Abstract
Megakaryocytes (MKs) undergo an endomitotic cell cycle, leading to polyploidy. We examined the expression of the flavoproteins and oxidative stress-promoting enzymes, NADPH oxidases (Nox's), in MKs because of their known role in promoting the cell cycle. Although the expression of Nox isoforms varies between cell types, they are induced at the mRNA level by mitogenic stimuli. Western blotting or reverse transcription–polymerase chain reaction of purified mouse MKs isolated from thrombopoietin (TPO)–treated bone marrow (BM) cultures indicated high expression of Nox1, a weak expression of Nox4, and no significant expression of Nox2. Immunofluorescence of freshly isolated MKs confirmed strong expression of Nox1 in one-third of MKs, whereas Nox1 staining was detected in nearly all MKs in TPO-stimulated BM cultures. Treatment of mouse BM cultures with Nox inhibitors resulted in accumulation of MKs with low DNA content levels and significant reduction of higher ploidy MKs. Purified, Nox-inhibited MKs showed a notable decrease in the level of the G1 phase cyclin E, a cyclin associated with MK polyploidy, and its up-regulation restored most of the effect of Nox inhibitors. Hence, this study shows the expression of Nox isoforms in MKs and highlights a potential role of flavoproteins in promoting polyploidization in this lineage.
Introduction
Megakaryocytes (MKs) are platelet precursors in the bone marrow (BM) that undergo a complex maturation process called megakaryopoiesis. A hallmark of MK development occurs after their commitment to the MK lineage, in which MKs go through iterative rounds of endomitosis to become large polyploid cells. These cells can contain DNA content of up to and greater than 128N, with 2N being normal diploid DNA content.1 The primary stimulus for progression of megakaryopoiesis is known to stem from thrombopoietin (TPO) signaling through the c-Mpl receptor2 ; however, despite this being the focus of many studies, much of TPO signaling in MKs remains unclear
Recently, investigations of reactive oxygen species (ROS), their generation, and downstream signaling effects have shown that low physiologic levels of ROS play a role in promoting cell proliferation in many cells types, such as fibroblast, prostate, macrophage, endothelial, and vascular smooth muscle cells (VSMCs).3-7 ROS-producing enzymes, such as a member of the NADPH oxidase (Nox) family, Nox1, have been described to be activated by mitogenic stimuli8 and to produce ROS that have been shown to induce and maintain G1 phase cyclin D1 expression in mouse lung epithelial cells.9 Similarly, Havens et al10 showed that ROSs increase in a cell cycle–dependent manner and oscillate with every cell division in human T98G glioblastoma cells, human leukemic T cells, and NIH 3T3 cells. Although, these investigators did not identify the source of ROS in these cells, they did show that on treatment with various antioxidants, cells undergo transient arrest and fail to make a timely G1-S phase progression.11-13 In addition, Havens et al10 described redox control over the activity of the anaphase promoting complex (APC) in association with Cdh1, mediating cyclin A degradation, a regulator of the G1/S transition. In the MK endomitotic cell cycle, cyclin D3 has been described as the predominant D-type cyclin that is up-regulated by TPO stimulation and induces high ploidy levels in cyclin D3 transgenic mice.14-16 Ablation of cyclin D3 in cultured MKs results in a notable decrease in MK ploidy level,16 although in vivo studies show a less dramatic effect on ploidy possibly because of compensation by other D cyclins.17 Cyclin E is another G1 phase cell-cycle regulator that has been suggested to play a role in MK ploidy promotion, notably inducing nonendomitotic megakaryoblastic K562 cells to undergo re-replication cycles.18 The importance of cyclin E in promotion of ploidy is also supported by evidence from the cyclin E1−/−E2−/−double knockout (KO) mice produced with the use of the tetraploid complementation rescue method.19 Interestingly, these mice developed normally with the exception of ploidy promotion in trophoblasts and MKs, showing a marked reduction in polyploid MKs even when cultured with TPO.
To date, investigation of ROS in MKs has been limited to studies that used human megakaryocytic leukemic cell lines, such as MO7e and B1647 cells.11,20-23 These studies have implicated ROS as second messengers involved in mediating signaling from growth factors, such as granulocyte macrophage-colony stimulation factor (GM-CSF) and TPO.11,20 With the use of a variety of oxidase inhibitors, these studies suggest that Nox's may be the source of ROS in these cell lineages.21 In addition, Seno et al24 has shown that human platelets and the MEG01 human megakaryocytic cell line both express Nox component subunits p22phox and p67phox at the protein level.24 The p22phox subunit was shown to be an essential part of the Nox complex in endothelial cells, VSMCs, phagocytes, and others.25 Similarly, p67phox was shown to be an inducible activator of the Nox, Nox2, although its ability to associate with Nox1 and Nox3 complexes remain undetermined.25 Interestingly, confirmation of expression of Nox1, Nox2, and Nox4 and their associated subunits has also been identified in human stem cells or progenitor cells from peripheral blood samples.26,27
In this study, we examined the expression of Nox1, Nox2, and Nox4 in primary murine MKs with the use of reverse transcription–polymerase chain reaction (RT-PCR) and Western blotting of magnetic bead–purified MKs as well as immunofluorescence, and we have shown that inhibition of these enzymes leads to a significant decrease in cyclin E and D3 levels and in polyploidy.
Methods
Mice
Wild-type (Wt) FVB or C57BL/6 mice (Taconic) were used for BM and fetal liver MK analyses as indicated. Nox2 KO mice28 and control strain (age- and sex-matched) C57BL/6 mice were purchased from The Jackson Laboratory. Nox1-deficient mice were previously described.29 All studies involving mice were approved by the Boston University Animal Care and Use Committees.
mRNA preparation and transcript analysis
Total RNA was harvested from freshly isolated MKs from Wt murine BM, Y10 cells, or primary vascular smooth muscle cells (VSMCs) prepared from mouse aorta as described previously,30 using TRIzol reagent according to the manufacturer's instructions (Invitrogen). Nox1, Nox2, and Nox4 transcripts were detected by RT-PCR as previously described.31 Primers used were Nox1 sense, 5′-gggatgaccataaggggagt-3′, and Nox1 antisense, 5′-cactccaggaaggaaatgga-3′; Nox4 sense, 5′-cagcttctacctacgcaata-3′, and Nox4 antisense, 5′-ggaaatgagcttggaacttggg-3′; CD68 sense, 5′-ggacccacaactgtcactcat-3′, and CD68 antisense, 5′-aagccccactttagctttacc-3′; Ly6G sense, 5′-atccttcttgtggtcctactgtgtg-3′, and Ly6G antisense, 5′-tgctcttgactttgcttctgtgag-3′. For BM MKs, transcripts were amplified with the following cycle numbers: Nox1, 32 cycles; Nox4, 35 cycles (38 cycles not shown); GAPDH, 20 cycles. For Y10 cells, transcripts were amplified with the following cycle numbers: Nox1, 33 cycles; Nox4, 38 cycles (41 cycles not shown); GAPDH 20 cycles. For BM, MK, and macrophages, transcripts were amplified with the following cell-cycle numbers: CD68, 30 cycles; Ly6G, 30 cycles. Real-time PCR was performed with the use of murine Nox1 TaqMan Gene expression primers and probes (Mm00549170_m1; Applied Biosystems). Reactions were run in triplicates. Samples were run on an Applied Biosystems Sequence Detection System 7300. Data were normalized to 18S rRNA (primer 18s rRNA; Applied Biosystems) and CD41 (Mm00439768_m1 Itga2b; Applied Biosystems) mRNA expression levels and analyzed with the δ-δ CT method.
MK enrichment by magnetic-activated cell sorting magnetic bead purification system
This method was used to purify MKs for analysis by Western blot. BM from 2 mice in each group was cultured for 3 days in the presence of TPO. Cells were washed twice with staining buffer (0.5% BSA, 2 mM EDTA, and PBS, pH 7.2). BM was then labeled with MK-specific antibody, anti–CD41-FITC (BD PharMingen). After 2 washes in staining buffer, BM cells were resuspended in staining buffer and anti–FITC-labeled microbeads (10 μL/10 × 106 cells; Miltenyi Biotec). Cells were loaded onto an equilibrated large-cell separation column (Miltenyi Biotec;) fitted with a 25-gauge needle for flow resistance. The bound CD41-FITC–labeled MKs were eluted with 1 mL buffer.
BM cultures and Y10/L8057 cells
Femoral BM was isolated as previously described32 and cultured with 25 ng/mL of TPO (murine PEG-rmMGDF; gift from Kirin Pharma Company) in IMDM media containing 10% bovine calf serum and 1% penicillin/streptomycin (Gibco). The murine megakaryocytic Y10/L8057 cells were cultured in F12 medium supplemented with 10% bovine calf serum and 1% penicillin/streptomycin. When stimulated with 25 ng/mL TPO, Y10/L8057 cells were cultured in IMDM-based media.
Western blot analysis
Cell pellets were lysed on ice with radioimmunoprecipitation assay buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, freshly supplemented with 1× protease inhibitor cocktail; Roche Applied Sciences). PVDF membranes were incubated with the following primary antibodies: Nox1 (rabbit, sc-25545; Santa Cruz), CD41 (mouse, sc-15328; Santa Cruz), cyclin E (rabbit, sc-481; Santa Cruz), cyclin D3 (rabbit, sc-182; Santa Cruz), human cyclin E (551160; BD PharMingen) GAPDH (sc-32233; Santa Cruz), and β-actin (mouse, A5441; Sigma) in 10% milk, PBS, and 0.05% Tween 20 overnight at 4°C. Membranes were then washed with PBS-Tween (0.05%) and incubated with appropriate secondary antibodies. Blots were developed with the use of the Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Immunofluorescence staining
BM cells suspended in PBS were spun down (2 × 105 cells/slide) on Colofrost Plus microscope slides (Fisher Scientific) by a Cytospin3 (Shandon) for 5 minutes at 120g. Cells were air-dried and fixed in 4% fresh paraformaldehyde at 4°C overnight. Slides were washed 3 times with PBS and permeabilized with 0.1% saponin, 0.4% bovine serum albumin (BSA) in PBS. Slides were blocked with 3% BSA in PBS followed by incubation with primary antibodies (Nox1, sc-25545) in staining buffer (0.5% BSA, PBS) at 4°C. After washes with 0.1% saponin, 0.4% BSA in PBS, slides were incubated with Alexa Fluor 594–conjugated donkey anti–rabbit IgG (Molecular Probes) in staining buffer. After washes in 0.1% saponin, 0.4% BSA in PBS, slides were washed once in PBS and then mounted with coverslips with Vectashield Mounting Medium for Fluorescence with DAPI (Vector Laboratories, Inc; catalog no. H-1200). For Nox2 immunofluorescence staining, slides were prepared as for Nox1 staining. After fixation, high-temperature antigen retrieval was performed as previously described.33,34 Primary Nox2 antibody (sc-5827; 1:50 dilution; Santa Cruz) was applied to slides in staining buffer (0.5% BSA, 0.25% Triton X-100, PBS), followed by washes and then incubation with secondary antibody (Alexa Fluor 488 rabbit anti–goat; dilution 1:1500; Molecular Probes). Some slides showed increased autofluorescence because of the antigen retrieval method as distinguished by comparison of the no staining control to the other slides and to secondary antibody control. Auto fluorescence was noted through both the red and green filters, whereas true staining with the Alexa Fluor 488 secondary antibody was not detected in the red filter. Thus, images were captured with the use of each filter, and composite overlays were created for each image.
All slides were observed with an Olympus ×70 inverted fluorescence microscope with a 60× objective (numeric aperture, 0.9), in addition to a 1.5× magnifier lens in some cases. Images were documented at 25°C with a Hamamatsu CCD camera C4742-95 (Hamamatsu Photonics) and analyzed with ImagePro software (Media Cybernetics Inc). Final images were prepared using Adobe Photoshop 7.0.
Ploidy analysis
Ploidy profile analyses were performed as previously reported by Nguyen et al.31 Analysis of S-phase in MKs was performed with the use of FlowJo cytometric analysis software Version 7.2.2 (TreeStar Inc).
ROS measurement
H2DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate) is a fluorogenic probe commonly used to detect cellular production of ROS. BM was harvested and cultured with TPO for 48 hours. Cells were then pretreated with 10 μM diphenylene iodonium (DPI) or DMSO control for 3 hours before labeling MKs with a CD41-PE–conjugated antibody (1:150 dilution). For MK labeling, cells were spun down and resuspended in staining buffer (3% BSA, PBS, CD41-PE antibody) for 15 minutes on ice. Cells were then washed twice with PBS and resuspended in phenol red–free IMDM, 10% BCS, 1% penicillin/streptomycin. Cells were incubated with 20 μM H2DCF-DA (prepared freshly in DMSO) for 15 minutes at 37°C, 5% CO2. After a wash with PBS, cells were resuspended in clear media and placed on ice until fluorescence-activated cell sorting (FACS), which was performed immediately. H2O2 (0.5 M) was added to the positive control before incubation on ice. H2O2 reacts directly with the H2DCF-DA dye. FACS analysis was performed on a BD FACScan. Live events were collected on the basis of forward and side scatter plots. MKs were gated by identification of CD41-PE–positive cells (FL2 channel). H2DCF-DA intensities were collected with the FL1 channel.
Platelet counts
Blood was collected via heart puncture, and platelet counts were performed with the use of the BD Unopette collection system (Becton Dickinson) and a Nebauer hemacytometer.
Generation of a PF4-cyclin E transgenic mouse model
The cDNA sequence corresponding to the human cyclin E1 (CCNE1) gene was cloned into a plasmid containing the 1.1-kilobase (kb) rat PF4 promoter sequence followed by a 1.7-kb fragment of the 3′ intron and poly A region of the human growth hormone, as we described previously.35 The PF4-cyclin E fragment was purified by electroelution and injected into the pronuclei of fertilized FVB/N strain mouse to produce transgenic mice. Insertion of the transgene was confirmed by PCR analysis. Expression of the transgene was confirmed by quantitative RT-PCR and Western blot analysis with the use of anti–cyclin E (BD PharMingen). All methods are as we described previously.35
Results
Identification of Nox mRNA and protein in murine MKs
RT-PCR analysis of cDNA from purified MKs was performed to determine whether Nox isoforms were expressed in MKs. Mouse BM was harvested and cultured in the presence of TPO for 3 days. MKs were isolated after magnetic bead purification with the use of Large Cell MACS (magnetic-activated cell sorting) columns as described in “Methods.” cDNA was prepared from MK RNA, and RT-PCR was used to detect Nox transcripts. Figure 1A (top) shows that Nox1 transcripts were easily detected in MKs and that a Nox4 transcript was detected at low level. Murine VSMC cDNA was used as a positive control for detection of Nox1 and Nox4 transcripts. To exclude the possibility of contamination of the MK-enriched fraction by other cells, which might contribute to the detected expression of Nox isoforms, we evaluated the purity of the MK fraction with the use of specific markers for neutrophils (Ly6G) and macrophages (CD68). As shown in Figure 1A (bottom), no significant contamination of the MK-enriched fraction was detected. RT-PCR analysis of the murine megakaryocytic Y10 cell line (derived from L8057 cells)14 showed similar results for Nox1 and Nox4 (Figure 1B). Interestingly, the addition of TPO to Y10 cells caused an increase in the amount of Nox1 transcript. Real-time PCR analysis from vehicle or TPO-treated BM cells further indicated the up-regulation of Nox1 in TPO-stimulated MKs (Figure 1C). Nox2 was not detected at appreciable levels in MKs, at base line, or after TPO treatment, as concluded also from immunostaining (supplemental Figures 1-2, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Expression of Nox mRNA in MKs. (A top) RT-PCR of Nox1 and Nox4 in magnetic bead–purified Wt MKs. RNA was prepared from purified MKs and BM fractions from cultured BM (+TPO; see purification image in Figure 2). Mouse VSMC cDNA was used as a positive control for detection of Nox1 (262 base pairs [bp]) and Nox4 (315 bp) transcripts. Omitting reverse transcriptase (RT) was used as a control for the PCR, whereas amplification of GAPDH was used as a control for RNA preparation (554 bp transcript). (A bottom) Determination of the purity of the MK-enriched fraction. RNA was prepared as described above. Amplifications of the neutrophil marker Ly6G and of macrophage/monocyte marker CD68 were used to exclude the possibility of contamination from different BM cell populations. Mac indicates macrophages. (B) Detection of Nox1 mRNA in the murine megakaryocytic Y10 cell line. RT-PCR was performed on Y10 cDNA from nontreated and TPO-treated (for 24 hours) cultures. VSMC cDNA was used as a positive control for detection of Nox1 and Nox4 transcripts. (C) Effect of TPO on Nox1 mRNA levels. Quantitative RT-PCR analysis was applied in RNA isolated form purified MKs after vehicle or TPO stimulation of the BM culture. 18s rRNA was used as an internal control, and Nox1 levels were normalized to CD41 expression levels (a MK marker). The graph represents the average of 3 independent experiments. Statistical significance (*P < .05) was determined using the Student t test.
Expression of Nox mRNA in MKs. (A top) RT-PCR of Nox1 and Nox4 in magnetic bead–purified Wt MKs. RNA was prepared from purified MKs and BM fractions from cultured BM (+TPO; see purification image in Figure 2). Mouse VSMC cDNA was used as a positive control for detection of Nox1 (262 base pairs [bp]) and Nox4 (315 bp) transcripts. Omitting reverse transcriptase (RT) was used as a control for the PCR, whereas amplification of GAPDH was used as a control for RNA preparation (554 bp transcript). (A bottom) Determination of the purity of the MK-enriched fraction. RNA was prepared as described above. Amplifications of the neutrophil marker Ly6G and of macrophage/monocyte marker CD68 were used to exclude the possibility of contamination from different BM cell populations. Mac indicates macrophages. (B) Detection of Nox1 mRNA in the murine megakaryocytic Y10 cell line. RT-PCR was performed on Y10 cDNA from nontreated and TPO-treated (for 24 hours) cultures. VSMC cDNA was used as a positive control for detection of Nox1 and Nox4 transcripts. (C) Effect of TPO on Nox1 mRNA levels. Quantitative RT-PCR analysis was applied in RNA isolated form purified MKs after vehicle or TPO stimulation of the BM culture. 18s rRNA was used as an internal control, and Nox1 levels were normalized to CD41 expression levels (a MK marker). The graph represents the average of 3 independent experiments. Statistical significance (*P < .05) was determined using the Student t test.
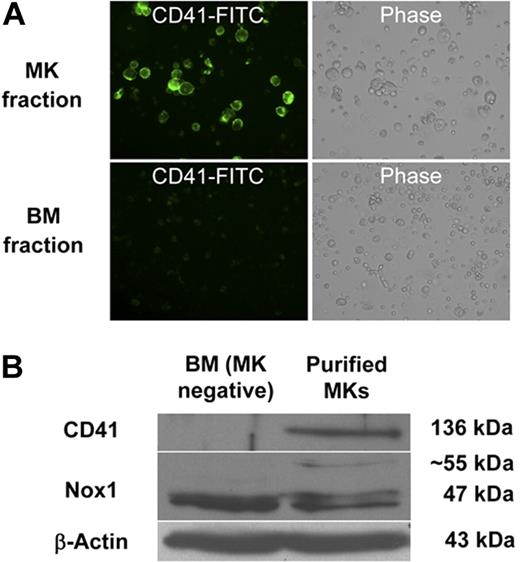
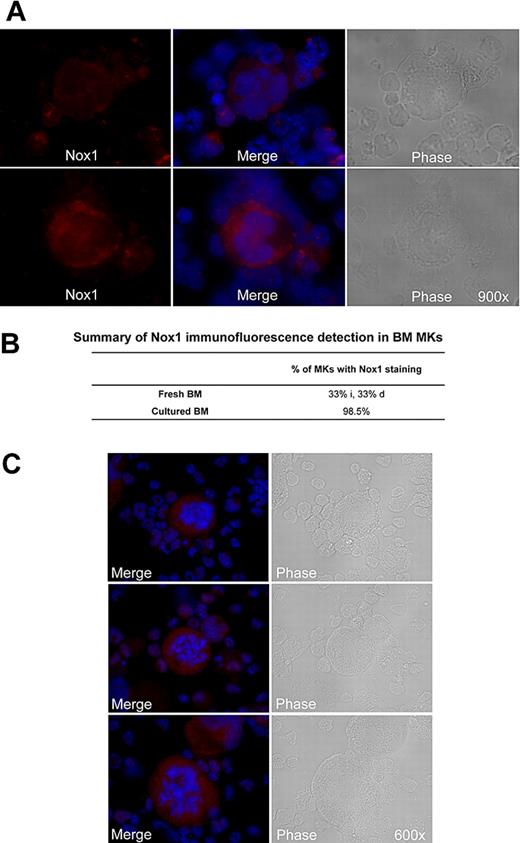
Because of the relative abundance of its transcript and its potential up-regulation by TPO, we first chose to confirm the presence of Nox1 protein in MKs. MKs from 3-day TPO-treated BM cultures were isolated with MACS magnetic bead column–based purification method, similar to the one applied in Figure 1C. Figure 2A shows a representative MK-purified cellular fraction (CD41-FITC positive) and the MK-negative cellular fraction (CD41-FITC negative) after purification. Purity of the MK fraction was estimated at 80% on the basis of CD41-FITC–positive cells and probably much greater on the basis of cell mass. Figure 2B shows a purified MK fraction and a MK-depleted fraction that were analyzed for Nox1 protein expression with Western blot analysis. A predominant and a weaker band at approximately 50 kDa and 55 kDa were identified in both fractions, further supporting the presence of Nox1 in MKs. Nox1 bands have also been reported at 55 and 63 kDa (in human samples).36 These may represent variations in posttranslational modifications, such as glycosylation, a common modification of Nox protein. Immunofluorescence staining of Nox1 was pursued to further confirm its expression in MKs and to establish a baseline for its cellular localization. TPO-stimulated BM cells were cytospun and stained for Nox1 expression as shown in Figure 3A through C. MKs were identified on the basis of size and high ploidy content. Figure 3A depicts a MK that showed dim cytosolic staining of Nox1, whereas the lower panel shows a MK that has intense cytosolic Nox1 staining. These images are representative of Nox1 staining and localization in MKs from both fresh and TPO-treated BM, respectively. Figure 3B summarizes Nox1 staining, highlighting that 66% of MKs are positive for Nox1 in fresh BM. One-half of the Nox1-positive MKs from fresh BM displayed dim staining, whereas the other half showed intense staining. Supportive of a role in TPO signaling, BM cultures stimulated with TPO showed Nox1 staining in 98.5% of MKs identified (Figure 3B). Interestingly, mitotic MKs, displaying condensed chromosomes, also showed intense Nox1 staining of its cytoplasm (Figure 3C).
Detection of Nox1 protein in magnetic bead–purified MKs. (A) Example of enrichment of CD41-FITC–labeled MKs after purification of TPO-stimulated BM cultures as described in “Methods.” Purity was assessed by fluorescence microscopy. Images are shown at ×400 magnification. (B) BM was isolated and cultured for 3 days (+TPO) before magnetic bead purification. Western blots were probed with antibodies for CD41, Nox1, and β-actin.
Detection of Nox1 protein in magnetic bead–purified MKs. (A) Example of enrichment of CD41-FITC–labeled MKs after purification of TPO-stimulated BM cultures as described in “Methods.” Purity was assessed by fluorescence microscopy. Images are shown at ×400 magnification. (B) BM was isolated and cultured for 3 days (+TPO) before magnetic bead purification. Western blots were probed with antibodies for CD41, Nox1, and β-actin.
Immunofluorescence detection of Nox1 in MKs stimulated with TPO. (A) Immunofluorescence was performed on cytospins of cultured mouse femoral BM cells. Nox1 (red) expression was observed in MKs with dim cytosolic staining (top), with intense cytosolic staining (bottom), or no staining (as summarized in panel B). DAPI counterstain was used to stain nuclei and is shown with Nox1 staining in the center. Images are shown at ×900 magnification. (B) Summary of the percentage of MKs detected with Nox1 staining. For fresh BM, the percentages of intensely stained (i) and dimly stained (d) MKs are noted. (C) Nox1 staining in mitotic MKs is intense and non-nuclear. Nox1 was also present in all mitotic MKs examined (n = 13), as noted by condensed chromosomes.
Immunofluorescence detection of Nox1 in MKs stimulated with TPO. (A) Immunofluorescence was performed on cytospins of cultured mouse femoral BM cells. Nox1 (red) expression was observed in MKs with dim cytosolic staining (top), with intense cytosolic staining (bottom), or no staining (as summarized in panel B). DAPI counterstain was used to stain nuclei and is shown with Nox1 staining in the center. Images are shown at ×900 magnification. (B) Summary of the percentage of MKs detected with Nox1 staining. For fresh BM, the percentages of intensely stained (i) and dimly stained (d) MKs are noted. (C) Nox1 staining in mitotic MKs is intense and non-nuclear. Nox1 was also present in all mitotic MKs examined (n = 13), as noted by condensed chromosomes.
Participation of Nox in MK polyploidization
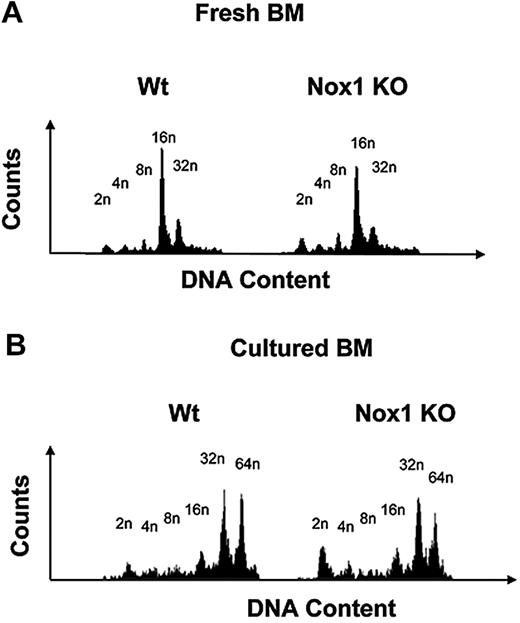
We first investigated whether Nox1-deficient mice displayed differences in MK polyploidy in comparison to Wt mice. Fresh BM was harvested and fixed before ploidy analysis. Figure 4 displays representative ploidy profiles from Wt and Nox1-deficient mice, showing a mild effect on MK ploidy at a basal level or on stimulation with TPO, but this was not statistically significant. Nox4 is expressed as low levels in MKs, but corresponding knockout (KO) mice are not available. Analysis of ploidy in MKs derived from Nox2-deficient mice (commercially available; see “Methods”) showed no effect on ploidy compared with control, which is expected because of the lack of significant expression of Nox2 in mouse MKs (supplemental Figure 3A; supplemental Table 1). The percentage of MKs in the BM was also similar in Wt and Nox2 KO mice (supplemental Figure 3B).
The ploidy profile in fresh and cultured MKs from Nox1 KO mice. (A) MK ploidy profiles from Wt and Nox1 KO mice. Fresh BM from Wt and Nox1 KO mice was fixed in 70% ethanol, washed, stained with CD41-FITC antibody, followed by propidium iodide (PI) staining before analysis on a BD FACScan. (B) MK ploidy profiles from Wt and Nox1 KO mice. BM from Wt and Nox1 KO mice was cultured and stimulated with TPO for 3 days. Cells were then fixed in 70% ethanol, washed, stained with CD41-FITC antibody, followed by PI staining before analysis on a BD FACScan. Each experiment was repeated 6 times.
The ploidy profile in fresh and cultured MKs from Nox1 KO mice. (A) MK ploidy profiles from Wt and Nox1 KO mice. Fresh BM from Wt and Nox1 KO mice was fixed in 70% ethanol, washed, stained with CD41-FITC antibody, followed by propidium iodide (PI) staining before analysis on a BD FACScan. (B) MK ploidy profiles from Wt and Nox1 KO mice. BM from Wt and Nox1 KO mice was cultured and stimulated with TPO for 3 days. Cells were then fixed in 70% ethanol, washed, stained with CD41-FITC antibody, followed by PI staining before analysis on a BD FACScan. Each experiment was repeated 6 times.
Considering the overlapping and compensatory functions of Nox in singly deleted Nox mice,28,29 and similar precedence with other proteins (eg, all, but not individual, cyclin E isoform KOs inhibit ploidy19 ), we evaluated the expression of Nox2 in the Nox1 KO mice. Quantitative RT-PCR analysis showed similar expression levels of Nox2 in Wt and Nox1 KO MKs. Similar results were obtained in BM and spleen, further suggesting that Nox2 is not compensating for the absence of Nox1 in various cell types (supplemental Figure 3C). There was also no compensation of Nox4 in Nox1 KO cells (data not shown).
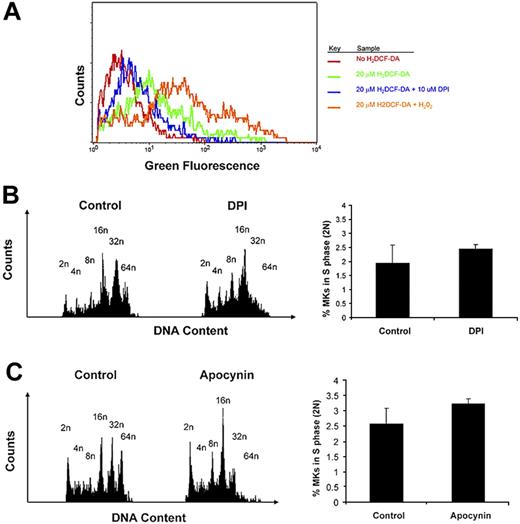
We then resorted to exploring the role of Nox in MKs using selective and specific inhibitors, DPI and apocynin, commonly used in the literature.8,26,27,29,37-41 First, to investigate whether Nox produce ROS in MKs, we measured ROS levels in MKs by FACS analysis with the fluorogenic probe H2DCF-DA. BM cultures were preincubated with DPI, followed by staining of MKs with CD41-PE antibody and loading of H2DCF-DA into cells, following conventional protocols.11,27 FACS analysis of CD41-labeled MKs indicated a decrease in H2DCF-DA fluorescence upon pretreatment of BM with Nox inhibitor DPI (Figure 5A). These results suggest that Nox proteins contribute to the production of ROS in MKs. To further examine effects on MK ploidy, we treated fresh BM cultures with a single low dose of 0.2 μM DPI or 200 μM apocynin and analyzed its effect on MK polyploidization in TPO-treated BM culture. These concentrations were selected on the basis of preliminary experiments with a concentration range, followed by focusing on low dosages that do not affect cell survival or apoptosis, as judged by trypan-blue exclusion and FACS analysis of less than 2n cells. DPI potently inhibited MK polyploidization, resulting in accumulation of MKs with a DNA content of 2n to 16n, and a decrease in higher ploidy MKs (32n-64n) in comparison to vehicle-treated control BM (Figure 5B left; Table 1). The Nox–specific inhibitor, apocynin, also showed a consistent pattern of ploidy inhibition, although it is known to have a lower binding constant25 (Figure 5C left; Table 2). Similar results were obtained with fetal liver cell cultures (data not shown). To determine whether the diminished percentage of high-ploidy MKs is due to a general reduction in cell cycling, we calculated the percentage of diploid MKs undergoing S phase, using FACS analysis (Figure 5B-C right). The results indicate a similar percentage of diploid MKs undergoing S phase in Nox-inhibited and control MKs. Collectively, these results suggest that Nox-mediated production of ROS is a regulator of MK polyploidization.
Nox inhibitors inhibit MK ploidy. (A) Nox inhibitor, DPI, decreases ROS production in MKs. The histogram depicts general ROS level detected by H2DCF-DA in CD41-PE–positive cells of cultured BM, as described in “Methods.” DPI was added as indicated, and H2O2 was added as a positive control, all as described in “Methods.” (B) DPI decreases ploidy of MKs in BM culture. (Left) BM cells were cultured (+TPO) for 3 days with or without 0.2 μM DPI. Cells were then labeled with CD41-FITC antibody followed by PI staining before FACS analysis on a BD FACScan. (Right) Percentage of MKs undergoing S phase. Cell-cycling cells were determined by selectively gating the area between 2N and 4N DNA peaks, as shown in the histogram profiles. Data were analyzed with FlowJo software (TreeStar). Statistical analysis was applied with the use of the t test for paired values. No significant difference was found (P > .05). (C) Apocynin decreases ploidy of MKs in BM culture. BM cells were cultured (+TPO) for 3 days with or without 200 μM apocynin. (Left) Cells were then labeled and analyzed as described above. (Right) Percentage of MKs undergoing S phase. Cell-cycling cells were determined as described above. Statistical analysis was applied with the use of the t test for paired values. No significant difference was found (P > .05).
Nox inhibitors inhibit MK ploidy. (A) Nox inhibitor, DPI, decreases ROS production in MKs. The histogram depicts general ROS level detected by H2DCF-DA in CD41-PE–positive cells of cultured BM, as described in “Methods.” DPI was added as indicated, and H2O2 was added as a positive control, all as described in “Methods.” (B) DPI decreases ploidy of MKs in BM culture. (Left) BM cells were cultured (+TPO) for 3 days with or without 0.2 μM DPI. Cells were then labeled with CD41-FITC antibody followed by PI staining before FACS analysis on a BD FACScan. (Right) Percentage of MKs undergoing S phase. Cell-cycling cells were determined by selectively gating the area between 2N and 4N DNA peaks, as shown in the histogram profiles. Data were analyzed with FlowJo software (TreeStar). Statistical analysis was applied with the use of the t test for paired values. No significant difference was found (P > .05). (C) Apocynin decreases ploidy of MKs in BM culture. BM cells were cultured (+TPO) for 3 days with or without 200 μM apocynin. (Left) Cells were then labeled and analyzed as described above. (Right) Percentage of MKs undergoing S phase. Cell-cycling cells were determined as described above. Statistical analysis was applied with the use of the t test for paired values. No significant difference was found (P > .05).
Low-dose DPI treatment decreases MK polyploidy
| Ploidy . | Control . | 0.2 μM DPI . | P . |
|---|---|---|---|
| 2n | 5.73 ± 1.642 | 10.027 ± 0.954 | .017 |
| 4n | 4.00 ± 0.516 | 9.25 ± 1.107 | < .001 |
| 8n | 6.22 ± 0.855 | 17.613 ± 2.938 | .003 |
| 16n | 26.077 ± 1.727 | 31.267 ± 1.407 | .016 |
| 32n | 30.307 ± 2.432 | 6.773 ± 1.329 | < .001 |
| 64n | 12.373 ± 1.183 | 0.567 ± 0.306 | < .001 |
| Ploidy . | Control . | 0.2 μM DPI . | P . |
|---|---|---|---|
| 2n | 5.73 ± 1.642 | 10.027 ± 0.954 | .017 |
| 4n | 4.00 ± 0.516 | 9.25 ± 1.107 | < .001 |
| 8n | 6.22 ± 0.855 | 17.613 ± 2.938 | .003 |
| 16n | 26.077 ± 1.727 | 31.267 ± 1.407 | .016 |
| 32n | 30.307 ± 2.432 | 6.773 ± 1.329 | < .001 |
| 64n | 12.373 ± 1.183 | 0.567 ± 0.306 | < .001 |
Summary of ploidy status of 0.2 μM DPI-treated MKs expressed as a percentage ± SD of total MKs collected (summary of 3 independent experiments). Subgroups were compared with the t test.
Nox inhibitor, apocynin, decreases MK polyploidy
| Ploidy . | Control . | 200 μM apocynin . | P . |
|---|---|---|---|
| 2n | 12.94 ± 2.84 | 14.39 ± 1.31 | .468 |
| 4n | 8.14 ± 1.13 | 8.56 ± 0.51 | .591 |
| 8n | 6.39 ± 0.15 | 13.95 ± 1.22 | < .001 |
| 16n | 18.80 ± 1.19 | 29.83 ± 1.02 | < .001 |
| 32n | 20.13 ± 1.67 | 12.72 ± 0.76 | .002 |
| 64n | 17.45 ± 2.61 | 1.24 ± 0.29 | < .001 |
| Ploidy . | Control . | 200 μM apocynin . | P . |
|---|---|---|---|
| 2n | 12.94 ± 2.84 | 14.39 ± 1.31 | .468 |
| 4n | 8.14 ± 1.13 | 8.56 ± 0.51 | .591 |
| 8n | 6.39 ± 0.15 | 13.95 ± 1.22 | < .001 |
| 16n | 18.80 ± 1.19 | 29.83 ± 1.02 | < .001 |
| 32n | 20.13 ± 1.67 | 12.72 ± 0.76 | .002 |
| 64n | 17.45 ± 2.61 | 1.24 ± 0.29 | < .001 |
Summary of MK ploidy status in apocynin-treated BM cultures expressed as a percentage ± SD of total MKs collected (summary of 3 independent experiments). Subgroups were compared with the t test.
Levels of G1 phase cyclins in Nox-inhibited MKs
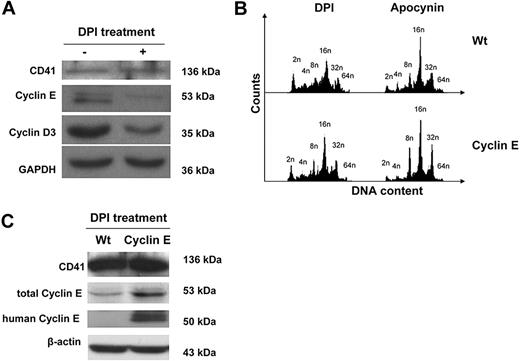
In search for mechanisms, we considered that during MK polyploidization, MKs undergo multiple rounds of an endomitotic cell cycle, the beginning of which is entrance into the G1 phase.42 Although many of the mechanisms of cyclins in G1 phase entrance and progression have been widely explored, it has more recently been shown that ROS, including those produced by Nox enzymes, are important for the expression of G1 phase cyclins.9,10,13,43 To determine whether ROS are involved in the control of G1 phase regulators in MKs, we analyzed expression of G1 cyclins D3 and E, which have been shown to be important for MK polyploidy.16,19,44,45 Fresh murine BM was cultured with TPO in the presence or absence of DPI, and MKs were then purified for protein analysis by Western blotting. Figure 6A indicates that treatment of MKs with DPI dramatically reduced levels of cyclin D3 and cyclin E. To further explore the mechanism by which NOX is implicated in the regulation of G1/S phase cyclins, we took advantage of a new cyclin E transgenic mouse model, in which the expression of the human cyclin E1 gene is targeted to MKs. Treatment of Wt and cyclin E transgenic MKs with DPI and apocynin showed that overexpression of cyclin E partially restored the ploidy profile of MKs that was affected by inhibition of Nox (Figure 6B). The fraction of 2N and 4N MKs decreased by 30.42% (± 2.82%; P < .01) and that of high-ploidy MKs (≥ 8N DNA content) increased by 29.69% (± 0.39%; P < .01) in DPI-treated cells with restored cyclin E, compared with the inhibitor alone. A similar trend was observed with apocynin treatment, including a 26.85% (± 17.85%) decrease in the 2N+4N MK fraction, and a 11.4% (± 1.17%; P < .01) increase in the 8N or greater MK fraction in cyclin E MKs compared with control MKs (P values were obtained based on t test applied to averages ± SDs; n = 3). Western blot analysis of DPI-treated MKs showed that the total levels of cyclin E are indeed up-regulated in the transgenic MKs compared with Wt MKs, further proving that cyclin E is responsible for the change in the ploidy profile (Figure 6C).
Effect of DPI on G1 phase cyclins and MK ploidy. (A) DPI decreases levels of G1 phase cyclins E and D3 in MKs. Wt BM was cultured (+TPO) for 3 days with or without 0.2 μM DPI. MKs were then purified from the cultures as in Figure 2, and lysates were subjected to Western blotting with the indicated antibodies. Data are representatives of 3 assays. An experiment performed with apocynin showed similar results (data not shown). (B) Expression of a cyclin E transgene partially restores the ploidy profile in Nox-inhibited MKs. Wt and cyclin E transgenic MKs were cultured (+TPO) for 3 days with 0.2 μM DPI or 200 μM apocynin. MKs were then subjected to ploidy analysis as described in “Methods” (data shown are representative of 3 experiments). (C) Cyclin E transgenic MKs have higher cyclin E levels compared with Wt on Nox inhibition. Wt and cyclin E transgenic MKs were cultured in the presence of 0.2 μM DPI and subjected to Western blot analysis with the indicated antibodies. Note that the levels of murine and human cyclin E are not comparable in this assay because they were probed with different antibodies. Similar increase in cyclin E levels in transgenic MKs compared with control was also observed in nontreated cells (not shown). Wt indicates wild-type MKs; cyclin E, cyclin E transgenic MKs. Data are representative of 3 assays.
Effect of DPI on G1 phase cyclins and MK ploidy. (A) DPI decreases levels of G1 phase cyclins E and D3 in MKs. Wt BM was cultured (+TPO) for 3 days with or without 0.2 μM DPI. MKs were then purified from the cultures as in Figure 2, and lysates were subjected to Western blotting with the indicated antibodies. Data are representatives of 3 assays. An experiment performed with apocynin showed similar results (data not shown). (B) Expression of a cyclin E transgene partially restores the ploidy profile in Nox-inhibited MKs. Wt and cyclin E transgenic MKs were cultured (+TPO) for 3 days with 0.2 μM DPI or 200 μM apocynin. MKs were then subjected to ploidy analysis as described in “Methods” (data shown are representative of 3 experiments). (C) Cyclin E transgenic MKs have higher cyclin E levels compared with Wt on Nox inhibition. Wt and cyclin E transgenic MKs were cultured in the presence of 0.2 μM DPI and subjected to Western blot analysis with the indicated antibodies. Note that the levels of murine and human cyclin E are not comparable in this assay because they were probed with different antibodies. Similar increase in cyclin E levels in transgenic MKs compared with control was also observed in nontreated cells (not shown). Wt indicates wild-type MKs; cyclin E, cyclin E transgenic MKs. Data are representative of 3 assays.
Discussion
ROS in BM cells were originally introduced with a rather notorious role of being harmful reactive molecules that were generated by neutrophils to kill invading pathogens. They have been recently redeemed by new studies that implicate their purposeful generation in the maintenance of particular redox environments or as second messengers in response to stimuli.46-48 In previous studies, Nox1 has been identified in hematopoietic stem cells,26 macrophages,49 as well as in the MO7e and B1647 erythro-megakaryocytic cell lines.22 Thus, although a few investigations have examined the role of ROS in MKs, they have been performed with megakaryocytic cell lines derived from leukemic BM.11,20-22,24 In this study we sought to examine the expression and activity of Nox in primary murine MKs. These investigations are the first to characterize the specific expression of Nox enzymes in MKs and their involvement in MK polyploidization.
Our initial transcript analysis indicated that Nox1 may be the most abundant Nox isoform in MKs and that Nox1 may also be up-regulated by the addition of TPO to these cultures. Nox4 levels were barely detectable. These results were further confirmed by Western blot analysis and by immunofluorescence that displayed high levels of Nox1 in TPO-treated MKs. Nox1 was localized largely in the cytosol in MKs. ROS levels of TPO-stimulated BM cultures were abrogated by Nox inhibitor, DPI, a selective inhibitor that covalently binds flavin-containing oxidases. Together, these results support the possibility that Nox1 may be the source of ROS and involved in mediating downstream signaling of TPO. The inducibility of ROS on TPO stimulation has been described in megakaryocytic cell lines, citing possible involvement of the MEK/ERK pathway and the PI3K/Akt pathways.11,50-52 It will be interesting to determine whether ROS are mediators of these pathways in the polyploidizing MKs.
Nox1-deficient mice displayed no differences in MK ploidy in comparison to Wt controls in both fresh and TPO-stimulated cultured BM. Analysis of commercially available Nox2 KO mice showed similar ploidy profiles in MKs at the basal level. In combination with our RT-PCR and Western blotting results for Nox2 in MKs, we conclude that Nox1 alone or Nox 2 alone is not crucial for MK polyploidization. The use of Nox inhibitors caused dramatic decreases in high-ploidy MKs. Further examination of the effect of Nox inhibitors on MK polyploidization showed that G1 cyclins D3 and E were down-regulated in contrast to vehicle-treated controls. This effect is also consistent with the proposed role of ROS in mediating the progression of G1-S phase, by regulation of expression of the G1 cyclin genes.10,12,13,43,53-56 In addition, up-regulation of cyclin E partially restores the ploidy levels in Nox-inhibited MKs, further supporting a role for Nox's in MK polyploidization by G1/S phase cyclin regulation. In summary, we report here the expression of Nox in MKs and its up-regulation in TPO-treated cells, as well as its importance for ploidy promotion, probably by the regulation of G1 phase cyclins.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Chihiro Yabe-Nishimura for his insight and provision of Nox1 knockout mice.
This work was supported by the National Heart, Lung, and Blood Institute (Bethesda, MD; grant HL80442; K.R.).
K.R. is an Established Investigator with the American Heart Association.
National Institutes of Health
Authorship
Contribution: D.J.M. designed and performed the experiments, interpreted the results, and contributed to writing the paper; A.E. designed and performed part of the experiments, interpreted the results, and contributed to writing the paper; M.M. performed part of the experiments; K.M. prepared the Nox1 knockout mouse samples and participated in related experiments; and K.R. designed the experiments, interpreted the results, oversaw the project, and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katya Ravid, Department of Biochemistry, K225, Boston University School of Medicine, 715 Albany St, Boston, MA 02118; e-mail: ravid@biochem.bumc.bu.edu.
References
Author notes
*D.J.M. and A.E. contributed equally to this paper.

![Figure 1. Expression of Nox mRNA in MKs. (A top) RT-PCR of Nox1 and Nox4 in magnetic bead–purified Wt MKs. RNA was prepared from purified MKs and BM fractions from cultured BM (+TPO; see purification image in Figure 2). Mouse VSMC cDNA was used as a positive control for detection of Nox1 (262 base pairs [bp]) and Nox4 (315 bp) transcripts. Omitting reverse transcriptase (RT) was used as a control for the PCR, whereas amplification of GAPDH was used as a control for RNA preparation (554 bp transcript). (A bottom) Determination of the purity of the MK-enriched fraction. RNA was prepared as described above. Amplifications of the neutrophil marker Ly6G and of macrophage/monocyte marker CD68 were used to exclude the possibility of contamination from different BM cell populations. Mac indicates macrophages. (B) Detection of Nox1 mRNA in the murine megakaryocytic Y10 cell line. RT-PCR was performed on Y10 cDNA from nontreated and TPO-treated (for 24 hours) cultures. VSMC cDNA was used as a positive control for detection of Nox1 and Nox4 transcripts. (C) Effect of TPO on Nox1 mRNA levels. Quantitative RT-PCR analysis was applied in RNA isolated form purified MKs after vehicle or TPO stimulation of the BM culture. 18s rRNA was used as an internal control, and Nox1 levels were normalized to CD41 expression levels (a MK marker). The graph represents the average of 3 independent experiments. Statistical significance (*P < .05) was determined using the Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/6/10.1182_blood-2008-12-195883/4/m_zh89990939500001.jpeg?Expires=1769873761&Signature=iAmBg~cPcugQNl6ZZp0uX9S7kaZrE8jbmX2~A~ZG6phdMHKED9txOL59TVarrVBLgN27Z4pCBgTwFhaHVKAtKHcudnf7UPBzfPkAsOKtUtJUtBDpj89xVs4k-pZwbOg-vQYQb4SWAMj5ex1LjEfyTTA5mUERBFckgNp45M4Kr18X4RZ6ujuXDTx5CCm6rac9~wx0Abw9dqy-YwQi32zBZR1IGW4D-5kTa5G1kgtarH-PpHAznLf1FUUG4CtSpApkJt1TMjQBs9ppNJC5NP0WAi0C0NYM4c7KLz3fHvFTU96DVOY8crmOWXRxQOKYZHxnG5HDb-Vfoaow5Crgw1LTsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal