic ISV outgrowth (Figure 4N).
Effects of small-molecular compounds on in vitro endothelial cell proliferation and tube formation
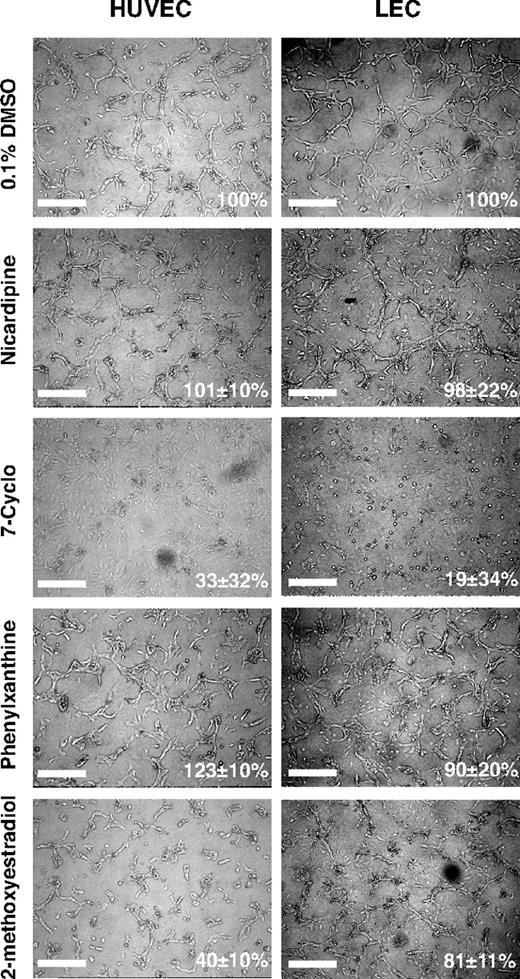
We next asked whether the in vivo activities of the compounds extended also to mammalian endothelia and whether the compounds interfered directly with endothelial cell functions. We selected 24 compounds and conducted proliferation and tube formation assays, 2 important steps in vessel formation, using human LEC and umbilical vein endothelial cell (HUVEC) cultures (Table 6).
Comparison between the in vivo and in vitro activities of small-molecule compounds
| Phenotype class in vivo/compound name . | HUVEC proliferation . | HUVEC tube formation . | LEC proliferation . | LEC tube formation . |
|---|---|---|---|---|
| Compounds affecting in vivo blood vessel development only | ||||
| Defective vasculogenesis | ||||
| Nocodazole | − | −− | − | −− |
| Podophyllotoxin | − | −− | − | −− |
| 2-Methoxyestradiol | O | −− | − | − |
| IC 261 | O | − | − | − |
| Tyrphostin AG 494 | O | O | O | − |
| Defective angiogenesis | ||||
| Retinoic acid | + | − | + | − |
| L-162,313 | + | − | O | − |
| Indirubin-3′-oxime | − | − | O | O |
| SU 5416 | O | O | O | − |
| Ectopic angiogenic sprouting | ||||
| GW5074 | O | O | + | − |
| VVN hypoplasia | ||||
| MK-886 | + | − | O | − |
| Compounds affecting in vivo blood and lymph vessel formation | ||||
| Defective blood and lymphangiogenesis | ||||
| Naphthyridine | −− | − | −− | − |
| 7-Cyclo | O | −− | O | −− |
| SP600125 | O | O | O | − |
| VVN hyperplasia and defective lymphangiogenesis | ||||
| 1,3-Diethyl-8-phenylxanthine | O | + | O | O |
| 4-Amino-1,8-naphthalimide | + | O | O | − |
| Genistein | O | − | O | − |
| Compounds affecting in vivo lymph vessel formation only | ||||
| Defective lymphangiogenesis | ||||
| Felodipine | O | − | O | O |
| Sobuzoxane | + | O | + | O |
| Nicardipine hydrochloride | O | O | O | O |
| GW2974 | + | − | + | − |
| SU 4312 | + | O | O | O |
| Compounds with no effect on vascular development in vivo | ||||
| Cyclosporin A | O | O | O | O |
| MRS 1845 | + | O | O | − |
| L-687,384 hydrochloride | + | − | O | − |
| Phenotype class in vivo/compound name . | HUVEC proliferation . | HUVEC tube formation . | LEC proliferation . | LEC tube formation . |
|---|---|---|---|---|
| Compounds affecting in vivo blood vessel development only | ||||
| Defective vasculogenesis | ||||
| Nocodazole | − | −− | − | −− |
| Podophyllotoxin | − | −− | − | −− |
| 2-Methoxyestradiol | O | −− | − | − |
| IC 261 | O | − | − | − |
| Tyrphostin AG 494 | O | O | O | − |
| Defective angiogenesis | ||||
| Retinoic acid | + | − | + | − |
| L-162,313 | + | − | O | − |
| Indirubin-3′-oxime | − | − | O | O |
| SU 5416 | O | O | O | − |
| Ectopic angiogenic sprouting | ||||
| GW5074 | O | O | + | − |
| VVN hypoplasia | ||||
| MK-886 | + | − | O | − |
| Compounds affecting in vivo blood and lymph vessel formation | ||||
| Defective blood and lymphangiogenesis | ||||
| Naphthyridine | −− | − | −− | − |
| 7-Cyclo | O | −− | O | −− |
| SP600125 | O | O | O | − |
| VVN hyperplasia and defective lymphangiogenesis | ||||
| 1,3-Diethyl-8-phenylxanthine | O | + | O | O |
| 4-Amino-1,8-naphthalimide | + | O | O | − |
| Genistein | O | − | O | − |
| Compounds affecting in vivo lymph vessel formation only | ||||
| Defective lymphangiogenesis | ||||
| Felodipine | O | − | O | O |
| Sobuzoxane | + | O | + | O |
| Nicardipine hydrochloride | O | O | O | O |
| GW2974 | + | − | + | − |
| SU 4312 | + | O | O | O |
| Compounds with no effect on vascular development in vivo | ||||
| Cyclosporin A | O | O | O | O |
| MRS 1845 | + | O | O | − |
| L-687,384 hydrochloride | + | − | O | − |
Scoring system for in vitro assays: O indicates inactive or marginal effects (85%-115% of control); −, moderate inhibition (50%-85% of control); −−, strong inhibition (< 50% of control); and +, moderate promotion (> 115%-150% of control).
HUVEC indicates human umbilical vein endothelial cell; LEC, lymphatic endothelial cell; VVN, vitelline vein network; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; and 7-Cyclo, 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine.
The cell proliferation assays were performed by treating the endothelial cell cultures with compounds at a screening dose of 10 μM in 0.1% DMSO for 48 hours (Figure 5A; Table 6). Nine compounds were scored as having marginal or no effects in the endothelial cell proliferation assays (85%-115% of control) regardless of the cell type tested. Nine compounds moderately promoted cell proliferation (> 115%-150% of control). Among these, 5 compounds (L-687,384, MK-886, MRS 1845, naphthalimide, and SU 4312) promoted proliferation in HUVEC but not LEC cultures; and one, GW5074, was selective for LEC cultures. Six compounds decreased endothelial cell proliferation in vitro. Interestingly, IC 261 and 2-methoxyestradiol preferentially inhibited LEC proliferation, whereas indirubin-3′-oxime selectively blocked HUVEC proliferation consistent with a previous report.30
Selective and cell-type specific in vitro responses of endothelial cells to treatment with small-molecule compounds. Twenty-four compounds were tested in vitro on human lymphatic (LECs; black bars) and blood vascular (HUVECs; open bars) endothelial cell cultures for effects on cell proliferation and tube formation. (A) Results of the compound screens using cell proliferation assays. Compounds were screened at a dose of 10 μM in 0.1% DMSO. Control cultures were treated with 0.1% DMSO. (B) Results of the compound screens using tube formation assays. Compounds were screened at a dose of 1 μM in 0.1% DMSO. Control cultures were treated with 0.1% DMSO. 7-Cyclo indicates 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; L-687,384, L-687,384 hydrochloride; Naphthalimide, 4-amino-1,8-naphthalimide; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; Nicardipine, nicardipine hydrochloride; Phenylxanthine, 1,3-diethyl-8-phenylxanthine. Bars represent mean values and SD of 3 independently performed assays.
Selective and cell-type specific in vitro responses of endothelial cells to treatment with small-molecule compounds. Twenty-four compounds were tested in vitro on human lymphatic (LECs; black bars) and blood vascular (HUVECs; open bars) endothelial cell cultures for effects on cell proliferation and tube formation. (A) Results of the compound screens using cell proliferation assays. Compounds were screened at a dose of 10 μM in 0.1% DMSO. Control cultures were treated with 0.1% DMSO. (B) Results of the compound screens using tube formation assays. Compounds were screened at a dose of 1 μM in 0.1% DMSO. Control cultures were treated with 0.1% DMSO. 7-Cyclo indicates 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; L-687,384, L-687,384 hydrochloride; Naphthalimide, 4-amino-1,8-naphthalimide; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; Nicardipine, nicardipine hydrochloride; Phenylxanthine, 1,3-diethyl-8-phenylxanthine. Bars represent mean values and SD of 3 independently performed assays.
The effects on endothelial tube formation were assessed after overnight treatment of LEC and HUVEC cultures at a compound concentration of 1 μM in 0.1% DMSO. Fourteen of 24 compounds tested had comparable effects in both cell types tested (Figures 5B, 6). These include 4 compounds (cyclosporin A, nicardipine, sobuzoxane, and SU 4312) with either no or only marginal effects on tube formation (85%-115% of control tube length), 7 compounds with moderate inhibition of tube formation (50%-85% of control), and 3 compounds (7-Cyclo, nocodazole, podophyllotoxin) strongly inhibiting of endothelial tube formation (< 35% of control). The remaining 10 compounds showed differential, cell-type specific effects on tube formation (Figures 5B, 6). Phenylxanthine promoted tube formation, whereas indirubin-3′-oxime, felodipine, and 2-methoxyestradiol acted as inhibitors of tube formation in HUVEC cultures. Six compounds (GW5074, MRS 1845, naphthalimide, SP600125, SU 5416, tyrphostin AG 494) preferentially blocked tube formation in LEC rather than HUVEC cultures (Figure 5B). A comparison of the results from the in vitro and in vivo compound screens is shown in Table 6.
Differential, cell-type specific effects of selected small-molecule compounds on endothelial tube formation. Confluent monolayers of HUVEC or LECs were overlaid with collagen type I gels containing the indicated compounds at a dose of 1 μM in 0.1% DMSO. Scale bars represent 100 μm.
Differential, cell-type specific effects of selected small-molecule compounds on endothelial tube formation. Confluent monolayers of HUVEC or LECs were overlaid with collagen type I gels containing the indicated compounds at a dose of 1 μM in 0.1% DMSO. Scale bars represent 100 μm.
Naphthyridine inhibits VEGFA-induced (lymph)angiogenesis in mice
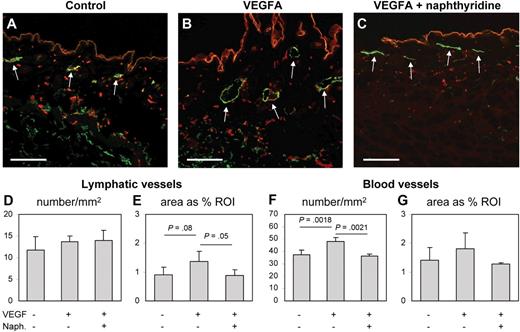
The adenosine A1 receptor antagonist naphthyridine was identified as a novel inhibitor of lymphatic and blood vessel formation in Xenopus tadpoles and in vitro cellular assays, where it acted antimitogenic and impaired endothelial tube formation. We determined that naphtyridine inhibited LEC tube formation with an IC50 of 1.3 μM plus or minus 0.1 μM (data not shown). To investigate whether naphthyridine might also inhibit mammalian angiogenesis and/or lymphangiogenesis in vivo, we implanted VEGFA-containing Matrigel plugs subcutaneously into adult mice and treated these mice systemically with naphthyridine (3 mg/kg per day) or with vehicle control for 6 days. In agreement with previous results,32 differential immunofluorescence analysis for the lymphatic-specific marker LYVE1 and the panvascular marker CD31 demonstrated lymphatic vessel enlargement in the skin surrounding VEGFA-containing Matrigels, compared with control Matrigels only containing phosphate-buffered saline (PBS; Figure 7A-B). Treatment with naphthyridine resulted in a reduction of blood vessel numbers and lymphatic vessel enlargement (Figure 7C). The quantitative image analyses shown in Figure 7D through G confirmed that the tissue area covered by lymphatic vessels surrounding VEGFA containing Matrigels was significantly reduced by treatment with naphthyridine compared with the PBS control (1.36% ± 0.36% vs 0.89% ± 0.18%; P = .05; Figure 7E), whereas the density of lymphatic vessels was unchanged by VEGFA or naphthyridine treatment (Figure 7D). Treatment with naphthyridine also reduced the number of VEGFA-induced blood vessels (36.49 ± 1.49 vs 47.93 ± 3.55; P = .002; Figure 7F) and resulted in a reduction of the tissue area covered by blood vessels (Figure 7G). We conclude that the ability of naphthyridine to inhibit lymph and blood vessel angiogenesis is conserved between Xenopus and mammals.
The adenosine A1 receptor antagonist naphthyridine inhibits VEGFA-induced angiogenesis and lymphatic vessel enlargement in mice. VEGFA-containing Matrigel plugs were implanted subcutaneously into adult mice, and the mice were subsequently treated systemically with naphthyridine or with vehicle control for 6 days. (A-C) Differential immunofluorescence analysis for the lymphatic-specific marker LYVE1 (green, examples highlighted by arrows) and the pan-vascular marker CD31 (red) demonstrated lymphatic vessel enlargement and enhanced numbers of blood vessels in the skin surrounding VEGFA-containing Matrigels (B), compared with Matrigels containing PBS (A). Treatment with naphthyridine resulted in a reduction of blood vessel numbers and lymphatic vessel enlargement (C). (D-G) Quantitative image analyses confirmed that the density of lymphatic vessels was unchanged by VEGFA alone or by combined VEGFA and naphthyridine treatment (D). In contrast, the tissue area covered by lymphatic vessels surrounding VEGFA-containing Matrigels was significantly reduced by treatment with naphthyridine (E). Treatment with naphthyridine also reduced the number of VEGFA-induced blood vessels (F) and the tissue area covered by blood vessels (G). Scale bars represent 100 μm.
The adenosine A1 receptor antagonist naphthyridine inhibits VEGFA-induced angiogenesis and lymphatic vessel enlargement in mice. VEGFA-containing Matrigel plugs were implanted subcutaneously into adult mice, and the mice were subsequently treated systemically with naphthyridine or with vehicle control for 6 days. (A-C) Differential immunofluorescence analysis for the lymphatic-specific marker LYVE1 (green, examples highlighted by arrows) and the pan-vascular marker CD31 (red) demonstrated lymphatic vessel enlargement and enhanced numbers of blood vessels in the skin surrounding VEGFA-containing Matrigels (B), compared with Matrigels containing PBS (A). Treatment with naphthyridine resulted in a reduction of blood vessel numbers and lymphatic vessel enlargement (C). (D-G) Quantitative image analyses confirmed that the density of lymphatic vessels was unchanged by VEGFA alone or by combined VEGFA and naphthyridine treatment (D). In contrast, the tissue area covered by lymphatic vessels surrounding VEGFA-containing Matrigels was significantly reduced by treatment with naphthyridine (E). Treatment with naphthyridine also reduced the number of VEGFA-induced blood vessels (F) and the tissue area covered by blood vessels (G). Scale bars represent 100 μm.
Discussion
In the present study, we have used a large-scale forward chemical genetics approach to identify novel bioactive compounds and to define several novel pathways acting during blood vascular development and lymphangiogenesis in Xenopus tadpoles. The use of Xenopus embryos for chemical library screening and drug discovery was previously proposed,18 and subsequently a small-scale pilot study provided further evidence for the feasibility.33 To our knowledge, this is the first large-scale chemical library screen for compounds modulating lymphatic vessel development in vivo.
Forward chemical genetics uses the screening of annotated libraries of small organic compounds with experimentally verified biologic mechanisms and activities to study biologic systems.34 This approach circumvents the well-known problems of target identification and lack of mechanistic understanding associated with active compounds recovered from screens using conventional chemical libraries.35 Forward chemical genetics has therefore become increasingly used in cell cultures to identify signaling pathways involved in cellular functions in vitro26,35,36 ; and more recently, whole organisms, such as Drosophila, Caenorhabditis elegans, and zebrafish, have been used for compound discovery.30,37,38 Importantly, chemical genetics using whole animals offers a complementary approach to loss-of-function mutations or knockdowns with siRNA or morpholino oligonucleotides in the analysis of complex biologic processes, such as organogenesis.
We now report here the establishment of a large-scale 2-step forward chemical genetics strategy in Xenopus embryos to efficiently recover compounds affecting vascular development in vivo. High-throughput, large-scale screening approaches require a functional phenotypic read-out that is easily detected and reliably predictive for vascular system defects. Edema is the major downstream phenotype caused by impairment of lymphatic vessel development25 but is also associated with disrupted development or function of the cardiovascular and excretory systems in tadpoles.39 We therefore decided to screen the LOPAC1280 chemical library by visual inspection for compounds that induce edema. There were several different types/locations of edema induced by different compounds. Remarkably, compounds targeting the same pathway often revealed similar phenotypes. For example, Ca2+-channel antagonists induced pericardial and ventral edemas, whereas retinoids caused cerebral and pronephric edemas (supplemental methods; supplemental Figure 1). Although most gene defects affecting lymphatic vessel development also cause edema formation, we cannot exclude the possibility that some compounds might have affected lymphatic (or blood) vessel development without causing edema formation. We therefore also scored for compounds causing late-stage lethality in response to compound treatment. A total of 66 compounds satisfying our screening criteria were recovered with a hit rate of 5%: 48 edema-inducing compounds and 18 compounds causing lethality. From a practical point of view, we were able to exclude 95% of the compounds as inactive on the basis of our simple, noninvasive phenotypic screening criteria.
A possible drawback of chemical screening in whole organisms, such as Xenopus or zebrafish, is that the uptake of compounds across the epidermis can vary; thereby, compounds may be scored as false negatives. The logarithm of the partition ratio between octanol and water (logP) values correlates well with a compound's membrane permeability. Typically, compounds with logP values greater than +1 are well absorbed.40,41 This general rule was recently confirmed by a mass spectroscopy study of compound uptake in zebrafish larvae.42 The penetration problem of hydrophilic compounds with logP values less than +1 can be largely eliminated by microinjection of the compound into the cytoplasm of fertilized eggs or the blood circulation of larvae.43 However, this approach is impractical in the context of large-scale chemical library screening. The drug penetration problem associated with waterborne drug administration can also be seen as an advantage of the screening approach as it allows for the selection of bioactive compounds with favorable permeability or uptake properties in whole organisms.
Edema formation and larval lethality may not only be caused by cardiovascular defects but could also be a consequence of renal dysfunction impairing fluid homeostasis. In the second screening step, compound-treated embryos were therefore subjected to semiautomated whole-mount in situ hybridizations using specific blood vascular and lymphatic marker genes. The analysis of vascular marker gene expression revealed that a total of 32 hits (24 of the 48 edema-inducing compounds and 8 of the 18 compounds associated with larval lethality) caused abnormal vascular development and morphogenesis in tadpoles. Collectively, the chemical screening strategy resulted in the recovery of compounds with an impressive 49.2% hit rate (32 of the 65 hits from the primary screen).
The LOPAC1280 library harbors 8 compounds (difluoromethylornithine, indirubin-3′-monoxime, 2-methoxyestradiol, minocycline hydrochloride, SU 4312, SU 5416, thalidomide, and tyrphostin AG1478) with known antiangiogenic activities.30,44 Three antiangiogenic compounds (difluoromethylornithine, minocycline hydrochloride, and thalidomide) are not considered because they are highly hydrophilic and therefore poorly penetrate embryos.30 A recent screen of the LOPAC1280 chemical library using transgenic zebrafish expressing a fluorescent vascular reporter gene resulted in the recovery of 3 of the 5 antiangiogenic compounds (indirubin-3′-monoxime, SU 4312, and tyrphostin AG1478).30 In Xenopus, all 5 compounds (indirubin-3′-monoxime, 2-methoxyestradiol, SU 4312, SU 5416, and tyrphostin AG1478) were scored as active. Importantly, the 2 VEGFR inhibitors SU4312 and SU5416 were identified as positive hits validating the screening procedure. Taken together, this analysis demonstrates that the present chemical library screening approach is capable of efficiently identifying known antiangiogenic compounds in Xenopus embryos.
We also identified 27 new compounds affecting vascular and/or lymphatic development in Xenopus embryos. A number of these compounds, such as nocodazole, podophyllotoxin, forskolin, and retinoic acid, are known to have broad effects on many cell types. Their antiangiogenic activities observed here are probably the result of pleiotropic effects. The predicted biologic functions of other recovered compounds implicate the requirement of various mechanisms, including hormone and cyclic nucleotide signaling, phosphorylation as well as K+ and Ca2+ channels for the normal development of blood vessels and/or lymphatics. Hits exhibiting only blood vascular system defects included compounds causing defective vasculogenesis, impaired angiogenesis, or VVN hypoplasia. Most interestingly, the Ca2+ATPase inhibitor calmidazolium chloride and Raf1 kinase inhibitor GW5074, 2 compounds not previously known to exhibit activities on the vascular system, were found to act pro-angiogenic by promoting ectopic, premature angiogenesis in Xenopus embryos. In addition, effects on the VVN were noticed. GW5074 was also tested in endothelial cell culture assays, where it promoted endothelial cell proliferation. On the basis of in vitro and in vivo evidence, these compounds imply Ca2+-ATPase and Raf kinase in the regulation of endothelial cell function. Furthermore, these small-molecule compounds may have the ability to substitute for mitogenic growth factors used to stimulate endothelial cell proliferation.
Several compounds inhibiting preferentially lymphatic vascular development were identified, including several tyrosine kinase inhibitors (SU 4312, SU 6656, and GW2974), and L-type calcium channel blockers (felodipine and nicardipine). Treatment with the VEGF- and platelet-derived growth factor-receptor antagonist SU 4312 had the most potent effect on lymphatic vessel formation in vivo as demonstrated by the absence of VEGFR3-positive structures of the lymphatic system in Xenopus embryos. Interestingly, the blood vasculature remained largely unaffected. These results suggest that, at the concentration of 20 μM used in the Xenopus assay, SU 4312 targets in vivo primarily the VEGFC/VEGFR3 signaling pathway, which regulates embryonic lymphangiogenesis in tadpoles and mouse embryos.13,25 At higher concentrations (> 30 μM), SU 4312 will also exhibit moderate antiangiogenic effects as was recently demonstrated in zebrafish.30 Collectively, these findings suggest that SU 4312 may act in vivo primarily on lymphatic vessels and therefore warrants further investigation. This also applies to the dual ErbB2 and EGF receptor tyrosine kinase inhibitor GW2974 and Src family kinase inhibitor SU 6656, which both exhibited antilymphatic activities in vitro and/or in vivo.
The role of calcium channels in lymphatic vessel formation and function has not been studied to date. The calcium channel blockers felodipine and nicardipine have vasodilatory effects and are therefore widely used to treat hypertension. However, one bothersome side effect of these older dihydropyridines is edema formation, especially in the feet, legs, and ankles,45 which could indicate dysfunction of the lymphatic vasculature. Our findings provided exciting novel clues awaiting now in-depth analysis in follow-up studies. Taken together, the Xenopus embryo-based chemical library screens offer a promising avenue for the identification of novel lead compounds to modulate normal or pathologic (lymph)angiogenesis in patients.
Compounds with activities on vascular or lymphatic development in Xenopus were also tested for effects on proliferation and/or tube formation of cultured human LECs and HUVECs. 7-Cyclo and naphthyridine, 2 compounds disrupting both blood and lymph vessel formation in vivo, also exhibited inhibitory activities for both HUVECs and LECs in vitro. Furthermore, the inhibition of HUVEC tube formation in vitro correlates well with the in vivo observation of defective ISV angiogenesis. Despite these compelling examples, many compounds with in vivo bioactivity failed to show comparable effects in cell culture models. For example, 4 of 5 compounds disrupting lymphangiogenesis in vivo were inactive in LEC tube assays. This indicates that in vitro assays fail to adequately reproduce all steps of vascular development. In contrast to traditional cell-based screens, high-throughput chemical screening in Xenopus tadpoles occurs in a physiologically more relevant context.
Naphthyridine, an adenosine A1 receptor antagonist, inhibited blood and lymphatic vessel formation in tadpoles, which could also be recapitulated using in vitro endothelial cell proliferation and tube formation assays. In a proof-of-principle study, we tested naphthyridine in an established mouse model of VEGFA-induced dermal neovascularization. Systemic treatment of mice with naphthyridine potently inhibited lymphatic vessel enlargement and angiogenesis, indicating that adenosine A1 receptors do not only act antagonistically on amphibian vascular development but also disrupt adult mammalian (lymph)angiogenesis. It will be of future interest to investigate whether inhibition of adenosine A1 receptors might also act under pathologic conditions inhibiting, for example, tumor-induced lymphangiogenesis and lymphatic metastasis. More generally, the conservation of the in vivo activities between amphibians and mammals validates Xenopus embryos as relevant screening tools in drug discovery for human diseases.
In conclusion, our work demonstrates that the Xenopus embryo model in combination with our 2-step screening methodology represents a promising, low-cost platform to identify novel pathways involved in angiogenesis and lymphangiogenesis, and to accelerate the discovery of novel drug-like small organic molecules. Despite the obvious differences in constitution and physiology between amphibians and humans, the Xenopus tadpole model represents a much needed tool to bridge the gap in drug discovery between traditional in vitro and preclinical animal models.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vasili Dabouras, Christophe Héligon, and Martin Kretz for performing initial pilot experiments with Xenopus embryos, Cornelius Fischer for advice with the Tecan liquid handling robot, and Jon Hall for critical reading of the manuscript.
This work was supported by a Roche Research Foundation fellowship (N.E.B.-T.); the National Institutes of Health (grant CA69184), the Swiss National Science Foundation (grant 3100A0-108207), the Austrian Science Foundation (grant S9408-B11), the Cancer League Zurich, and the European Community (LSHC-CT-2005-518178; M.D.); and the Swiss National Science Foundation (3100A0-114102) and the European Community (EuReGene LSHG-CT-2004-005085; A.W.B.).
National Institutes of Health
Authorship
Contribution: A.W.B. and M.D. conceived the project; R.E.K. and A.W.B. designed and performed the Xenopus experiments; N.E.B.-T. and M.D. designed the experiments with mammalian cells and the Matrigel plug implantation assays; N.E.B.-T. performed the in vitro assays with mammalian cells and the mouse experiments; and R.E.K., N.E.B.-T., M.D., and A.W.B. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: André W. Brändli, Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zürich, Wolfgang-Pauli-Strasse 10, CH-8093 Zürich, Switzerland; e-mail: brandli@pharma.ethz.ch.
References
Author notes
*R.E.K. and N.E.B.-T. contributed equally to this study.

![Figure 3. Compounds affecting blood and lymph vessel development in Xenopus tadpoles. Control DMSO- (A-B) and compound-treated (C-J) Xenopus embryos were analyzed by whole-mount in situ hybridization for expression of the blood vascular marker gene apj at stage 35/36 and the lymphatic marker gene vegfr3 at stage 42. Panels of the embryonic blood vasculature (apj) are accompanied by close-up views illustrating ISV angiogenesis and VVN development in the embryonic trunk. The panels visualizing the developing lymphatic system (vegfr3) include close-ups of the head and midtrunk region (middle panels) for the ALSs and the ALHs, and enlargements (left panels) of the tail for PLVs. (A) Normal ISVs (arrowhead) and VVN (*). (B) Normal ALSs (arrow), ALHs (*), and PLVs (arrowheads). (C) Stunted ISVs (arrowhead), normal VVN (*). (D) Hypoplastic PLV (arrowheads), impaired ALS (arrow), and ALH (*) lymphatics. (E) Stunted ISVs (arrowhead), hypoplastic VVN (*). (F) Stunted ALS lymphatics (arrow), dysplastic ALH lymphatics (*), hypoplastic PLVs (arrowheads). (G) Normal ISVs (arrowhead), hyperplastic VVN. (H) Hypoplastic PLVs (arrowheads), impaired ALS (arrow), and ALH (*) lymphatics. (I) Stunted ISVs (arrowhead), hyperplastic VVN. (J) Complete lack of ALS (arrow), ALH (*) and tail lymphatics. Note that vegfr3 expression persists in the VVN (arrowhead). (K) Scheme of the blood vasculature (blue) of the stage 35/36 embryo. (L) Scheme of the lymphatic vasculature (orange) of the stage 42 embryo. 7-Cyclo indicates 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; Naphthalimide, 4-amino-1,8-naphthalimide; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; PS; pronephric sinus; DPLV, dorsal posterior lymph vessel; and VPLV, ventral posterior lymph vessel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/5/10.1182_blood-2009-03-211771/5/m_zh89990939660003.jpeg?Expires=1765922202&Signature=t~SfjP6IhA0NAAaOq7rhz9cM2aqTSmHVPvyshl~c-bokvI3bcglpBNEpoc7YCX2Baz7lU1dkDd9kXKM~EdSOqRGhN9dKh0ZJ9Fzw4a9L3hddPtJEEcn92yBWbnrqiq1VmaX5XweyVhEjYHjhladfIaUpkt8PL4MNAAkJh2Xqll1kwr3CtcGJyztUzFjpUKqm88ZDENbU9MNSOEJIYvCahTMK4BO933NvcsOUJCIHM8PUHKBHFQ0UuMu0DiAdVh3cS2kHrixjhprUqbXn4Onma0YrPyYf8mVtUIWE9C8xWfUtHD2NZpVLvtB3dnGioMnzOIdh-FjLVh6xHFpzebNvjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Selective and cell-type specific in vitro responses of endothelial cells to treatment with small-molecule compounds. Twenty-four compounds were tested in vitro on human lymphatic (LECs; black bars) and blood vascular (HUVECs; open bars) endothelial cell cultures for effects on cell proliferation and tube formation. (A) Results of the compound screens using cell proliferation assays. Compounds were screened at a dose of 10 μM in 0.1% DMSO. Control cultures were treated with 0.1% DMSO. (B) Results of the compound screens using tube formation assays. Compounds were screened at a dose of 1 μM in 0.1% DMSO. Control cultures were treated with 0.1% DMSO. 7-Cyclo indicates 7-cyclopentyl-5-(4-phenoxy)phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; L-687,384, L-687,384 hydrochloride; Naphthalimide, 4-amino-1,8-naphthalimide; Naphthyridine, 7-chloro-4-hydroxy-2-phenyl-1,8-naphthyridine; Nicardipine, nicardipine hydrochloride; Phenylxanthine, 1,3-diethyl-8-phenylxanthine. Bars represent mean values and SD of 3 independently performed assays.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/5/10.1182_blood-2009-03-211771/5/m_zh89990939660005.jpeg?Expires=1765922202&Signature=m9Klgsq0Bfn2KR9jQ2u-dcNf9JS8Cr1cBtulPR8ujTbbXn88i9Oflp4FAxyF78KH6FBKWVAx-XQweP6erk3tD~L7zTPj~ojLPhEDWzfGIbXspnC0vhEPrxW0RFIzQnIDFSQENQbqSNDkV3Dg60pIrZjsjEVeDPawN2o9jvxZ8qnfFrEwbiqsKlTRBVjJqYyn8xCTndULsJDVaRpFFiw95EuOqrWkgBoafDkUN9lqYAtjd7u8aa6xGQLnl3wq~zEpXNlDCMoeO~LmKW9r5i9t9LUiv28Iim4be9o-ftbkPgIRg6YNe48uXKQMO9ssxz7e0p3cjqauRfcjJKKKjvvLwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal