A hematopoietic cell transplantation regimen was adapted from a preclinical model that used reduced-intensity conditioning (RIC) and protected against graft-versus-host disease (GVHD) by skewing residual host T-cell subsets to favor regulatory natural killer T cells. One hundred eleven patients with lymphoid (64) and myeloid (47) malignancies received RIC using total lymphoid irradiation (TLI) and antithymocyte globulin (ATG) followed by the infusion of granulocyte colony-stimulating factor-mobilized grafts. Included were 34 patients at least 60 years of age, 32 patients at high risk of lymphoma relapse after disease recurrence following prior autologous transplantation, and 51 patients at high risk of developing GVHD due to lack of a fully human leukocyte antigen (HLA)–matched related donor. Durable chimerism was achieved in 97% of patients. Cumulative probabilities of acute GVHD (grades II-IV) were 2 and 10% of patients receiving related and unrelated donor grafts. Nonrelapse mortality (NRM) at 1 year was less than 4%. Cumulative incidence of chronic GVHD was 27%. The 36-month probability of overall and event-free survival was 60% and 40%, respectively. Disease status at start of conditioning and the level of chimerism achieved after transplantation significantly impacted clinical outcome. The high incidence of sustained remission among patients with active disease at time of transplantation suggests retained graft-versus-tumor reactions. Active trial registration currently at clinicaltrials.gov under IDs of NCT00185640 and NCT00186615.
Introduction
Reduced-intensity conditioning (RIC) for allogeneic hematopoietic cell transplantation (HCT) has allowed for the treatment of patients who are otherwise ineligible for conventional high-dose preparatory approaches due to older age and/or comorbid medical conditions.1 Yet, despite RIC, clinical outcomes have remained limited by the risks of acute graft-versus-host disease (GVHD) and nonrelapse mortality (NRM). Following RIC, the incidence of acute GVHD (grades II-IV) ranged from 20% to 65%,1,,,,,,,,,,,,–14 accounting for approximately half of NRM.1,3,,–6,8,15 Prior efforts to reduce acute GVHD focused on the depletion of donor, mature T cells, as this subset is responsible for inducing acute GVHD. Depletion of donor T cells by ex vivo manipulation of the graft or by administration of anti-T-cell antibodies shortly before transplantation with myeloablative conditioning reduced acute GVHD but was associated with increased incidences of tumor relapse, opportunistic infections, and graft failure.16,17 Overall, patient survival was not improved.16,17
In an effort to reduce acute GVHD without compromising efficacy of HCT, we developed a novel reduced-intensity, preparatory regimen based upon a murine model of bone marrow transplantation using fractionated total lymphoid irradiation (TLI) and antithymocyte serum (ATS). In this model, TLI and ATS altered the host immune profile to favor regulatory natural killer T (NKT) cells that suppress GVHD by polarizing donor conventional T cells toward secretion of noninflammatory cytokines such as interleukin-4 (IL-4), and by promoting expansion of donor CD4+CD25+FoxP3+ T regulatory cells.18,,–21 This skewing was a result of NKT-cell resistance to radiation-induced apoptosis due to increased expression of antiapoptotic genes.18,21,22 The favorable ratio of host NKT cells did not affect donor CD8+ T-cell cytolytic function and graft antitumor activity was preserved.18,20
We translated TLI and ATS to a human protocol and in a series of 37 patients with lymphoid malignancies and acute leukemia, receiving human leukocyte antigen (HLA)–matched allogeneic HCT following TLI and antithymocyte globulin (ATG) conditioning, acute GVHD developed in only 2 patients, or 5%.23 The limited sample size prevented accurate determination of acute GVHD and NRM rates, and due to short duration of follow-up, we were unable to report if this regimen compromised clinical outcomes of chronic GVHD, event-free survival (EFS), and overall survival (OS).
We now report the outcomes of 111 patients treated with TLI and ATG followed by allogeneic HCT with follow-up sufficient to determine risk of acute and chronic GVHD, relapse, EFS, and OS. TLI and ATG conditioning was associated with low rates of acute and chronic GVHD, NRM, invasive viral or fungal infections, and hospital admissions following HCT. The high incidence of sustained complete remission (CR) among patients with active disease at the time of transplantation suggests the presence of retained graft-versus-tumor reactions.
Methods
Patients
Between December 28, 2001, and September 6, 2007, 111 consecutive patients provided informed consent in accordance with the Declaration of Helsinki and were enrolled in a transplant protocol approved by the Stanford University Institutional Review Board. Censoring date was the last clinic visit before October 1, 2008, allowing for a minimum follow-up of 12 months. Patients enrolled had a diagnosis of malignant lymphoid or myeloid disease. All patients were ineligible for conventional full-dose conditioning due to age greater than 55 years, younger age yet with significant medical comorbidities, or prior therapies such as autologous HCT. Patients were not excluded based on disease status at time of enrollment, response to chemotherapy, or history of prior bacterial, fungal, or viral infection (other than human immunodeficiency virus). Patients with a corrected pulmonary-diffusion capacity less than 35%, cardiac ejection fraction less than 30%, Karnosfky performance status less than 50%, decompensated liver disease, or who were pregnant, were excluded. Disease status at the start of conditioning was assigned according to consensus guidelines.24,25
Transplant regimen
TLI and ATG conditioning was performed as previously reported.23 In brief, ATG (thymoglobulin; Genzyme, Boston, MA) was infused intravenously at 1.5 mg/kg per day starting 11 days before transplantation for 5 consecutive days. TLI was administered from a 15-MeV linear accelerator with photon beam at a dose of 0.8 Gy per day starting on day −11 daily through day −7, inclusive, and from day −4 through day −1, inclusive, with 2 fractions delivered on day −1 for a total dose of 8 Gy. Fields used for radiation were similar to those previously described.26 All patients were infused with unmanipulated granulocyte colony-stimulating factor (G-CSF; Amgen) “mobilized” blood mononuclear cells on day 0. Prophylactic immunosuppressive therapy included oral cyclosporine and mycophenolate mofetil.1 Cyclosporine was tapered to discontinuation between days 56 and 180 and mycophenolate mofetil was stopped on day 28 for recipients of related donor grafts. For recipients of unrelated donor grafts, cyclosporine was tapered to discontinuation between days 100 and 180, and mycophenylate mofetil was tapered between days 42 and 96. Bacterial, viral, and fungal prophylaxis was administered to all patients following generally accepted standards. Patients were monitored weekly for cytomegalovirus (CMV) and Epstein-Barr virus infections with serum polymerase chain reaction assays.1,5 Any CMV copy number of 600/mL was treated with valgancyclovir induction followed by maintenance.
Molecular typing and graft content
Patients with related and unrelated donors were matched for HLA-A, -B, -Cw, -DRB1, and -DQB1 by high-resolution DNA typing. CD34, CD3, CD4, and CD8 cell content of harvested cells in the graft were determined.23
Donor and host cells
Host T-cell populations were monitored in 14 randomly selected patients before TLI and ATG, and immediately after conditioning yet before donor cell infusion for changes in the absolute number of host CD3+, CD4+, CD8+, and invariant NKT cells (CD3+CD161hiVα24+Vβ11+) by flow cytometric analysis (Becton Dickinson). Donor cell chimerism was evaluated in all patients by DNA genotyping of simple sequence-length polymorphic markers that encode short tandem repeats.27 Beginning 28 days after transplantation and at designated time points thereafter, chimerism was evaluated on whole blood and on cell subsets using immunomagnetic beads (Dynal Biotech/Invitrogen) coated with monoclonal antibodies against CD3, CD15, CD19, and CD56. Complete chimerism was defined as the attainment of at least 95% donor-type peripheral blood CD3+ cells, and primary graft failure was defined as the failure to exceed 5% donor CD3+ cells at any time after HCT. Mixed chimerism is inclusive of all chimerism status not defined by complete chimerism or primary graft failure (ie, 5%-95% donor type engraftment).28
Clinical outcome assessment
Diagnosis and grading of acute29 and chronic30 GVHD were done according to established criteria. Immunosuppressive therapy for acute (grades II-IV) and chronic GVHD (extensive) typically consisted of starting prednisone or solumedrol 1 to 2 mg/kg per day. Hospital admission rate and length of stay during the first 100 days after transplantation were recorded. All patients were assessed for NRM. Patients in CR at the time of HCT who subsequently developed relapsed disease were designated as relapse, while patients with active disease (partial remission [PR], stable disease [SD], or progressive disease [PD]) at time of transplantation who subsequently developed progression of disease without first obtaining remission were designated as PD. Therapy for relapse or PD was based on the discretion of the attending physician with therapy and response to treatment recorded for all patients. Donor lymphocyte infusions (DLI) were administered only as treatment for relapse or PD.
Statistical analysis
We performed descriptive statistical analyses constructing actuarial EFS and OS with 95% confidence intervals (CIs) estimated according to the Kaplan-Meier method with stratification by disease category, disease status at transplantation, patient age, and degree of donor chimerism achieved after transplantation. EFS was defined as first observation of relapse, disease progression, or death. The cumulative incidence and probabilities of acute (grades II-IV) and chronic (extensive) GVHD, NRM, and chimerism were estimated according to previously described methods.31 Cumulative incidence of acute and chronic GVHD was calculated with competing risks of relapse, death, and primary graft failure. Cumulative incidence of NRM was calculated with relapse as a competing risk. We had 2 hypotheses: first, among patients with non-Hodgkin lymphoma (NHL) disease status of CR or PR at the start of conditioning would predict better OS and EFS compared with patients with SD or PD, and among patients with de novo acute myeloid leukemia (AML) CR1 or CR2 at the start of HCT would predict for better outcomes compared with patients beyond CR2. Second, NHL patients in CR or PR and de novo AML patients in CR1 or CR2 at the time of transplantation, who achieve complete chimerism would have better outcomes compared with patients achieving mixed chimerism. The corresponding null hypotheses were tested with the Cox proportional-hazard ratios. Fold decrease in CD3, CD4, CD8, and invariant NKT-cell populations pre- and postconditioning with TLI and ATG were compared by Wilcoxon signed rank test. Statistical analysis was performed using open-source software R (www.r-project.org).
Results
Patient characteristics
Table 1 summarizes the clinical features of 111 patients enrolled, 64 with lymphoid and 47 with myeloid malignancies. Notable features included: 34 patients 60 years of age or older, 32 patients at high risk of lymphoma relapse after failing a prior autologous HCT, 85 patients with advanced-stage disease, and 51 patients at high risk of developing GVHD due to lack of availability of an HLA-matched related donor. At the time of last evaluation, the median follow-up for all 111 patients was 665 days and was 882 days among the 67 patients alive, of which 40.3% were beyond 3 years following HCT.
Patient and disease characteristics
| Characteristics . | No. of patients = 111 . |
|---|---|
| Median age, y (range) | 54 (21-67) |
| Follow-up | |
| Median follow-up days for all 111 patients (range) | 665 (32-2408) |
| Median follow-up days for 67 patients alive at last follow-up (range) | 882 (379-2408) |
| Diagnosis, no. (%) | |
| Lymphoid | 64 (57.7) |
| Non-Hodgkin lymphoma | 57 (89.1%) |
| De novo diffuse large B-cell NHL | 14 (21.9) |
| Diffuse large B-cell NHL with evidence of transformation from follicular NHL | 9 (14.1) |
| Mantle-cell lymphoma | 13 (20.3) |
| Follicular small-cleaved NHL | 10 (15.6) |
| Small lymphocytic leukemia/chronic lymphocytic leukemia or prolymphocytic leukemia | 7 (10.9) |
| Angioimmunoblastic lymphoma | 2 (3.1) |
| Peripheral T-cell lymphoma | 1 (1.6) |
| NKT-cell lymphoma | 1 (1.6) |
| Hodgkin lymphoma | 5 (7.8) |
| Pre-B acute lymphoblastic leukemia | 2 (3.1) |
| Advanced-stage disease* | 55 (85.9) |
| Prior autologous transplantation | 32 (50.0) |
| Myeloid | 47 (42.3) |
| De novo AML | 38 (80.9) |
| Favorable cytogenetic risk | |
| t(8:21), inv(16), or t(16:16) | 2 (5.3) |
| t(15:17) APL | 2 (5.3) |
| Intermediate cytogenetic risk | |
| Normal cytogenetics | 16 (42.1) |
| +8 | 1 (2.6) |
| Cytogenetics unknown | 11 (28.9) |
| Unfavorable cytogenetic risk | |
| del5q, t(6:9) −7, or complex | 6 (15.8) |
| AML evolved from MDS | 3 (6.4) |
| Myelodysplastic syndrome–RAEB | 2 (4.3) |
| Treatment-related AML | 1 (2.1) |
| Accelerated-phase CML | 3 (6.4) |
| Advanced-stage disease† | 30 (63.8) |
| Donor, no. (%) | |
| Related | 61 (54.9) |
| Fully matched | 60 (98.4) |
| 1-allele mismatched related donor | 1 (1.6) |
| Unrelated | 50 (45.0) |
| Fully matched | 45 (90.0) |
| 1-allele mismatched unrelated donor | 5 (10.0) |
| Sex mismatch, no. (%) | |
| M→M | 35 (31.5) |
| M→F | 24 (21.6) |
| F→M | 30 (27.0) |
| F→F | 22 (19.8) |
| Cytomegalovirus serologic status, no. (%) | |
| Donor, recipient, or both seropositive | 74 (66.7) |
| Donor and recipient seronegative | 24 (21.6) |
| Disease status at start of TLI and ATG, no. (%) | |
| Lymphoid | |
| Complete remission | 17 (26.6) |
| Partial remission | 38 (59.4) |
| Stable disease or progressive disease | 9 (14.1) |
| Myeloid | |
| 1st complete remission | 26 (55.3) |
| 2nd complete remission | 10 (21.3) |
| ≥ CR2, PR, SD, or PD | 11 (23.4) |
| Characteristics . | No. of patients = 111 . |
|---|---|
| Median age, y (range) | 54 (21-67) |
| Follow-up | |
| Median follow-up days for all 111 patients (range) | 665 (32-2408) |
| Median follow-up days for 67 patients alive at last follow-up (range) | 882 (379-2408) |
| Diagnosis, no. (%) | |
| Lymphoid | 64 (57.7) |
| Non-Hodgkin lymphoma | 57 (89.1%) |
| De novo diffuse large B-cell NHL | 14 (21.9) |
| Diffuse large B-cell NHL with evidence of transformation from follicular NHL | 9 (14.1) |
| Mantle-cell lymphoma | 13 (20.3) |
| Follicular small-cleaved NHL | 10 (15.6) |
| Small lymphocytic leukemia/chronic lymphocytic leukemia or prolymphocytic leukemia | 7 (10.9) |
| Angioimmunoblastic lymphoma | 2 (3.1) |
| Peripheral T-cell lymphoma | 1 (1.6) |
| NKT-cell lymphoma | 1 (1.6) |
| Hodgkin lymphoma | 5 (7.8) |
| Pre-B acute lymphoblastic leukemia | 2 (3.1) |
| Advanced-stage disease* | 55 (85.9) |
| Prior autologous transplantation | 32 (50.0) |
| Myeloid | 47 (42.3) |
| De novo AML | 38 (80.9) |
| Favorable cytogenetic risk | |
| t(8:21), inv(16), or t(16:16) | 2 (5.3) |
| t(15:17) APL | 2 (5.3) |
| Intermediate cytogenetic risk | |
| Normal cytogenetics | 16 (42.1) |
| +8 | 1 (2.6) |
| Cytogenetics unknown | 11 (28.9) |
| Unfavorable cytogenetic risk | |
| del5q, t(6:9) −7, or complex | 6 (15.8) |
| AML evolved from MDS | 3 (6.4) |
| Myelodysplastic syndrome–RAEB | 2 (4.3) |
| Treatment-related AML | 1 (2.1) |
| Accelerated-phase CML | 3 (6.4) |
| Advanced-stage disease† | 30 (63.8) |
| Donor, no. (%) | |
| Related | 61 (54.9) |
| Fully matched | 60 (98.4) |
| 1-allele mismatched related donor | 1 (1.6) |
| Unrelated | 50 (45.0) |
| Fully matched | 45 (90.0) |
| 1-allele mismatched unrelated donor | 5 (10.0) |
| Sex mismatch, no. (%) | |
| M→M | 35 (31.5) |
| M→F | 24 (21.6) |
| F→M | 30 (27.0) |
| F→F | 22 (19.8) |
| Cytomegalovirus serologic status, no. (%) | |
| Donor, recipient, or both seropositive | 74 (66.7) |
| Donor and recipient seronegative | 24 (21.6) |
| Disease status at start of TLI and ATG, no. (%) | |
| Lymphoid | |
| Complete remission | 17 (26.6) |
| Partial remission | 38 (59.4) |
| Stable disease or progressive disease | 9 (14.1) |
| Myeloid | |
| 1st complete remission | 26 (55.3) |
| 2nd complete remission | 10 (21.3) |
| ≥ CR2, PR, SD, or PD | 11 (23.4) |
AML indicates acute myeloid leukemia; APL, acute promyelocytic leukemia; MDS, myelodysplastic syndrome; RAEB, refractory anemia with excess blasts; NHL, non-Hodgkin lymphoma; NOS, not otherwise specified; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; and MMF, mycophenolate mofetil.
Advanced-stage lymphoid malignant disease was defined as disease status beyond second complete remission or not in remission at the start of transplant conditioning.
Advanced-stage myeloid malignant disease was defined as a high-risk cytogenetic abnormality and age beyond 55 years at diagnosis, myelodysplastic syndrome with progression to acute myeloid leukemia AML, treatment-related AML, or disease.
Transplantation conditioning and donor graft
All patients were hospitalized for 5 days (days −11 through −7) for the administration of ATG. Total lymphoid irradiation was delivered as 10 fractions at 0.8 Gy per dose (day −11 through day −1) and was completed in all 111 patients as an outpatient. Following completion of TLI, all patients received an intravenous infusion of G-CSF–mobilized blood mononuclear cells from related (n = 61) and unrelated donors (n = 50). Of the 61 related donor transplants, 1 had a single-allele mismatch, and of the 50 unrelated donor transplants, 5 had a single-allele mismatch. The mean absolute number of CD34+ and CD3+ cells infused did not differ significantly between related and unrelated donor grafts (Table 2; P = .24 and P = .54, respectively).
Graft cell dose and chimerism
| Characteristics . | N = 111 . | Related donor grafts, n = 61 . | Unrelated donor grafts, n = 50 . |
|---|---|---|---|
| Mean cell dose infused (range) | |||
| CD3 cells × 108/kg | 3.2 (0.02-10) | 2.97 (0.02-6.50) | 3.76 (1.30-10) |
| CD34 cells × 106/kg | 7.3 (1.1-20.5) | 6.65 (2.1-12.8) | 8.02 (1.1-20.5) |
| Chimerism, no. (%) | |||
| Complete chimerism (≥ 95%) | 86 (77.5) | 46 (75.4) | 40 (80.0) |
| Mixed chimerism (5%-94%) | 21 (18.9) | 14 (23.0) | 7 (14.0) |
| Primary graft failure (< 5%) | 4 (3.6) | 1 (1.6) | 3 (6.0) |
| Characteristics . | N = 111 . | Related donor grafts, n = 61 . | Unrelated donor grafts, n = 50 . |
|---|---|---|---|
| Mean cell dose infused (range) | |||
| CD3 cells × 108/kg | 3.2 (0.02-10) | 2.97 (0.02-6.50) | 3.76 (1.30-10) |
| CD34 cells × 106/kg | 7.3 (1.1-20.5) | 6.65 (2.1-12.8) | 8.02 (1.1-20.5) |
| Chimerism, no. (%) | |||
| Complete chimerism (≥ 95%) | 86 (77.5) | 46 (75.4) | 40 (80.0) |
| Mixed chimerism (5%-94%) | 21 (18.9) | 14 (23.0) | 7 (14.0) |
| Primary graft failure (< 5%) | 4 (3.6) | 1 (1.6) | 3 (6.0) |
MRD indicates matched related donor; and MUD, matched unrelated donor.
Alterations in host T-cell subsets
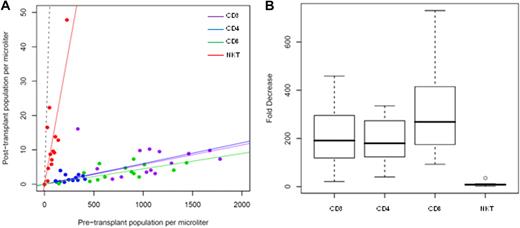
Murine models of bone marrow transplantation showed that after conditioning with TLI and ATS, regulatory invariant NKT cells became the predominant residual host T-cell subset and were responsible for protection against GVHD.18,19 In the preclinical model, analyses were performed on cells isolated from murine spleens, yet in humans, only blood was available for similar studies. In the current protocol, the effect of TLI and ATG on circulating host T-cell subsets was monitored by analysis of peripheral blood samples at 24-hours pre-TLI and immediately post-TLI, yet before infusion of donor cells, for changes in absolute numbers of T-cell subsets (Figure 1A). The median decrease in CD3+, CD4+, and CD8+ T cells was 191-fold, 180-fold, and 268-fold, respectively (Figure 1B). In contrast, the median-fold decrease (8.6-fold) in invariant NKT cells was significantly less than that observed for conventional T-cell subsets (P = .001). This resulted in a significant increase in the percentage of NKT cells in the blood among gated CD3+ (T-cell receptor [TCR] αβ+) cells; whereas NKT cells accounted for only 0.01% of all CD3+ cells before TLI and ATG administration, 0.5% of T cells expressed the invariant NKT-cell TCR Vα24Vβ11 after TLI. Notably, 2 of the 14 patients evaluated lacked measurable invariant NKT-cell populations pre-TLI or post-TLI and ATG.
Effect of TLI + ATG on circulating T-cell subsets. Absolute T-cell subset population size pre-TLI (x-axis) and immediately post-TLI (y-axis) with plotted linear regression (solid lines, constrained through 0,0) for CD3, CD4, CD8, and NKT-cell subsets. Dashed line representative of a population with no change (y = x) in population size between pre-TLI and immediately post-TLI (A). Boxplot of fold decrease in CD3, CD4, CD8, and NKT-cell populations (median, thick line; quartiles, box; range, whiskers; outliers, circle; B).
Effect of TLI + ATG on circulating T-cell subsets. Absolute T-cell subset population size pre-TLI (x-axis) and immediately post-TLI (y-axis) with plotted linear regression (solid lines, constrained through 0,0) for CD3, CD4, CD8, and NKT-cell subsets. Dashed line representative of a population with no change (y = x) in population size between pre-TLI and immediately post-TLI (A). Boxplot of fold decrease in CD3, CD4, CD8, and NKT-cell populations (median, thick line; quartiles, box; range, whiskers; outliers, circle; B).
Engraftment
Multilineage donor hematopoietic cell engraftment occurred within 28 days of HCT in 107 (96%) patients. Complete chimerism was achieved in 86 (78%) patients during study follow-up and occurred by 90 days after transplantation; 21 (19%) patients achieved stable mixed chimerism (Table 2). No additional interventions were administered to convert mixed to complete chimerism in the absence of relapse or PD. Achievement of mixed or complete chimerism did not differ significantly between recipients of related and unrelated grafts (P = .43). Neither disease type (myeloid vs lymphoid malignancies) nor disease status at time of HCT significantly correlated with the likelihood of achieving complete or mixed chimerism. Four cases of primary graft failure occurred, all of whom had evidence of marrow relapse or PD 10-47 days after HCT. Dose of CD34+ cells infused did not differ significantly between those achieving complete chimerism, mixed chimerism, or primary graft failure (P = .21). Three patients received less than 2.0 × 106 CD34+ cells/kg body weight, and all 3 achieved complete chimerism. Similarly, CD8+ and CD3+ cell dose did not differ by chimerism achieved (data not shown).
Tolerability of TLI and ATG
Following outpatient donor cell infusion, 30 (27%) patients were readmitted to the hospital during the first 100 days with a median length of stay of 3.5 days (Table 3). The majority of hospitalizations, 19 (63%), were for investigation of fevers, including febrile neutropenia in 8 patients. Invasive viral infections led to 2 hospitalizations (one case of pulmonary respiratory syncytial virus [RSV] and one case of CMV enteritis) during the first 100 days. Remarkably, only 1 of the 111 patients was hospitalized for acute GVHD.
Patient outcomes
| Outcome . | No. of patients = 111 . |
|---|---|
| Posttransplant admission within 100 days, n (%) | 30 (27.0) |
| Posttransplant admission diagnosis, n (%) | |
| Febrile, nonneutropenic | 11 (36.7) |
| Febrile neutropenia | 8 (26.7) |
| Cyclosporine toxicity | 5 (16.7) |
| Other* | 6 (20.0) |
| Posttransplant admission duration, median days (range) | 3.5 (1-21) |
| Cause of death, n (%) | 44 (39.6) |
| Disease progression | |
| Relapse | 25 (56.8) |
| cGVHD | 2 (4.5) |
| aGVHD | 1 (2.3) |
| Cyclosporine microangiopathy | 1 (2.3) |
| Infection (bacterial line infection) | 1 (2.3) |
| Cardiac disease | 1 (2.3) |
| Cerebrovascular accident | 1 (2.3) |
| Patient characteristics at last follow-up, n (%) | |
| Alive | 67 (60.4) |
| Duration of follow-up | |
| 1-3 y | 40 (59.7) |
| > 3 y | 27 (40.3) |
| Karnofsky performance status | |
| 100% | 46 (68.7) |
| 90% | 16 (23.9) |
| 80% | 4 (6.0) |
| 70% | 1 (1.5) |
| Immunosuppression, number of medications | |
| No immunosuppression | 46 (68.7) |
| 1 medication | 13 (19.4) |
| 2 medications | 5 (7.5) |
| 3 medications | 3 (4.5) |
| Alive, relapse or disease progression | 20 (29.9) |
| Disease-free interval, median days (range) | 670 (17-2239) |
| Current disease status | |
| CR | 13 (65.0) |
| PR | 6 (30.0) |
| PD | 2 (10.0) |
| Outcome . | No. of patients = 111 . |
|---|---|
| Posttransplant admission within 100 days, n (%) | 30 (27.0) |
| Posttransplant admission diagnosis, n (%) | |
| Febrile, nonneutropenic | 11 (36.7) |
| Febrile neutropenia | 8 (26.7) |
| Cyclosporine toxicity | 5 (16.7) |
| Other* | 6 (20.0) |
| Posttransplant admission duration, median days (range) | 3.5 (1-21) |
| Cause of death, n (%) | 44 (39.6) |
| Disease progression | |
| Relapse | 25 (56.8) |
| cGVHD | 2 (4.5) |
| aGVHD | 1 (2.3) |
| Cyclosporine microangiopathy | 1 (2.3) |
| Infection (bacterial line infection) | 1 (2.3) |
| Cardiac disease | 1 (2.3) |
| Cerebrovascular accident | 1 (2.3) |
| Patient characteristics at last follow-up, n (%) | |
| Alive | 67 (60.4) |
| Duration of follow-up | |
| 1-3 y | 40 (59.7) |
| > 3 y | 27 (40.3) |
| Karnofsky performance status | |
| 100% | 46 (68.7) |
| 90% | 16 (23.9) |
| 80% | 4 (6.0) |
| 70% | 1 (1.5) |
| Immunosuppression, number of medications | |
| No immunosuppression | 46 (68.7) |
| 1 medication | 13 (19.4) |
| 2 medications | 5 (7.5) |
| 3 medications | 3 (4.5) |
| Alive, relapse or disease progression | 20 (29.9) |
| Disease-free interval, median days (range) | 670 (17-2239) |
| Current disease status | |
| CR | 13 (65.0) |
| PR | 6 (30.0) |
| PD | 2 (10.0) |
CR indicates complete remission; PR, partial remission; and PD, progressive disease.
CMV enteritis, n = 1; arrhythmia, n = 1; chest pain, n = 1; acute GVHD, n = 1; GI bleed, n = 1; RSV infection, n = 1.
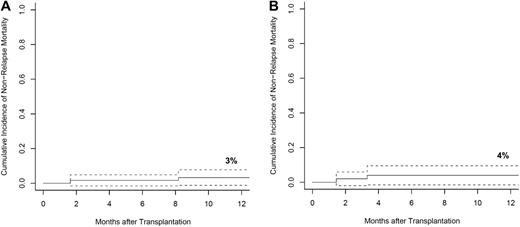
Four of the 111 patients suffered NRM during the first year after transplantation accounting for a 1-year NRM cumulative risk of 3% for related and 4% for unrelated donor transplants (Figure 2A-B). GVHD was responsible for 2 of the 4 deaths; 1 due to acute GVHD and 1 in a patient with extensive chronic GVHD. Cyclosporine microangiopathy and septic shock associated with a bacterial line infection accounted for the remaining 2 deaths. No deaths occurred as a result of invasive fungal or viral infections. Karnofsky performance status (KPS) was assessed in all patients before transplantation and at interval evaluations during follow-up (Table 3). At last follow-up, 93% of living patients had a performance status of 90% or greater, and no patients had a performance status of less than 70%. This result compared favorably with the pretransplantation KPS of these same patients of whom 79% had a performance status of 90% or greater, 15% were at 80%, and 6% were 70% or below.
NRM. Cumulative incidence of NRM, with 95% CIs, among patients who received matched-related donor (A) and matched-unrelated donor (B) grafts with competing risks of relapse.
NRM. Cumulative incidence of NRM, with 95% CIs, among patients who received matched-related donor (A) and matched-unrelated donor (B) grafts with competing risks of relapse.
Graft-versus-host disease
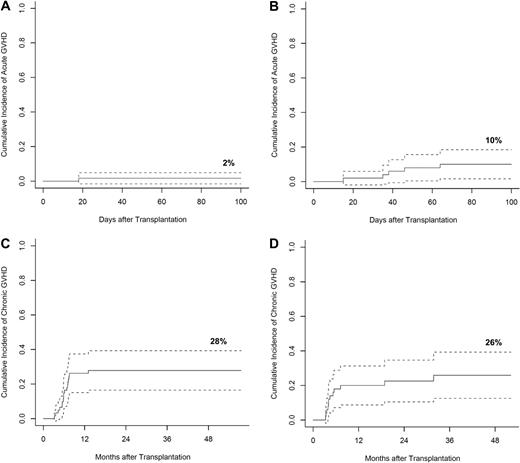
The probability of acute GVHD (grades II-IV) by day 100 was 2% among related donor transplants and 10% among unrelated donor transplant recipients (Figure 3A-B). Severity of acute GVHD among the entire cohort included 3 patients with grade II, 2 with grade III, and one with grade IV. It is noteworthy that the 12 patients who demonstrated a post-TLI and ATG skewing of residual host T-cell subsets favoring invariant NKT cells did not develop acute GVHD, yet the 2 patients without detectable invariant NKT cells developed acute GVHD.
GVHD. Cumulative incidence of acute GVHD with 95% CIs, among patients who received matched-related donor (A) and matched-unrelated donor (B) grafts. Cumulative incidence of chronic GVHD with 95% CIs, among patients who received matched-related donor (C) and matched-unrelated donor (D) grafts. Cumulative incidence of acute and chronic GVHD were calculated with competing risks including relapse, death, and primary graft failure.
GVHD. Cumulative incidence of acute GVHD with 95% CIs, among patients who received matched-related donor (A) and matched-unrelated donor (B) grafts. Cumulative incidence of chronic GVHD with 95% CIs, among patients who received matched-related donor (C) and matched-unrelated donor (D) grafts. Cumulative incidence of acute and chronic GVHD were calculated with competing risks including relapse, death, and primary graft failure.
The cumulative risk of extensive chronic GVHD at 3 years among recipients of related and unrelated grafts was 28% (95% confidence interval [CI], 16%-39%) and 26% (95% CI, 13%-39%), respectively (Figure 3C-D). All but one patient with acute GVHD developed progressive chronic GVHD. With a minimum follow-up of 1 year, (range 1-7 years) 69% of patients at last follow-up were on no immunosuppressive therapy (Table 3).
OS and EFS
The 36-month probability of OS and EFS for all 111 patients was 60% (95% CI, 51%-71%) and 40% (95% CI, 31%-52%), respectively (supplemental Figure 1A, available on the Blood website [see the Supplemental Materials link at the top of the online article], and Table 4). OS and EFS for recipients of related and unrelated donor grafts are shown in supplemental Figure 1B and C. Supplemental Figure 1D and E shows probability of OS and EFS for patients with lymphoid and myeloid malignant diseases. Of the 64 patients with lymphoid malignancies, 44 were alive at last follow-up, and of the 47 patients with myeloid malignancies, 23 were alive at last follow-up.
Overall and event-free survival by patient group
| Patient group . | Overall survival,3-year % (95% CI) . | Event-free survival,3-year % (95% CI) . |
|---|---|---|
| All | 60 (51-71) | 40 (31-52) |
| Donor graft | ||
| Related | 70 (58-83) | 47 (35-63) |
| Unrelated | 47 (33-67) | 32 (20-52) |
| Diagnosis | ||
| Lymphoid | 69 (58-82) | 42 (30-56) |
| Myeloid | 47 (33-67) | 40 (27-59) |
| Age | ||
| Lymphoid | ||
| < 55 y | 65 (51-82) | 42 (29-61) |
| ≤ 55 y | 80 (61-100) | 41 (22-78) |
| Myeloid | ||
| < 55 y | 47 (29-78) | 30 (15-59) |
| ≤ 55 y* | 48 (31-76) | 49 (32-75) |
| Disease status at the start of conditioning | ||
| Non-Hodgkin lymphoma | ||
| CR | 92 (79-100) | 36 (13-96) |
| PR | 76 (63-92) | 53 (38-73) |
| SD or PD* | 0 | 12 (2-78) |
| De novo AML | ||
| CR1 | 50 (33-77) | 48 (31-73) |
| CR2 | 37 (14-100) | 50 (27-93) |
| Other† | 20 (3-100) | 0 |
| Chimerism | ||
| NHL in CR or PR at start of TLI and ATG | ||
| Complete donor chimerism | 81 (69-93) | 74 (57-91) |
| Mixed donor chimerism | 72 (36-100) | 0 |
| De novo AML in CR1 or CR2 at start of TLI and ATG | ||
| Complete donor chimerism | 64 (42-86) | 64 (43-84) |
| Mixed donor chimerism | 25 (0-55) | 25 (0-56) |
| Primary graft failure | 0 | 0 |
| Prior autologous HCT | ||
| NHL with prior autologous HCT | 75 (58-95) | 41 (25-69) |
| NHL without prior autologous HCT | 73 (56-95) | 46 (27-76) |
| Patient group . | Overall survival,3-year % (95% CI) . | Event-free survival,3-year % (95% CI) . |
|---|---|---|
| All | 60 (51-71) | 40 (31-52) |
| Donor graft | ||
| Related | 70 (58-83) | 47 (35-63) |
| Unrelated | 47 (33-67) | 32 (20-52) |
| Diagnosis | ||
| Lymphoid | 69 (58-82) | 42 (30-56) |
| Myeloid | 47 (33-67) | 40 (27-59) |
| Age | ||
| Lymphoid | ||
| < 55 y | 65 (51-82) | 42 (29-61) |
| ≤ 55 y | 80 (61-100) | 41 (22-78) |
| Myeloid | ||
| < 55 y | 47 (29-78) | 30 (15-59) |
| ≤ 55 y* | 48 (31-76) | 49 (32-75) |
| Disease status at the start of conditioning | ||
| Non-Hodgkin lymphoma | ||
| CR | 92 (79-100) | 36 (13-96) |
| PR | 76 (63-92) | 53 (38-73) |
| SD or PD* | 0 | 12 (2-78) |
| De novo AML | ||
| CR1 | 50 (33-77) | 48 (31-73) |
| CR2 | 37 (14-100) | 50 (27-93) |
| Other† | 20 (3-100) | 0 |
| Chimerism | ||
| NHL in CR or PR at start of TLI and ATG | ||
| Complete donor chimerism | 81 (69-93) | 74 (57-91) |
| Mixed donor chimerism | 72 (36-100) | 0 |
| De novo AML in CR1 or CR2 at start of TLI and ATG | ||
| Complete donor chimerism | 64 (42-86) | 64 (43-84) |
| Mixed donor chimerism | 25 (0-55) | 25 (0-56) |
| Primary graft failure | 0 | 0 |
| Prior autologous HCT | ||
| NHL with prior autologous HCT | 75 (58-95) | 41 (25-69) |
| NHL without prior autologous HCT | 73 (56-95) | 46 (27-76) |
CI indicates confidence interval; HCT, hematopoietic cell transplantation; AML, acute myeloid leukemia; NHL, non-Hodgkin lymphoma; CR, complete remission; PR, partial remission; SD, stable disease; and PD, progressive disease.
The OS falls below the EFS as an artifact of censoring subject(s) between the times of another subject's relapse and death.
Other, beyond 2nd CR, partial response, stable disease, or progressive disease.
Impact of disease status
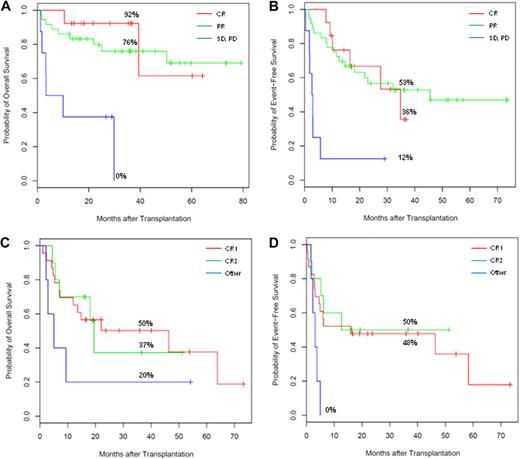
The status of disease at the start of conditioning significantly influenced OS and EFS (Figure 4A-D and Table 4). For patients with NHL (n = 57), chemotherapy-insensitive or chemotherapy-refractory disease (defined as SD or PD) significantly increased risk of death and relapse/progression compared with patients transplanted in CR or PR (hazard ratios of 9.1 [95% CI, 1.81-46.2] and 5.9 [95% CI, 1.92-18.3] for OS and EFS, respectively; Figure 4A,B). The 36-month actuarial OS and EFS for patients in CR or PR at the time of transplantation were similar, and with follow-up extending to 7 years, the median OS had not been reached for either of these groups of patients. In contrast, transplant recipients who had SD or PD at the start of TLI and ATG had 36-month actuarial OS and EFS of less than 12%.
Effect of disease status at transplant on OS and EFS. Kaplan-Meier OS (A) and EFS (B) curve estimates among patients with NHL stratified by disease status at time of transplantation (first CR, PR, or SD and PD). Kaplan-Meier OS (C) and EFS (D) curve estimates among patients with de novo AML stratified by disease status at time of transplantation (first CR, second CR, or beyond second remission including persistent disease). Corresponding 36-month OS or EFS noted. The OS curve for patients with de novo AML falls below the EFS at 19.5 months as an artifact of censoring of 2 subjects between the times of another subject's relapse and death.
Effect of disease status at transplant on OS and EFS. Kaplan-Meier OS (A) and EFS (B) curve estimates among patients with NHL stratified by disease status at time of transplantation (first CR, PR, or SD and PD). Kaplan-Meier OS (C) and EFS (D) curve estimates among patients with de novo AML stratified by disease status at time of transplantation (first CR, second CR, or beyond second remission including persistent disease). Corresponding 36-month OS or EFS noted. The OS curve for patients with de novo AML falls below the EFS at 19.5 months as an artifact of censoring of 2 subjects between the times of another subject's relapse and death.
Ten of the 111 patients in the current study had follicular lymphoma that was chemosensitive to salvage therapy. Two of these 10 had relapse of disease after prior autologous transplantation, 9 were in PR at the time of allogeneic transplantation, 1 was in CR, and 4 received their graft from an unrelated donor. With a median follow-up of 5.4 years, 9 of 10 patients were alive and in CR. Seven had no history of GVHD and were successfully removed from posttransplantation immune suppression medications.
Patients with de novo AML not in CR1 or CR2 had increased risk of death and relapse compared with patients who received transplants in first or second remission (hazard ratios of 2.5 [95% CI, 0.80-7.87] and 3.7 [95% CI, 1.22-11.3] for OS and EFS, respectively; Figure 4C-D).
Impact of chimerism
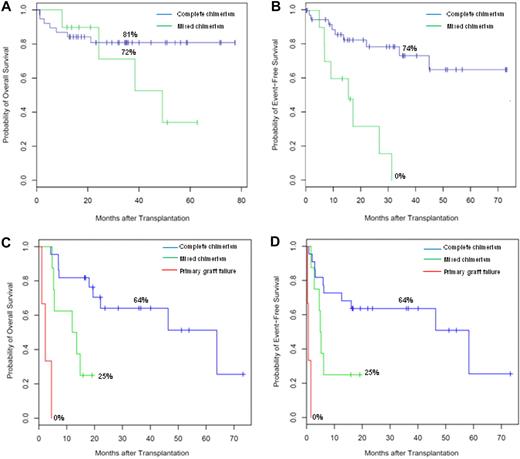
As hypothesized, NHL patients with SD or PD and de novo AML patients beyond CR2 had uniformly poor outcomes. Therefore, we evaluated the impact of chimerism only among patients with variable outcomes, specifically NHL patients in CR or PR and de novo AML patients in CR1 or CR2 at the start of HCT (Figure 5A-D and Table 4). For patients with NHL who achieved complete chimerism, the median OS and EFS were not reached (Figure 5A-B), yet the median OS and EFS were 50.4 and 16.4 months, respectively, for patients with sustained mixed chimerism (hazard ratios of 2.2 [95% CI, 0.6-7.4] and 6.29 [95% CI, 2.23-17.8], for OS and EFS, respectively).
Effect of chimerism on OS and EFS. Kaplan-Meier OS (A) and EFS (B) curve estimates among patients with NHL enrolled with CR or PR stratified by chimerism (complete or mixed chimerism, no graft failures occurred). Kaplan-Meier OS (C) and EFS (D) curve estimates among patients with de novo AML enrolled with disease control, CR1 or CR2 stratified by chimerism (complete, mixed, or primary graft failure). Corresponding 36-month OS or EFS noted.
Effect of chimerism on OS and EFS. Kaplan-Meier OS (A) and EFS (B) curve estimates among patients with NHL enrolled with CR or PR stratified by chimerism (complete or mixed chimerism, no graft failures occurred). Kaplan-Meier OS (C) and EFS (D) curve estimates among patients with de novo AML enrolled with disease control, CR1 or CR2 stratified by chimerism (complete, mixed, or primary graft failure). Corresponding 36-month OS or EFS noted.
Among patients with de novo AML in CR1 or CR2 at the start of conditioning, the median OS and EFS were significantly improved with attainment of complete chimerism, 63.9 and 58.4 months, compared with patients with mixed chimerism, 12.0 and 4.9 months (hazard ratios of 5.0 [95% CI, 1.5-16.9] and 3.3 [95% CI, 1.09-9.60], for OS and EFS, respectively; Figure 5C-D). OS and EFS among patients with primary graft failure were as expected poor at 2.3 and 0.6 months, respectively.
Age
Forty-eight percent (n = 53) of the 111 patients were 55 years of age or older, 31% (n = 34) were 60 years or older, and 7% (n = 8) were over age 65 at the start of transplantation. The clinical outcomes of OS, EFS, NRM, acute and chronic GVHD, and level of donor chimerism achieved appeared similar among older and younger age recipients with either lymphoid or myeloid diseases (OS and EFS shown in supplemental Figure 2A-D and Table 4).
Prior autologous transplantation
Thirty-two of the 64 patients with lymphoid malignant diseases had relapse of lymphoma following autologous transplantation before TLI and ATG conditioning, and these patients did not have adverse risk of graft failure, relapse, or death. Median OS among NHL patients with prior autologous transplant was 50.3 and 39.4 months without prior autologous HCT (supplemental Figure 3A-B and Table 4).
Relapse and antitumor reactions
Of the 111 patients in this study, 44 died (Table 3). The cumulative risks of relapse and disease progression at 48 months for all patients (NRM as a competing risk) were 60% (95% CI, 44%-77%; supplemental Figure 4A) and 53% (95% CI, 39%-68%; supplemental Figure 4B), respectively. Levels of chimerism impacted the cumulative risk of relapse or disease progression. Patients who achieved mixed chimerism had a 100% (95% CI, 77%-100%) risk of disease relapse/progression at 36 months, whereas those who achieved complete chimerism had a cumulative incidence of relapse/progression of 43% (95% CI, 31%-54%; supplemental Figure 4C). Thirteen of 20 patients whose disease had relapsed or progressed after transplantation achieved a CR after either infusion of donor lymphocytes or cytokine-induced killer cells.32
At last follow-up, 67 patients were alive of whom 59 (88%) were in CR. Among those in CR, 58% (n = 34) had measurable disease at the start of transplantation including 31 patients (53%) in PR and 3 (5%) with PD. The contribution of TLI versus the antitumor activity of the graft to these responses is difficult to discern, except in 20 of the 34 patients who achieved a CR had clearing of tumor that was outside the field of the total lymphoid irradiation. Fourteen of these patients had bone marrow involvement, and 6 patients had other extranodal sites of disease.
Discussion
The objective of the current study was to determine the incidence of acute and chronic GVHD, NRM, tumor relapse, EFS and OS in 111 patients with lymphoid and myeloid malignant diseases who underwent HLA-matched HCT using RIC with TLI and ATG. This study included 34 patients considered too old (at least 60 years of age) for myeloablative transplantation, 32 patients at high risk of lymphoma relapse after disease recurrence following prior autologous HCT, and 51 patients at high risk of developing GVHD due to lack of availability of a fully HLA-matched related donor.
The incidence of acute GVHD, grade II or higher, in this study appeared considerably lower than reported in other single institution or multicenter studies.2,,,,,,,,,,,–14 In these latter studies, rates of acute GVHD were 22% to 78% following RIC with sublethal total body irradiation (TBI) and/or chemotherapeutic agents. Two groups of investigators have reported rates of acute GVHD lower than 20%. Khouri et al reported an incidence of grade II or higher acute GVHD of 11% following conditioning with fludarabine, cyclophosphamide, and rituximab.33 Yet in this study, 96% of patients received G-CSF–mobilized peripheral blood mononuclear cells or bone marrow from an HLA-matched related donor. The incidence of acute GVHD when applied to a population of recipients of unrelated donor grafts remains unknown. Mackinnon and colleagues used an alemtuzumab-containing reduced-intensity regimen and reported that 15% of 88 patients developed acute GVHD grade II or higher.34 Yet 41% of patients required an infusion of donor lymphocytes for persistent low levels of donor chimerism, and 28% of these patients developed clinically significant GVHD following DLI. In our study, only 5.4% of 111 patients developed grade II or higher acute GVHD, of whom 46% received grafts from someone other than a fully HLA-matched related donor, including 6 recipients of HLA-mismatched grafts.
It is unlikely that the low incidence of acute GHVD was explained by the persistence of ATG associated with in vivo donor T-cell depletion. Efforts by other investigators to reduce acute GVHD by the addition of ATG, for the purpose of in vivo T-cell depletion, to standard RIC regimens, were associated with GVHD rates of 26% to 64%.9,–11,35,36 Further, we previously showed that biologically active ATG was not detectable in the serum of transplant recipients beyond 7 days following transplantation.23 Rather, based on our preclinical studies, the more important effect of ATG was to deplete conventional host T cells that may reject the donor graft, without depletion of regulatory T cells.18,,–21 In the animal model, the preponderance of NKT cells that developed after TLI was critical for GVHD protection, because NKT cells polarized donor T cells to a Th2 phenotype with expansion of donor T regulatory cells via an IL-4–dependent mechanism.18,,–21 This polarization markedly reduced the ability of donor T cells to mediate GVHD. As demonstrated in the animal model, we previously showed human chimeric donor CD4+ T cells had a Th2 pattern of cytokine production, yet chimeric CD4+ T cells obtained from patients conditioned with TBI instead of TLI and ATG did not.23 In the current study, we now confirmed that TLI and ATG resulted in an alteration of host T-cell subsets that strongly favored invariant NKT cells, as the ratio of host invariant NKT cells to conventional T cells significantly increased following TLI and ATG administration. We submit that the Th2 polarization previously described,23 resulted from the increased ratio of regulatory host invariant NKT cells that skewed IL-4 production following TLI and ATG administration. It is noteworthy that among the 14 patients in whom we measured T-cell subsets, 2 patients without measurable invariant NKT cells developed acute GVHD, yet 12 patients who displayed an increased ratio of invariant NKT cells did not. It remains unclear why these patients lacked an invariant NKT-cell population; perhaps this may have related to cytotoxic treatment before starting TLI and ATG.
Animal models confirmed that both Th1 and Th2 type donor CD4 T cells are implicated in the pathogenesis of chronic GVHD.37 The incidence of chronic GVHD observed in the current study did not differ for recipients of matched related and unrelated donor grafts, and among patients who developed chronic GVHD, the majority of patients were successfully removed from immunosuppressive therapy. These rates compare favorably with reported rates of chronic GVHD following RIC transplantation of 33% to 78%.1,–3,12,–14,38,,,,,,–45 Addition of ATG in standard RIC regimens may be associated with a reduction in rates of chronic GVHD, however, not all investigators have confirmed this finding.10,35,44,45 The relatively low rate of chronic GVHD we observed may result, in part, from the low rate of acute GVHD, as acute GVHD is a significant risk factor for development of chronic GVHD.30
It is difficult to discern the antitumor activity contributed by 8 Gy of TLI from alloreactive graft-versus-tumor reactions mediated by donor immune cells. Prior studies suggested that doses higher than 15 Gy were required for long-term control of limited-site disease in follicular lymphoma and higher than 35 Gy for aggressive lymphoma.46 Of note, in the current study, 34 patients with measurable lymphoma at the start of TLI and ATG achieved and have maintained a CR, and 20 of these patients had clearing of tumor that was outside the field of the lymphoid irradiation. Similarly, the 36-month EFS of patients with myeloid malignant diseases in second remission at the start of TLI and ATG was 50%, suggesting beneficial graft antitumor reactions in this patient population who have fared poorly with standard therapies.
Tumor response to salvage chemotherapy was an important pretransplantation characteristic that predicted risk of posttransplantation disease relapse or progression. Patients with lymphoid malignancies who prior to TLI and ATG failed to display chemosensitivity to salvage treatment had posttransplantation rates of disease progression in excess of 85%. In these cases it was presumed that the kinetics of tumor progression outpaced and overwhelmed graft antitumor reactions. A history of lymphoma relapse after prior autologous transplantation did not adversely affect survival outcomes or relapse rate, and this has been reported by others.47 Patients with chemosensitive follicular lymphoma had excellent outcomes, a finding consistent with that of other groups suggesting that this disease entity is particularly sensitive to effects of graft-versus-tumor activity.33
Advanced patient age, and low (≤ 80%) pretransplantation KPS predicted increased NRM and/or poor OS following allogeneic HCT using RIC in some studies yet not in others.9,12,42 This variability may reflect that, in general, young patients receiving RIC were selected because they had comorbidities that were not controlled for and outweighed the impact of advanced age and low KPS. Our observed 1-year NRM of 3% was remarkable especially in light of the following: 21% of patients had a pretransplantation KPS of 80% or less, 31% of patients were age 60 or older, 29% had relapse of lymphoma after a prior autologous transplantation, and 46% received a graft from someone other than an HLA-matched sibling. All patients received their donor cell infusion as an outpatient, yet only 27% were admitted to hospital at any point in the first 100 days after transplantation with a median duration of hospitalization of 3.5 days.
The low NRM and overall excellent tolerability of TLI and ATG may, in part, be explained by the low incidence of acute and chronic GVHD. Without the need for high-dose immune suppression medication to control GVHD, patients were at less risk for the development of clinically significant infections. Of the 111 transplant recipients, only 3 died from GVHD and one from an infection during the entire follow-up period that extended to almost 7 years. The low NRM makes this transplantation procedure attractive for patients with nonmalignant diseases who may benefit from allogeneic HCT.48
The limitation to TLI and ATG conditioning that must be addressed was the observation that 19% of patients developed mixed chimerism after transplantation who failed to convert to complete donor hematopoietic cell chimerism. Mixed chimerism was associated with a significantly higher incidence of disease relapse or progression irrespective of whether the patient had a lymphoid or myeloid malignancy. “Mega-dose” CD34+ cell infusion has been associated with improved engraftment.49 In our study, the mean absolute number of CD34+ cells infused was 7.3 ± 3.5 × 106/kg, and attempts to further increase this number would require the inconvenience of additional days of donor apheresis. Rather, an alternate approach is to increase the dose of TLI administered. In the animal model, increased doses of TLI more extensively depleted host conventional immune cells that interfered with donor cell engraftment and increased further the ratio of host invariant NKT cells to conventional T cells that were critical for GVHD prevention.50 We previously showed that 12 Gy fractionated TLI combined with ATG is safe and well-tolerated in humans48 and anticipate this modification will increase the number of patients with complete chimerism. It remains to be determined if this strategy will, like in the preclinical model, afford GVHD protection above and beyond what we currently report.
In conclusion, with extended patient follow-up and increased sample size, we confirmed that TLI and ATG conditioning was well tolerated and associated with low rates of acute GVHD and NRM, irrespective of whether the graft was from a related or unrelated donor. For patients beyond 1 year from transplantation, the majority were withdrawn from immune suppression medication as none were required for the control of chronic GVHD. Despite attenuation of graft-versus-host reactions, TLI and ATG preserved graft-versus-tumor reactions. Over 50% of patients with lymphoid malignancies who had residual disease at the start of transplantation achieved and maintained conversion to a CR. For patients with high risk myeloid malignancies in second CR, the 36-month EFS was 50%. Future efforts will confirm if, as in the preclinical model, an increased dose of TLI will reduce further the risk of tumor relapse by enhancing chimerism. These outcomes will likely translate into improved OS for cancer patients who require allogeneic HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant nos. P01 CA049605, P01 HL075462, and Cancer Center Support grant no. 1PO30CA124435-01.
National Institutes of Health
Authorship
Contribution: R.L., J.A.S., and S.S. designed the research protocol; H.E.K. and R.L. collected data, analyzed and interpreted data, and wrote the manuscript; B.B.T. and P.W.L. performed statistical analysis, analyzed, and interpreted data; and K.H., J.A.S., G.G.L., D.B.M., L.J.J., S.A., W.W., R.T.H., R.S.N., K.G.B., and R.L. entered patients on study, verified patient data, and reviewed the manuscript.
Conflict-of-interest disclosure: R.L., D.B.M., R.S.N., and S.S. report receiving lecture and consulting fees from Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Robert Lowsky, Stanford University Medical Center, 300 Pasteur Dr, Rm H3249, Stanford, CA 94305; e-mail: rlowsky@stanford.edu.