Abstract
Hereditary thrombocythemia is a rare autosomal dominant disorder caused by mutations in either the thrombopoietin gene (TPO) or its receptor c-MPL. TPO mutations described so far lead to thrombopoietin overproduction through increased translation of m-RNA. Unilateral transverse reduction limb defects are usually sporadic and generally thought to be caused by vascular disruptions. Reports of inherited unilateral limb defects are extremely rare. In the present study, we describe a family with segregation of G185T TPO mutation in the 5′ UTR region in 4 subjects with thrombocythemia. Three of these patients also present congenital transverse limb defects. Association of these events gives a strong hint of the in vivo involvement of thrombopoietin in vasculogenesis, confirming the role of TPO in human development of the hemangioblast, the embryonic progenitor of the hematopoietic and endothelial lineages. This is the first report showing that vascular disruptions could be secondary to specific gene derangements.
Introduction
Hereditary thrombocythemia is a rare autosomal dominant heterogeneous disorder. Mutations have been identified within the thrombopoietin gene (TPO) in a few families.1-4 An activating mutation in the TPO receptor gene (c-MPL) has been identified in additional families.5,6 TPO and c-MPL mutations have been excluded in some families, suggesting involvement of at least one additional gene.7 All TPO germline mutations identified to date alter the 5′ untranslated region (UTR), removing the inhibitory upstream open reading frames thus leading to increased translation of the TPO m-RNA and overproduction of platelets.1-4,8
Limb defects are among the most common congenital anomalies in the human population, but most cases, especially unilateral transverse reduction defects, are usually sporadic.9 Disruptive vascular pathogenesis was recognized in some cases, as the result of an intrauterine vascular accident or of an ischemic process leading to interruption of blood supply to the developing structure during the fourth to sixth week of development.10 Rare cases of vertical transmission of unilateral limb defects have been reported,11 but it is not clear if this is the result of a monogenic disorder or of a multifactorial predisposition. A possible role of familial defects in anticoagulation has been hypothesized.12 Recently, a 2-year-old boy with hereditary thrombocythemia and skeletal anomalies was reported, but the molecular cause was not identified.13
Endothelial and hematopoietic cells have a common precursor, the hemangioblast, that was identified in cultures of mouse embryonic stem cells14 and later detected in mouse embryos.15 Abundant indirect evidence corroborates the existence of a human hemangioblast as well.16 Understanding of the molecular mechanisms that govern early development of hematopoietic and endothelial progenitors is limited, but TPO was identified as a critical factor for the proliferation of hemangioblasts.17,18
Methods
Analysis of TPO gene exons and flanking intronic sequences was performed by direct sequencing of DNA extracted from peripheral blood in the proband (primer sequences available on request). TPO gene variants were analyzed on DNA extracted from blood or chorionic villous from other family members.
Array-comparative genomic hybridization (CGH) was performed using a whole-genome microarray with a resolution of approximately 75 Kb. The test DNA and a sex-match reference DNA were double-digested and labeled with different fluorochromes. Finally they were hybridized on the same slide and the results were provided, after scanning, by 2 dedicated softwares, calculating the log ratio of fluorescence emissions and other statistical data.
All microarray data have been deposited at ArrayExpress under accession number E-MEXP-2214.19
All the family members involved in the study signed a specific informed consent in accordance with the Declaration of Helsinki, approved by the Ethical Review Board of Policlinico Sant'Orsola-Malphigi, stating their consent for scientific use of results.
The proband (II-2 in Figure 1) has a unilateral congenital transverse defect of his right upper (absence of forearm and hand) and right lower (absence of foot) limbs. The proband has a high platelet count (ranging from 650 to 800 × 109/L in the absence of therapy) and a bone marrow biopsy showed several megakaryocytes.
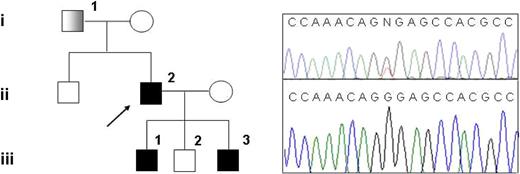
Family pedigree and mutation detection. (Left) Pedigree of the family with hereditary thrombocythemia; ■: thrombocythemia and unilateral limb defect;  : thrombocythemia only. (Right top) Sequencing chromatogram showing the G185T heterozygous mutation in subject III-1. (Right bottom) Sequencing chromatogram showing normal sequence in subject III-2.
: thrombocythemia only. (Right top) Sequencing chromatogram showing the G185T heterozygous mutation in subject III-1. (Right bottom) Sequencing chromatogram showing normal sequence in subject III-2.
Family pedigree and mutation detection. (Left) Pedigree of the family with hereditary thrombocythemia; ■: thrombocythemia and unilateral limb defect;  : thrombocythemia only. (Right top) Sequencing chromatogram showing the G185T heterozygous mutation in subject III-1. (Right bottom) Sequencing chromatogram showing normal sequence in subject III-2.
: thrombocythemia only. (Right top) Sequencing chromatogram showing the G185T heterozygous mutation in subject III-1. (Right bottom) Sequencing chromatogram showing normal sequence in subject III-2.
During his wife's first pregnancy, a first-trimester ultrasound scan revealed that the fetus was affected by a skeletal anomaly of the right foot. The mother did not report any substance abuse, did not have gestational diabetes, and underwent chorionic villus sampling at 12 weeks (cytogenetic analysis showed a normal male karyotype). After delivery, it was confirmed that the child (III-1) had a milder lower limb defect, with the upper limbs being spared (Figure 2A). Blood examination showed that the child also has thrombocythemia (> 1000 × 109/L) and TPO concentration was elevated in serum (1385 pg/mL).
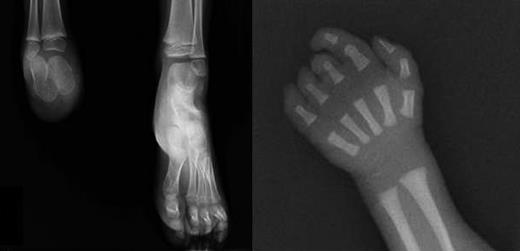
X-rays of limb defects in patients. (Left) Foot x-ray of subject III-1, showing absence of the right foot; the calcaneus and astragalus, that are absent in the father, are present although smaller than the controlateral ones. (Right) X-ray of right hand in subject III-3, showing absence of the last phalange of 2° digit and last 2 phalanges of 3° to 5° digits.
X-rays of limb defects in patients. (Left) Foot x-ray of subject III-1, showing absence of the right foot; the calcaneus and astragalus, that are absent in the father, are present although smaller than the controlateral ones. (Right) X-ray of right hand in subject III-3, showing absence of the last phalange of 2° digit and last 2 phalanges of 3° to 5° digits.
The couple had a second child, a boy with normal platelet count and no limb defects (III-2). During the third pregnancy, chorionic villus sampling was performed at 12 weeks of gestation for advanced maternal age and a unilateral foot defect was detected by ultrasound scan at the time. Cytogenetic analysis showed a normal male karyotype. After delivery, a unilateral digital defect was noted (Figure 2B) and the left foot defect was confirmed (III-3). The platelet count was normal at birth, but increased to more than 700 × 109/L 2 months after delivery.
The father's proband (I-1) is affected by thrombocythemia, with no limb defects on clinical examination, and he has 6 unaffected siblings.
Results and discussion
We detected a G185T heterozygous mutation (Figure 1) in the 5′ UTR exon 2, according to NCBI reference sequence NM_000460.2, in the proband's DNA. This mutation has already been described in a Japanese family (referred to as G516T), where it is associated with autosomal dominant isolated thrombocythemia and proven to cause TPO overproduction.3 The proband was also heterozygous for a common polymorphism in exon 6 (rs6141 in dbSNP), and a duplication in intron 4 (IVS4-18dupCTTC) that is not reported in public SNP databases. Analysis of blood or fetal tissue from other family members (parents and 3 children of the proband) showed that the G185T mutation segregates with disease, whereas the intronic duplication and the A allele of rs6141 are in trans with regard to the causative mutation. No abnormalities were identified on a workup for thrombophilic factors in subjects II-2 and III-1 (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Array-CGH analysis did not show any cryptic chromosome rearrangements in the proband.
To our knowledge, there are no previous reports of families with thrombocythemia associated with limb defects where a genetic cause was identified. In the family that we describe, 3 of 4 members carrying the TPO G185T mutation have a transverse limb defect. Subject I-1 has thrombocythemia but no limb defect: x-rays of hands and feet were not performed, but the results of the clinical examination were normal. None of his 6 siblings is reported to have thrombocythemia or limb defect.
One possible explanation is that I-1 has a de novo mutation in a mosaic form not causing a limb phenotype. No other tissues are available from the patient to investigate this hypothesis. On the other hand, absence of limb defects in 4 families with thrombocythemia due to TPO overproduction1-4 and variable severity of limb phenotype in our family suggest that TPO overproduction may be causative for the limb defect on a specific genetic background. This hypothesis would be further supported by the description of additional similar cases. In fact, a patient with hereditary thrombocythemia and skeletal defects was recently described, but TPO mutation screening was not performed, although TPO concentration was markedly elevated (2119.8 pg/mL).13 Possible associated factors, such as genetic predisposition to thrombophilia and cryptic chromosome rearrangements, were excluded in our family. A last possibility is de novo onset of a mutation in a gene unrelated to TPO in the proband, causing the limb phenotype, which segregates independently in his children. This is highly unlikely, because families with a definitive autosomal dominant phenotype of isolated limb reduction defects have not been described so far.
TPO was established to be present at the earliest stages of hematopoietic development in the yolk sac17 and was implicated as a critical physiologic regulator of the hemangioblast, being one of optimized hemangioblast growth factors in vitro, together with interleukin-6 and FGF2/heparin sulfate.18 Developmental biology of the early stages of human development is almost inaccessible to investigation, but strong clues to the identification of relevant factors can be obtained from human pathology. We report the first hint of TPO involvement in human disorders of vasculogenesis, suggesting that vascular disruptions leading to limb defects can be secondary to a specific gene derangement.
Families with hereditary thrombocythemia should be warned about the risk of congenital limb defects and, in case of pregnancy, an accurate prenatal scan should be performed to exclude this possibility. The same concern may apply to families with activating c-MPL mutations. The assessment of subjects affected by a transverse limb defect should include a platelet count and a follow-up program to exclude myeloproliferative disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kerry Rhoden and Dr Tommaso Pippucci (both in the Medical Genetics Unit, University of Bologna) for invaluable advice, and Dr Patrizia Noris (Clinica Medica III, Fondazione IRCCS Policlinico San Matteo), who performed TPO dosage. We are indebted to all members of the family we describe for their cooperation. M.S. is supported by Italian Ministry of Health, Strategic Program RFPS-4-631972 “Genetic Basis of Birth Defects.”
Authorship
Contribution: C.G. and M.S. initiated the study, counseled the family in every step of the analysis, interpreted results, and wrote the paper; S.C. and E.P. designed and performed the TPO analysis and interpreted the results; F.M. and A.P. performed clinical follow-up for every member of the family, and organized clinical and radiographic examinations; P.M. performed and interpreted array-CGH analysis; and G.R. was responsible for overview of the genetic analysis and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio Graziano, U. O. Genetica Medica, Policlinico Sant'Orsola-Malpighi, Università degli Studi di Bologna, Via Massarenti 9, 40138 Bologna, Italy; e-mail: claudio.graziano@unibo.it.