Abstract
Using different mouse monoclonal and human antiphospholipid (aPL) antibodies, we developed a new animal model of renal injury that shares many features with thrombotic microangiopathy (TMA). We found that more than 1 mechanism/signaling pathway is involved in glomerular injury induced by aPL antibodies in this model. Both complement-dependent and complement-independent pathways were identified that lead to glomerular endothelial cell damage and renal function impairment. We also found that C5a-C5aR interaction is a crucial step for the activation of the coagulation cascade and glomerular injury induced by complement-activating antibodies. In addition, our studies demonstrated complement-independent mechanisms in which reactivity with β2 glycoprotein I (β2GPI) plays an important role in aPL-induced glomerular damage and renal failure. Independently of the mechanism responsible for aPL-induced TMA, mice that express low levels of tissue factor (TF) were protected from glomerular injury. That genetic reduction of TF prevents renal injury induced by different aPL antibodies indicates that TF is a common mediator of glomerular damage and a possible target for selective pharmacologic intervention. Treatment with pravastatin, which down-regulates glomerular TF synthesis, prevents aPL-induced TMA in this mouse model, thus emphasizing that targeting TF might be a good therapeutic intervention in patients with TMA.
Introduction
Deposition of fibrin, endothelial injury, and thrombi formation within the renal microvasculature and consequent tissue ischemia are an important finding in the series of events resulting from thrombotic microangiopathy (TMA).1 Glomerular capillaries are especially vulnerable2 to endothelial injury and thombosis causing irreversible kidney damage with serious clinical implications, such as renal failure, hypertension, and its sequelae. TMA can be observed in a wide spectrum of disorders, including thrombotic thrombocytopenic purpura, hemolytic-uremic syndrome, malignant hypertension, postpartum renal failure, preeclampsia, and autoimmune diseases, including antiphospholipid syndrome (APS).2-5 Acute and/or chronic renal thrombotic manifestations are frequently observed in patients with antiphospholipid antibodies (aPLs).6 To date, therapy for the renal involvement in TMA is limited and shows little clinical effect.
Animal models of TMA are an invaluable tool to investigate the molecular and cellular events that lead to microvascular injury and thrombosis. A few animal models of TMA have been previously described.7-10 Most of these models require uninephrectomy and direct intraarterial injection of antibodies.7-9 Another model involves gamma-ray irradiation of the kidneys; however, signs of renal impairment in this model are not observed until 40 weeks of treatment.10
We developed a mouse model of TMA that has the advantage of using smaller amounts of antibodies and fast development of disease. This model closely reproduces the human pathophysiology of an early stage of TMA as it is induced by aPLs, frequently associated with renal vascular thrombosis, rather than using xenospecific antibodies. This model will allow us identify the molecular and cellular events that determine renal injury and identify targets for therapy.
aPL is a term that often encompasses distinct coexisting antibodies of multiple specificities, including lupus anticoagulant, anticardiolipin antibodies, and antibodies against β2-glycoprotein I (β2GPI; plasma phospholipids binding protein component of the macromolecular complex).11 Because aPL antibodies are a heterogeneous group of antibodies, we considered the possibility that more than one mechanism/signaling pathway may be involved in endothelial injury and activation of the coagulation cascade in the kidneys. Using this model of aPL-antibody induced TMA, we identified complement-dependent and complement-independent pathways that lead to endothelial injury and glomerular damage.
Tissue factor (TF) is the primary cellular initiator of blood coagulation and plays a key role in hemostasis. In view of previous studies showing that aberrant TF expression may be responsible for thrombotic disorders and fibrin deposition observed in many clinical conditions,12 we studied the role of TF in this model of TMA. We found that TF is a common mediator of glomerular injury induced by aPL antibodies that activate complement and by anti β2GPI aPL antibodies that do not activate complement. These data indicate that TF may be a possible target that offers potential opportunities for selective pharmacologic intervention in patients with TMA.
Methods
Mice
Adult C57BL/6 males (7-8 weeks old) were used for all experiments (The Jackson Laboratory). C5a receptor (C5aR)–deficient mice, generated by homologous recombination technology, were obtained from Dr Craig Gerard (Harvard Medical School).13 Low-TF mice were generated as described and express very low levels of human TF (hTF) from a transgene in the absence of murine TF(mTF−/−/hTF+).14 mTF+/−/hTF+ mice crossed with mTF+/−/hTF+ mice were used as controls. All animal studies were approved by the Institutional Animal Care and Use Committee of the Hospital for Special Surgery.
Preparation of antibodies for in vivo studies
Human IgG containing aPL antibodies (aPL-IgG) were obtained from 6 patients with APS with high titers of aPL-IgG antibodies (> 80 GPL units) identified through the Autoimmune Registry and Repository of the Rheumatic Disease Service at the Hospital for Special Surgery. The Institutional Review Board of the Hospital for Special Surgery approved the collection and use of samples for research purposes. IgG was purified by affinity chromatography using Protein G-Sepharose chromatography columns (GE Healthcare). Control human IgG from a healthy nonautoimmune person was purified by an identical method. Human antibodies were analyzed by immunoassay for reactivity against cardiolipin using the anticardiolipin antibody enzyme-linked immunosorbent assay (ELISA) test kit (Louisville APL Diagnostics) and β2GPI using an ELISA kit from INOVA Diagnostics.
Mouse monoclonal autoantibodies FB1, FC1, and FD1 were developed from spleens of NZW3BXSBF1 mice, which spontaneously develop a systemic lupus erythematosus-like syndrome.15,16 The mouse monoclonal antibodies were purified from hybridoma supernatants using Protein G columns. Monoclonal antibodies FB1 and FD1 are both anticardiolipin antibodies, and they do not bind to β2GPI (Table 1). FC1 does not bind to cardiolipin and binds to β2GPI (Table 1). FB1, an IgG2b, activates complement and FD1 (IgG1) and FC1 (IgG1) do not (Table 1). All IgG samples were treated to deplete endotoxin with Centriprep ultrafiltration devices (Millipore) and determined to be free of endotoxin contamination by the limulus amebocyte lysate assay to a sensitivity of 0.06 EU/mL (Associates of Cape Cod).
Clinical and laboratory features of the 6 patients used as a source of human polyclonal aPL (aPL-IgG) and specificity of mouse monoclonal antibodies
| Patient no. . | Age, y/sex . | aCL (> 80 GPL) . | LA . | Anti-β2GPI . | Complement activation* . | Clinical features . |
|---|---|---|---|---|---|---|
| 1 | 57/M | + | + | − | + | LN |
| 2 | 25/M | + | − | − | + | DVT |
| 3 | 55/F | + | − | + | + | CVA Pregnancy loss |
| 4 | 26/F | + | + | + | + | DVT LN |
| 5 | 48/M | + | + | − | + | CVAs |
| 6 | 50/F | + | + | + | +/− | Hepatic, renal, cardiac infarction; renal TMA; pregnancy loss |
| Mouse mAbs | ||||||
| FB1 | + | − | − | + | ||
| FC1 | − | − | + | − | ||
| FD1 | + | − | − | − |
| Patient no. . | Age, y/sex . | aCL (> 80 GPL) . | LA . | Anti-β2GPI . | Complement activation* . | Clinical features . |
|---|---|---|---|---|---|---|
| 1 | 57/M | + | + | − | + | LN |
| 2 | 25/M | + | − | − | + | DVT |
| 3 | 55/F | + | − | + | + | CVA Pregnancy loss |
| 4 | 26/F | + | + | + | + | DVT LN |
| 5 | 48/M | + | + | − | + | CVAs |
| 6 | 50/F | + | + | + | +/− | Hepatic, renal, cardiac infarction; renal TMA; pregnancy loss |
| Mouse mAbs | ||||||
| FB1 | + | − | − | + | ||
| FC1 | − | − | + | − | ||
| FD1 | + | − | − | − |
LA indicates lupus anticoagulant; LN, lupus nephritis; DVT, deep vein thrombosis; CVAs, cerebrovascular accidents; +, positive; and −, negative.
+ indicates a number of C3b positive cells higher than 10%; +/−, 5% to 10% of the cells were C3b positive; and −, less than 5% of the cells were C3b positive.
Table 1 gives the relevant clinical and laboratory features of the 6 patients used as a source of aPL-IgG. The specificity of the mouse monoclonal antibodies used in these studies is also presented in Table 1. The capacity of human and mouse aPL antibodies to activate complement activation is also shown in Table 1. To assess C3 activation, human choriocarcinoma cells (BeWo cells) rich in phospholipids, were incubated with FB1, FC1, FD1, or mouse IgG (500 μg/mL) for 30 minutes at 37°C in the presence of 10% mouse serum. BeWo cells were also incubated with aPL-IgG (500 μg/mL) from the 6 different patients. C3b deposition on the cell surface was quantified by fluorescence-activated cell sorter analysis using a fluorescein isothiocyanate (FITC)–conjugated anti–mouse C3 antibody (Cappel ICN). If the number of C3b-positive cells was higher than 10% the sample was classified as positive. If 5% to 10% of the cells were C3b-positive the sample was classified as plus or minus, and if less than 5% of the cells were C3b-positive the sample was considered negative.
Mouse model of TMA: renal function studies and histologic studies
Wild-type mice and genetically modified mice were injected on day 0 with mouse monoclonal aPL antibodies (FB1, FC1, or FD1) or mouse IgG as control treatment (50 μg intravenously). A group of mice was treated with aPL-IgG antibodies from 6 patients with APS or normal human IgG from 5 healthy donors as control (1 mg, intravenously) A second dose of antibodies was administered 72 hours later. This schedule of administration was repeated for 3 weeks. Mice were killed after 1, 2, and 3 weeks. To study the role of statins on aPL-induced TMA, mice were treated with pravastatin (Sigma-Aldrich; 5 μg/mouse, intraperitoneally) 18 hours before aPL injections. Blood and urine samples were collected weekly to follow up kidney function (blood urea nitrogen [BUN], creatinine and albumin in urine determinations). BUN was measured using a direct colorimetric method (Berthelot reaction). Blood samples were collected into 3.8% sodium citrate at a ratio of 9:1, and plasma levels of thrombin-antithrombin III (TAT) complex were measured using a commercial ELISA kit (Enzygnost TAT; Dade Behring). Albumin-to-creatinine ratio (ACR) in random urine specimens (accepted alternative to 24-hour urine collections) were used to monitor kidney function. Urinary albumin was determined by ELISA (Albuwell M, Exocell). Creatinine in urine was quantified with the Creatinine Companion kit (Exocell), based on the Jaffé reaction of alkaline picrate with creatinine. On day 7, 14, and 21, the animals were killed. Kidneys were frozen or paraffin embedded for immunohistochemical analysis. C3 was detected with specific antibodies (Cappel ICN). Human or mouse aPL-Abs deposition in the kidneys was detected by immunohistochemistry using FITC-anti–human or anti–mouse IgG antibodies (eBioscience). Fibrin deposition was analyzed with polyclonal rabbit anti–human fibrinogen antibody (Dako North America). TF was determined with a rabbit anti–mouse TF antibody (generously provided by Wolfram Ruf, Scripps Institute). A group of kidneys were fixed in 2% paraformaldehye/2% glutaraldehyde in 0.1 M phosphate buffer for electron microscopy (EM). EM was evaluated in a semiquantitative way. Severity of the vascular lesions was graded in the involved glomeruli based on the extent of loss of fenestrations, endothelial swelling, and detachment of endothelial cells from the glomerular basement membrane. Glomerular vascular lesions involving less than 25% of the glomeruli were scored as 1, involving 25% to 50% of the glomeruli as 2, and involving more than 50% of the glomeruli as 3. A total of 25 glomeruli per mice were scored. These scores were obtained by independent study by 2 scientists, and the scoring of all tissues was conducted in a blind manner.
Glomeruli isolation
Glomeruli were isolated using a modified sieving method according to Misra.17 The capsules of the kidneys (10 kidneys/group) were removed, and each kidney was bisected longitudinally. The medulla was removed by macroscopic dissection, and the cortical tissue was minced and gently pushed through a 315-μm sieve. The suspension was then sieved stepwise through 280-μm, 200-μm, and 125-μm sieves. The glomeruli were recovered on a 63-μm sieve. Purity of the preparation was assessed by optical microscopy. Glomeruli preparations (< 5% tubular contamination) were used for TF mRNA expression analysis.
TF mRNA expression analysis
For real-time PCR, total RNA from glomeruli preparations was extracted using an RNeasy Mini kit (QIAGEN), and 1 μg total RNA was reverse-transcribed using a First Strand cDNA Synthesis kit (Fermentas). Relative quantification of gene expression was performed by real-time PCR using iQ SYBR-Green Supermix on the iCycler iQ thermal cycler (Bio-Rad) following the manufacturer's protocols. Primer sequences were as follows: mouse GAPDH, sense primer 5′-tggagaaacctgccaagtatg-3′, antisense primer 5′-gttgaagtcgcaggagacaac-3′; mouse TF, sense primer 5′-catggagacggagaccaact-3′, antisense primer 5′-ccatcttgttcaaactgctga-3′.18 Relative expression was normalized for levels of GAPDH. The generation of only the correct size amplification products was confirmed using agarose gel electrophoresis.
Image acquisition
Images were acquired using a Nikon Eclipse E400 microscope fitted with Plan Apo objective lenses (4×/0.75, 10×/0.75, 20×/0.75, and 40×/0.75) and a Nikon DXM 1200 digital camera with ACT-1 Version 2 image-acquisition software.
Results
Mouse monoclonal and human aPL antibodies bind to glomeruli
Male C57BL mice were injected with mouse monoclonal antibodies (FB1, FC1, and FD1) or mouse IgG as isotype control antibody. Another group of mice was injected with aPL-IgG isolated patients with APS or NH-IgG from healthy persons. Six different aPL-IgG samples and 5 NH-IgG samples were studied. Four hours after aPL antibody injections, animals were killed, and the kidneys were washed through cardiac perfusion and then frozen for immunohistochemical detection of IgG using FITC-conjugated antimouse or antihuman IgG. After in vivo administration, FB1, FC1, and FD1 antibodies bind to glomeruli (Figure 1Ai-iii). A significant amount of fluorescence was observed in glomeruli from mice treated with mouse monoclonal Abs FB1, FC1, and FD1. aPL-IgG antibodies isolated from 6 different patients also bind to glomeruli (Figure 1Av). Neither mouse IgG nor NH-IgG binds to glomeruli (Figure 1Aiv-vi). Human and mouse aPL antibodies bind robustly to placenta,19,20 but in nonpregnant mice they were only found in kidney tissue. The absence of other organ involvement is another advantage of this new mouse model of TMA.
FB1, FC1, and aPL-IgG induced glomerular histologic lesions characteristic of TMA. (A) Binding of antibody FB1 (i), FC1 (ii), FD1 (iii), mouse IgG (iv), aPL-IgG (v), and NH-IgG (vi) to renal glomeruli after in vivo administration. Note that all mouse aPL antibodies and human aPL antibodies bind to glomeruli. (B) Transmission electron micrographs of glomeruli (original magnification ×7000). Swelling of glomerular capillary loops endothelial cells (*) and loss of fenestrations (#) were observed in glomeruli from FB1- (i), FC1- (ii), and aPL-IgG–treated mice (iii). Note the numerous red blood cells (RC) trapped between swollen endothelial cells. No signs of endothelial injury, well-preserved fenestrations (—), and widely patent glomerular capillary lumina are observed in kidneys from mouse IgG- (iv) and NH-IgG-treated mice (v; control groups). Similar to kidneys from mouse IgG-treated mice, no signs of endothelial injury were observed in glomeruli from FD1-treated mice. In FD1-treated mice (vi), glomerular capillary lumina are patent, fenestrations are well preserved (—), and endothelial cells are not swollen. Six or 7 mice were studied in each experimental group. (C) Glomerular injury scores. EM was evaluated in a semiquantitative way. These scores were obtained by independent study by 2 scientists, and the scoring of all tissues was conducted in a blinded manner. FB1, FC1, and aPL indicate that IgG-treated mice showed the higher glomerular injury scores compared with control mice.
FB1, FC1, and aPL-IgG induced glomerular histologic lesions characteristic of TMA. (A) Binding of antibody FB1 (i), FC1 (ii), FD1 (iii), mouse IgG (iv), aPL-IgG (v), and NH-IgG (vi) to renal glomeruli after in vivo administration. Note that all mouse aPL antibodies and human aPL antibodies bind to glomeruli. (B) Transmission electron micrographs of glomeruli (original magnification ×7000). Swelling of glomerular capillary loops endothelial cells (*) and loss of fenestrations (#) were observed in glomeruli from FB1- (i), FC1- (ii), and aPL-IgG–treated mice (iii). Note the numerous red blood cells (RC) trapped between swollen endothelial cells. No signs of endothelial injury, well-preserved fenestrations (—), and widely patent glomerular capillary lumina are observed in kidneys from mouse IgG- (iv) and NH-IgG-treated mice (v; control groups). Similar to kidneys from mouse IgG-treated mice, no signs of endothelial injury were observed in glomeruli from FD1-treated mice. In FD1-treated mice (vi), glomerular capillary lumina are patent, fenestrations are well preserved (—), and endothelial cells are not swollen. Six or 7 mice were studied in each experimental group. (C) Glomerular injury scores. EM was evaluated in a semiquantitative way. These scores were obtained by independent study by 2 scientists, and the scoring of all tissues was conducted in a blinded manner. FB1, FC1, and aPL indicate that IgG-treated mice showed the higher glomerular injury scores compared with control mice.
FB1, FC1, and aPL-IgG induce glomerular injury in mice
EM analysis was performed to identify endothelial injury in aPL-treated mice. EM examination of renal glomerular capillaries in FB1-treated mice showed endothelial swelling with reduction of endothelial fenestrations (Figure 1B). Complete loss of endothelial fenestrations was also observed in some glomeruli in FB1-treated mice. Swollen glomerular endothelial cells (GECs) occupied a wide area of the capillaries lumina and clumping of red cells and platelet aggregation were also found occluding the capillary lumina in FB1-treated mice. Actual separation of the GECs from the GBM was also noted in some glomerular capillaries from FB1-treated mice. Neither subendothelial nor subepithelial deposits were detected by EM. No thickening of the GBM was observed in mice treated with FB1. Extensive glomerular injury was also observed in kidneys from FC1-treated mice, characterized by loss of fenestrations, GEC swelling, and clusters of red cells occluding the glomerular capillaries (Figure 1B). There was no evidence of renal arterial vessel injury in the kidneys of aPL-treated mice (FB1, FC1, or human polyclonal aPL) by routine light microscopy. However, increased luminal staining for fibrin was observed in only 3 of 34 kidneys in these mice, affecting approximately 20% to 30% of the small arteries and arterioles (data not shown).
No signs of GEC damage were observed in glomeruli from mice treated with mouse IgG, FD1, or NH-IgG (Figure 1B). EM analysis of glomeruli from these mice revealed normal configuration of glomerulus. The capillary lumina were not occluded, and there are no signs of endothelial injury. The endothelium is thin, and the fenestrations are abundant and well preserved. Glomerular injury, evaluated in a semiquantitative way considering the percentage of endothelial cells showing loss of fenestrations and endothelial swelling, the area of the capillaries lumens occupied by endothelial cells and detachment of endothelial cells from the glomerular basement membrane, was graded from 0 to 3+ (Figure 1C). Glomeruli from FB1-, FC1-, and aPL-IgG treated mice showed the highest scores (Figure 1C).
Increased fibrin deposition in glomeruli from FB1-, FC1-, and aPL-IgG–treated mice
Glomerular intracapillary fibrin thrombi can be difficult to detect by conventional hematoxylin and eosin staining, especially in the early stages of disease. To show the presence of fibrin deposits, we performed immunohistochemistry with specific antibodies against fibrin (Figure 2A). We found increased fibrin deposition in glomeruli from mice treated with FB1, FC1, and aPL-IgG antibodies as early as one week after treatment. Increased fibrin staining was also observed at 2 and 3 weeks. No increase in fibrin staining was observed in glomeruli from mice treated with FD1, mouse IgG, or NH-IgG (Figure 2A). That FD1 does not increase fibrin deposition suggests that direct binding of aPL antibodies is not sufficient to activate the coagulation cascade and induce fibrin formation.
Detection of fibrin, TF, and C3 in glomeruli from mouse aPL antibodies (FB1, FC1, and FD1) and human aPL antibodies (aPL-IgG). (A) Fibrin staining in glomeruli from mice treated with FB1, FC1, FD1, and aPL-IgG antibodies after 1, 2, and 3 weeks of treatment. Mouse IgG and NH-IgG were used as control antibodies. (B) Immunohistochemical studies performed in kidneys from FB1-, FC1-, and aPL-IgG–treated mice showed increased TF expression. Increased C3 deposition was only observed in mice treated with FB1 and aPL-IgG.
Detection of fibrin, TF, and C3 in glomeruli from mouse aPL antibodies (FB1, FC1, and FD1) and human aPL antibodies (aPL-IgG). (A) Fibrin staining in glomeruli from mice treated with FB1, FC1, FD1, and aPL-IgG antibodies after 1, 2, and 3 weeks of treatment. Mouse IgG and NH-IgG were used as control antibodies. (B) Immunohistochemical studies performed in kidneys from FB1-, FC1-, and aPL-IgG–treated mice showed increased TF expression. Increased C3 deposition was only observed in mice treated with FB1 and aPL-IgG.
Immunohistochemical analysis of kidneys from mice treated with FB1 showed increased TF staining and increased C3 deposition in the glomeruli (Figure 2B). Mice treated with FC1 also showed increased TF staining, but no C3 deposition was observed in glomeruli in these mice. Minimal TF and C3 staining were present in kidneys from mice treated with FD1 or mIgG (Figure 2B). Mice treated with human polyclonal aPL induced increased TF expression and C3 deposition in glomeruli.
It has been described that a priming proinflammatory factor is necessary to induce thrombosis in anti-β2GPI-treated mice.21 In our studies, GEC activation and fibrin formation by anti-β2GPI FC1 antibody or complement-activating aPL antibody FB1 did not require any priming stimulus. However, administration of a subclinical dose of bacterial lipopolysaccharide (LPS; 2 μg/g, intraperitoneally) as a proinflammatory priming stimulus along with the FC1 or FB1 antibody injection accelerated the induction of the disease. Mice treated with LPS and FB1 or FC1 developed TMA as early as one week after treatment (data not shown). The LPS-primed, aPL-treated mice group is parallel to human disease where viral and bacterial infections or other environmental stimuli can amplify inflammation and tissue injury in patients with circulating autoantibodies.
Abnormal renal function in FB1-, FC1-, and aPL-IgG–treated mice
FB1, FC1, and aPL-IgG induced an increase in ACR 3 weeks after treatment, compared with animals receiving FD1, mIgG, or NH-IgG (Figure 3A). The rise in ACR observed in these mice correlates with the loss of endothelial fenestrations in glomerular capillaries observed in these mice. Hematuria was negative in FB1-, FC1-, and aPL-IgG–treated mice.
Impaired renal function and increased TAT levels in mice treated with FB1, FC1, and aPL-IgG. (A) Urinary ACR. FB1, FC1, and aPL-IgG induced albuminuria compared with mIgG- and NH-IgG-treated mice. FD1-treated mice did not show increased ACR compared with mIgG. (B) BUN. BUN levels in FB1-, FC1-, and aPL-IgG -treated mice were higher than in mIgG- or NH-IgG-treated mice. Mice treated with FD1 did not show increased BUN levels compared with mIgG-treated mice. (C) TAT complex. TAT levels were increased in FB1-, FC1-, and aPL-IgG–treated mice compared with control mice (mIgG- and NH-IgG-treated mice). *Different from mIgG-treated mice (P < .01). #Different from NH-IgG-treated mice (P < .01).
Impaired renal function and increased TAT levels in mice treated with FB1, FC1, and aPL-IgG. (A) Urinary ACR. FB1, FC1, and aPL-IgG induced albuminuria compared with mIgG- and NH-IgG-treated mice. FD1-treated mice did not show increased ACR compared with mIgG. (B) BUN. BUN levels in FB1-, FC1-, and aPL-IgG -treated mice were higher than in mIgG- or NH-IgG-treated mice. Mice treated with FD1 did not show increased BUN levels compared with mIgG-treated mice. (C) TAT complex. TAT levels were increased in FB1-, FC1-, and aPL-IgG–treated mice compared with control mice (mIgG- and NH-IgG-treated mice). *Different from mIgG-treated mice (P < .01). #Different from NH-IgG-treated mice (P < .01).
Elevated BUN levels were found in FB1-, FC1-, and aPL-IgG–treated mice 3 weeks after treatment, indicating impaired renal function (Figure 3B). BUN levels within the normal range for mice with normal renal function were observed in FD1-, mIgG-, and NH-IgG-treated mice (Figure 3B).
High plasma levels of TAT complexes are frequently associated with thrombotic events. We observed increased levels of plasma TAT levels in FB1-, FC1-, and aPL-IgG–treated mice that developed features of TMA after 3 weeks of treatment (Figure 3C). These results correlate with the increased fibrin deposition and GEC damage observed in mice treated with FB1-, FC1-, and aPL-IgG–treated mice.
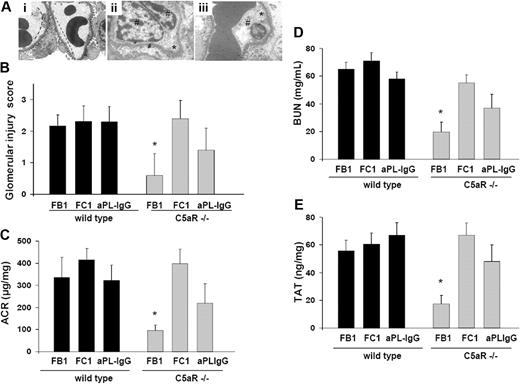
C5a-C5aR interaction is required for FB1-induced renal injury
Because C5a up-regulates procoagulant activities on the endothelial cells and on inflammatory cells (neutrophils and monocytes),22,23 we investigated the contribution of the C5a-C5aR interaction to renal injury induced by aPL antibodies. As shown by EM studies, glomerular injury was almost completely inhibited in C5aR-deficient mice treated with FB1 (Figure 4A-B). Glomerular injury scores in FB1-treated C5aR−/− mice were markedly lower than in wild-type mice treated with FB1 (Figure 4B). In addition, FB1-induced renal failure was ameliorated in C5aR−/− mice. BUN and ACR levels in FB1-treated C5aR−/− mice were not different from mouse IgG-treated wild-type mice (Figure 4C-D). TAT levels in C5aR−/− mice treated with FB1 were significantly lower than those observed in wild-type mice, indicating that C5aR is required for the prothrombotic phenotype observed in FB1-treated mice. Accordingly, less fibrin deposition was observed in glomeruli from FB1-treated mice (data not shown). These results indicate that complement-activating antibody FB1-induced glomerular injury requires C5a-C5aR interaction. On the other hand, C5aR genetic deletion resulted only in partial protection in aPL-IgG–treated mice. Renal failure and albuminuria were ameliorated in aPL-IgG C5aR-deficient mice compared with wild-type mice. However, these values were higher than control values (Figure 4C-D). Signs of endothelial injury were seen in glomerular vessels from aPL-IgG–treated C5aR mice (Figure 4A). These results can be explained by the fact that human aPL-IgG antibodies are a mixture of complement-activating antibodies and other antibodies that do not activate complement. Anti-β2GPI antibody FC1-induced renal failure and glomerular injury were not prevented by the genetic deletion of C5aR (Figure 4A-D). In addition, the absence of C5aR did not prevent FC1-induced TAT increase or fibrin deposition either (Figure 4E).
Genetic deletion of C5aR prevents glomerular injury and renal failure in FB1-treated mice. (A) Transmission electron micrograph of glomeruli from C5aR-deficient mice treated with FB1 (i), FC1 (ii), and aPL-IgG (iii). In C5aR−/− mice treated with FB1, no signs of glomerular endothelial injury were found. The capillary lumina were patent, the endothelial cells were normal in appearance, and the fenestrations were abundant and well preserved (—). However, C5aR deficiency did not prevent FC1- or aPL-IgG–induced endothelial injury. Glomerular capillary loops show endothelial cell swelling (#) and absence of fenestrations (*) in these mice (original magnification ×7000). Six or 7 mice were studied in each experimental group. (B) Glomerular injury scores. (C) Urinary ACR. FB1-induced, increased ACR was not observed in C5aR-deficient mice compared with wild-type mice. *Different from wild-type mice (P < .001). C5aR deficiency did not prevent increased ACR in FC1-treated mice. Albuminuria was ameliorated in C5aR−/− mice treated with aPL-IgG, but ACR values in these mice were still different from control values. (D) BUN. BUN levels in FB1-treated C5aR−/− mice were lower than in FB1-treated wild-type mice. *Different from wild-type (P < .005). C5aR−/− mice were not protected from FC1-induced BUN increase. A partial protection was observed in aPL-IgG–treated, C5aR-deficient mice. (E) TAT complex. TAT levels were diminished in FB1-treated C5aR−/− mice. *Different from wild-type (P < .01). FC1-treated C5aR−/− mice showed high TAT levels comparable with wild-type mice. aPL-treated C5aR−/− mice showed a slight diminution in TAT values compared with wild-type mice.
Genetic deletion of C5aR prevents glomerular injury and renal failure in FB1-treated mice. (A) Transmission electron micrograph of glomeruli from C5aR-deficient mice treated with FB1 (i), FC1 (ii), and aPL-IgG (iii). In C5aR−/− mice treated with FB1, no signs of glomerular endothelial injury were found. The capillary lumina were patent, the endothelial cells were normal in appearance, and the fenestrations were abundant and well preserved (—). However, C5aR deficiency did not prevent FC1- or aPL-IgG–induced endothelial injury. Glomerular capillary loops show endothelial cell swelling (#) and absence of fenestrations (*) in these mice (original magnification ×7000). Six or 7 mice were studied in each experimental group. (B) Glomerular injury scores. (C) Urinary ACR. FB1-induced, increased ACR was not observed in C5aR-deficient mice compared with wild-type mice. *Different from wild-type mice (P < .001). C5aR deficiency did not prevent increased ACR in FC1-treated mice. Albuminuria was ameliorated in C5aR−/− mice treated with aPL-IgG, but ACR values in these mice were still different from control values. (D) BUN. BUN levels in FB1-treated C5aR−/− mice were lower than in FB1-treated wild-type mice. *Different from wild-type (P < .005). C5aR−/− mice were not protected from FC1-induced BUN increase. A partial protection was observed in aPL-IgG–treated, C5aR-deficient mice. (E) TAT complex. TAT levels were diminished in FB1-treated C5aR−/− mice. *Different from wild-type (P < .01). FC1-treated C5aR−/− mice showed high TAT levels comparable with wild-type mice. aPL-treated C5aR−/− mice showed a slight diminution in TAT values compared with wild-type mice.
FB1, FC1, and aPL-IgG increase TF synthesis in glomeruli
Increased glomerular TF expression was observed in FB1- and FC1- and aPL-IgG–treated mice. To substantiate this finding, we measured TF synthesis in glomeruli from FB1-, FC1-, and aPL-IgG–treated mice. Notably, an 18-fold and a 21.1-fold increase in TF mRNA was observed in glomeruli from FB1- and aPL-IgG treated mice, respectively (Table 2). Increased TF synthesis was also found in glomeruli from mice treated with FC1 (16-fold increase in TF RNA; Table 2). TF synthesis in mIgG- and NH-IgG–treated mice was not different from untreated mice (data not shown). TF up-regulation in glomeruli was induced by complement-dependent (FB1) and complement-independent (FC1) mechanisms.
2−DDCT data analysis
| Description . | Average CT, TF . | Average CT, GAPDH . | DCT . | DDCT . | 2−DDCT . | Expression difference* . |
|---|---|---|---|---|---|---|
| Glomeruli from untreated mice | 31.5 | 20.3 | 11.2 ± 1.0 | 0 ± 0.65 | — | — |
| Glomeruli from FB1-treated mice | 28.3 | 21.3 | 7.0 ± 1.5 | −4.2 ± 0.6 | 18.4 ± 1.0 | ×18.4 (2 > 1)† |
| Glomeruli from FC1-treated mice | 28.7 | 21.5 | 7.2 ± 1.8 | −4 ± 1.0 | 16.0 ± 0.8 | ×16.0 (3 > 1)† |
| Glomeruli from aPL-IgG-treated mice | 27.9 | 22.1 | 6.8 ± 1.8 | −4.4 ± 1.2 | 21.1 ± 1.3 | ×21.1 (4 > 1)† |
| Description . | Average CT, TF . | Average CT, GAPDH . | DCT . | DDCT . | 2−DDCT . | Expression difference* . |
|---|---|---|---|---|---|---|
| Glomeruli from untreated mice | 31.5 | 20.3 | 11.2 ± 1.0 | 0 ± 0.65 | — | — |
| Glomeruli from FB1-treated mice | 28.3 | 21.3 | 7.0 ± 1.5 | −4.2 ± 0.6 | 18.4 ± 1.0 | ×18.4 (2 > 1)† |
| Glomeruli from FC1-treated mice | 28.7 | 21.5 | 7.2 ± 1.8 | −4 ± 1.0 | 16.0 ± 0.8 | ×16.0 (3 > 1)† |
| Glomeruli from aPL-IgG-treated mice | 27.9 | 22.1 | 6.8 ± 1.8 | −4.4 ± 1.2 | 21.1 ± 1.3 | ×21.1 (4 > 1)† |
Relative quantification of TF between glomeruli from FB1- and FC1-treated mice and glomeruli from untreated mice. The comparison was performed in pairs using the same target gene and the same glomeruli samples.
CT indicates threshold value; DCT, average target CT − average GAPDH CT; DDCT (for the same target gene), average DCT glomeruli from FB1- or FC1-treated mice − average DCT glomeruli from untreated mice (DDCT = 0 when the glomeruli suspension of interest is used as calibrator); —, not applicable; and 2−DDCT, normalized target gene amount (TF) relative to target gene amount in glomeruli from untreated mice.
P < .001; n = 4 to 8 mice/group.
Different from glomeruli from untreated mice (P < .001).
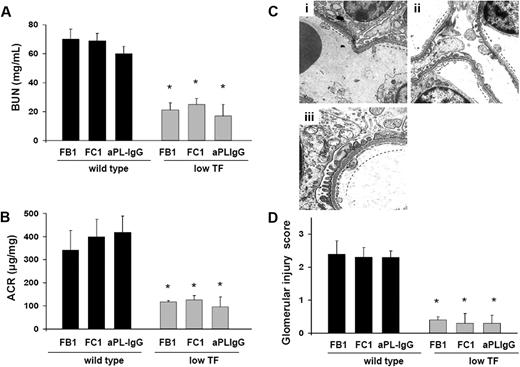
Low TF expressing mice are protected from FB1-, aPL-IgG–, and FC1-induced TMA
To further analyze the importance of TF in aPL-induced renal injury, we studied renal function and renal histology in mice expressing low levels of TF (mTF−/−,hTF+ [low TF mice]).14 We found that low TF mice treated with FB1, FC1, or aPL-IgG were protected from renal failure compared with wild-type mice. BUN levels and ACR levels in FB1-, FC1-, or aPL-IgG–treated low TF mice were lower than in wild-type mice (Figure 5A-B). Serum t1/2 and peak levels of FB1, FC1, and aPL-IgG antibodies were comparable in low TF mice and wild-type mice (data not shown). These data exclude the possibility that differences in the binding of aPL antibodies to glomeruli in the 2 strains of mice account for differences in aPL-mediated renal injury. The reduction of TF activity to less than 1% of normal levels completely prevented glomerular endothelial damage induced by FB1, FC1, and aPL-IgG, as assessed by EM (Figure 5C). The high glomerular injury scores observed in FB1-, FC1-, and aPL-IgG–treated wild-type mice were not observed in low TF mice (Figure 5D). Patent capillary lumina, thin endothelium with few mild swollen endothelial cells having abundant and well-preserved fenestrations were observed in FB1-, FC1-, and aPL-IgG–treated low TF mice (Figure 5C). The reduction in renal injury observed in low TF mice treated with FB1, FC1, and human aPL-IgG demonstrates that TF is a crucial effector in aPL-induced TMA.
Low TF expression prevents glomerular injury in mouse aPL antibody (FB1 and FC1)– and human aPL antibody (aPL-IgG)–treated mice. (A) BUN levels. Mice expressing low levels of TF were protected from BUN increase induced by FB1, FC1, and aPL-IgG antibodies. *Different from wild-type (P < .005). (B) Genetic diminution of TF prevented ACR increase in FB1-, FC1-, and aPL-IgG–treated mice. *Different from wild-type (P < .01). (C) Transmission electron micrograph of a glomerulus from low TF mice treated with FB1 (i), FC1 (ii), and aPL-IgG (iii). Low expression of TF prevented glomerular injury in these mice. Well-preserved endothelial cells with abundant fenestrations were observed (—). Six or 7 mice were studied in each experimental group. (D) Glomerular injury scores. FB1-, FC1-, and aPL-IgG–treated mice showed glomerular injury scores significantly lower than wild-type mice. *Different from wild-type mice (P < .005).
Low TF expression prevents glomerular injury in mouse aPL antibody (FB1 and FC1)– and human aPL antibody (aPL-IgG)–treated mice. (A) BUN levels. Mice expressing low levels of TF were protected from BUN increase induced by FB1, FC1, and aPL-IgG antibodies. *Different from wild-type (P < .005). (B) Genetic diminution of TF prevented ACR increase in FB1-, FC1-, and aPL-IgG–treated mice. *Different from wild-type (P < .01). (C) Transmission electron micrograph of a glomerulus from low TF mice treated with FB1 (i), FC1 (ii), and aPL-IgG (iii). Low expression of TF prevented glomerular injury in these mice. Well-preserved endothelial cells with abundant fenestrations were observed (—). Six or 7 mice were studied in each experimental group. (D) Glomerular injury scores. FB1-, FC1-, and aPL-IgG–treated mice showed glomerular injury scores significantly lower than wild-type mice. *Different from wild-type mice (P < .005).
Pravastatin prevents glomerular endothelial injury in FB1-, FC1-, and aPL-IgG–treated mice
Because TF expression is crucial to glomerular thrombosis in FB1-, FC1-, and aPL-IgG–treated mice and statins suppressed TF expression in various cell types, including blood monocytes in patients with nephritic syndrome24 and neutrophils from aPL-treated mice,25 we hypothesized that treatment with pravastatin might reduce aPL-induced TF synthesis and thus prevent glomerular injury and kidney failure. As expected, pravastatin down-regulated TF synthesis in glomeruli from FB1-, FC1-, and aPL-IgG–treated mice (2−DDCT: FB1 = 18.4 ± 1.0 vs FB1 + pravastatin = 1.5 ± 0.9, P < .001; FC1 = 16.0 ± 0.8 vs FC1 + pravastatin = 1.3 ± 0.8, P < .001; aPL-IgG = 21.1 ± 1.3 vs aPL-IgG + pravastatin = 1.2 ± 0.7, P < .001). In addition, pravastatin stabilized renal function as reflected by BUN and ACR levels. FB1 + pravastatin-, aPL-IgG + pravastatin-, and FC1 + pravastatin-treated mice showed BUN and ACR values not different from mIgG- or NH-IgG treated mice (Figure 6A-B). Down-regulation of TF by pravastatin correlated with the lower TAT levels observed in FB1 + pravastatin-, FC1 + pravastatin-, and aPL-IgG + pravastatin-treated mice compared with FB1-, FC1-, or aPL-IgG–treated mice (Figure 6C). These data are consistent with EM studies that showed no signs of TMA in kidneys from FB1-, FC1-, and aPL-IgG -treated mice that received pravastatin (Figure 6D). Glomerular injury scores in FB1 + pravastatin-, FC1 + pravastatin-, and aPL-IgG–treated mice were substantially lower than in FB1-, FD1-, or aPL-IgG–treated mice (Figure 6E). Glomerular capillaries were patent, and the endothelium was not swollen in mice treated with pravastatin and the different aPL antibodies. Fenestrations were well preserved in FB1-, FD1-, and aPL-IgG–treated mice that received pravastatin explaining the absence of albuminuria in these mice (Figure 6B).
Pravastatin prevents glomerular injury in FB1-, FC1-, and aPL-IgG–treated mice. (A) BUN levels. Mice treated with pravastatin exhibited a significant decrease in BUN levels compared with FB1-, FC1-, and aPL-IgG–treated mice, respectively. *P < .005. (B) Urinary ACR. Pravastatin treatment prevented the development of albuminuria in FB1-, FC1-, and aPL-IgG–treated mice. *P < .005. (C) TAT complex levels. FB1-, FC1-, and aPL-IgG–treated mice that received pravastatin treatment showed decreased TAT levels compared with mice did not receive pravastatin. *P < .001. Pravastatin diminished TAT values in FB1-treated mice. *Different from mIgG-treated mice (P < .001). (D) Transmission electron micrograph of glomeruli. Patent capillary lumina, thin endothelium with abundant and well-preserved fenestrations (—) is observed in FB1- (i), FC1- (ii), and aPL-IgG–treated mice (iii) that received pravastatin treatment. (E) Glomerular injury scores. Pravastatin treatment diminished glomerular injury scores in FB1-, FC1-, and aPL-IgG–treated mice. *P < .01.
Pravastatin prevents glomerular injury in FB1-, FC1-, and aPL-IgG–treated mice. (A) BUN levels. Mice treated with pravastatin exhibited a significant decrease in BUN levels compared with FB1-, FC1-, and aPL-IgG–treated mice, respectively. *P < .005. (B) Urinary ACR. Pravastatin treatment prevented the development of albuminuria in FB1-, FC1-, and aPL-IgG–treated mice. *P < .005. (C) TAT complex levels. FB1-, FC1-, and aPL-IgG–treated mice that received pravastatin treatment showed decreased TAT levels compared with mice did not receive pravastatin. *P < .001. Pravastatin diminished TAT values in FB1-treated mice. *Different from mIgG-treated mice (P < .001). (D) Transmission electron micrograph of glomeruli. Patent capillary lumina, thin endothelium with abundant and well-preserved fenestrations (—) is observed in FB1- (i), FC1- (ii), and aPL-IgG–treated mice (iii) that received pravastatin treatment. (E) Glomerular injury scores. Pravastatin treatment diminished glomerular injury scores in FB1-, FC1-, and aPL-IgG–treated mice. *P < .01.
Discussion
In the present work, we describe a mouse model for thrombotic microangiopathy induced by antiphopholipid antibodies with rapid development of renal morphologic features similar to those noted in humans. This model allowed us to study the mechanisms that lead to the development of endothelial injury and renal failure. Because aPLs are a heterogeneous group of antibodies, it was not surprising to find that different mechanisms were involved in kidney injury induced by these antibodies. We found complement-dependent and complement-independent mechanisms responsible for endothelial activation and renal failure in this model. Table 3 summarizes the effects of mouse aPL antibodies and human aPL antibodies on glomerular injury and renal function. FB1, FC1, and human aPL-IgG induced glomerular injury accompanied by albuminuria and decline in renal function indicated by rise in BUN. Glomerular findings were characterized by endothelial swelling with extensive loss of fenestrations and detachment from the glomerular basement membrane; and renal function abnormalities were characterized by increased BUN levels and appearance of albuminuria. Because complement-activating antibody FB1, and FC1, which does not activate complement, induce glomerular injury, we can conclude that multiple mechanisms are involved in the pathogenesis of aPL-induced TMA.
Effects of aPL antibodies and control antibodies on renal histology and function
| Ab . | ↑ glomerular deposition/expression . | EM . | Thrombin generation and renal function . | Amelioration of GEC injury and renal failure . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C3 . | Fibrin . | TF . | GEC damage . | ↑TAT . | ↑BUN . | ↑ACR . | C5aR−/− mice . | Low TF mice . | Pravastatin . | |
| FB1 (C′ activating Ab) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| FC1 (anti-B2GPI Ab) | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| FD1 | No | No | No | No | No | No | No | NA | NA | NA |
| aPL-IgG | Yes | Yes | Yes | No | Yes | Yes | Yes | Partial | Yes | Yes |
| NH-IgG | No | No | No | No | No | No | No | NA | NA | NA |
| Ab . | ↑ glomerular deposition/expression . | EM . | Thrombin generation and renal function . | Amelioration of GEC injury and renal failure . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C3 . | Fibrin . | TF . | GEC damage . | ↑TAT . | ↑BUN . | ↑ACR . | C5aR−/− mice . | Low TF mice . | Pravastatin . | |
| FB1 (C′ activating Ab) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| FC1 (anti-B2GPI Ab) | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| FD1 | No | No | No | No | No | No | No | NA | NA | NA |
| aPL-IgG | Yes | Yes | Yes | No | Yes | Yes | Yes | Partial | Yes | Yes |
| NH-IgG | No | No | No | No | No | No | No | NA | NA | NA |
Effects of FC1, FB1, FD1, aPL-IgG, and NH-IgG on glomerular C3 and fibrin deposition, TF expression and synthesis, plasma thrombin antithrombin III (TAT) levels, and renal function (BUN and ACR). The last 3 columns represent the effect of genetic deletion of C5aR, genetic diminution of TF, and pravastatin treatment on endothelial injury and renal failure.
C′ indicates complement; Ab, antibody; GEC, glomerular endothelial cell; and NA, not applicable.
Because FD1, which binds to glomeruli with equal affinity to FB1, did not activate the coagulation cascade and induce renal failure, we can conclude that direct binding of antibodies to cardiolipin is not sufficient to activate endothelial cells and induce thrombus formation.
The experiments performed in C5aR-deficient mice identified complement split product C5a as an important mediator of endothelial injury, glomerular damage, and renal thrombosis in FB1 and human aPL-IgG treated mice. C5aR-deficient mice were protected from FB1-induced renal failure and glomerular injury (Table 3). Studies performed in a rat model of thrombotic glomerulonephritis induced by antiglomerular basement membrane antibodies support our findings. Glomerular thrombosis in the rat model was also dependent on anaphylatoxin C5a.26 That C5a is required for aPL-induced thrombus formation was also demonstrated in an in vivo model where a standardized thrombogenic injury ensued in the femoral vein. In this model, aPL antibodies contributed toward a significant increase in thrombus size and endothelial cell activation that depends on the activation of C3 and C5.27 Moreover, C5aR-deficient mice did not show increased thrombus formation after aPL treatment.28 C5a can induce thrombosis by increasing TF expression on endothelial cells, monocytes, and neutrophils.29,30 Increased TF expression was observed in glomeruli from FB1-treated mice. Moreover, mice that express lower levels of TF were protected from FB1-induced TMA. We previously demonstrated that aPL antibody-induced complement activation and downstream signaling via C5a receptors in neutrophils in mice leads to the induction of TF.20 Ritis et al reported similar results in humans.31 On the other hand, TF expression on GECs as a consequence of C5a-C5aR interaction is another possible mechanism explaining the link between complement activation and TF in this model of TMA. Only partial protection from glomerular injury and renal failure was observed in C5aR-deficient mice injected with aPL-IgG. This can be explained by the fact that different antibodies (complement-activating and non–complement-activating) can be found in human aPL-IgG samples.
C5aR deficiency did not prevent renal injury in FC1-treated mice. This is not surprising considering that FC1 is an antibody that does not activate complement.
Antibodies reactive with β2GPI (FC1) induced glomerular endothelial injury in this study. The formation of β2GPI immune complex also appears to play a role in the development of arterial thrombosis in an animal model of photochemically induced vessel damage.32 Although this model was shown to be complement-independent (F(ab′)2 fragments were capable of inducing thrombosis), Fischetti et al showed that activation of coagulation by antibodies to β2GPI is complement-dependent and requires LPS as a priming factor.21 They identified the membrane attack complex (MAC) as a mediator of thrombosis in the mesenteric vessels.21 We did not see complement deposition in glomeruli from mice treated with β2GPI-dependent FC1 antibodies. In addition, C6-deficient mice that cannot assembly the MAC were not protected from FB1- or FC1-induced TMA (data not shown), suggesting that MAC formation is not required for glomerular thrombosis induced by FB1 or FC1. The results obtained in C5aR- and C6-deficient mice treated with FC1 indicate that complement activation does not play a role in glomerular injury induced by FC1 antibodies that react with β2GPI.
It was interesting to observe that genetic reduction of TF levels prevented TMA induced by both complement-activating antibodies (FB1) and non–complement-activating β2GPI reactive antibodies (FC1) in this study. These data indicate that TF is a common downstream mediator of both mechanisms. The source of TF responsible for glomerular thrombosis in this model can be the GECs or the inflammatory cells. The presence of infiltrating macrophages in glomeruli from FB1- and FC1-treated mice (data not shown) suggests that TF on inflammatory cells can be responsible for endothelial activation and thrombus formation. Many studies describe increased TF expression on monocytes in response to aPL antibodies.33-35 However, induction of TF on GECs can also contribute toward local activation of the coagulation cascade in glomerular capillaries leading to fibrin deposition. Anti-β2GPI antibodies can induce TF on endothelial cells by several mechanisms.36-38 Several in vitro studies have shown that antibodies directed against β2GPI induce the expression of TF on human umbilical vein endothelial cells and that p38 mitogen-activated protein kinase activation is required.37 In addition, it has been shown that anti-β2GPI aPL antibodies can dysregulate the fibrinolytic system by cross-linking with annexin 2 (profibrinolytic endothelial cell surface receptor) on the endothelial surface inducing increased expression of TF.38 As annexin 2 is not a transmembrane protein, this interaction may require an “adaptor” protein, which is able to transduce intracellular signaling. It was suggested that toll-like receptor 4 may be involved in β2GPI aPL antibody-induced thrombosis.39,40 Our results cannot entirely identify the source of TF but provide strong evidence that TF is a key mediator of glomerular injury in this model of aPL-induced TMA.
In conclusion, here we have described a new model for TMA in mice with renal morphologic features similar to those noted in humans. This model is induced by aPL antibodies, frequently associated with glomerular and microvascular injury and fibrin microthrombi. Using this model, we identified complement-dependent and complement-independent pathways that lead to GEC damage and renal function impairment. We also demonstrated that TF expression is a common mediator of glomerular damage in this model and a possible target that offers potential opportunities for selective pharmacologic intervention in patients with TMA. Considering the beneficial effects of pravastatin in our animal studies, we postulate that pravastatin may be a good therapy to prevent glomerular injury in patients with aPL antibodies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr B. Paul Morgan (School of Medicine, Cardiff University) for making C6-deficient mice available for our studies, Dr Craig Gerard (Children's Hospital, Harvard Medical School) for generously providing the C5aR deficient mice, and Dr Wolfram Ruf (Scripps Institute) for the anti-TF antibody.
This work was supported by grants from Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery (G.G.) and the Alliance for Lupus Research (G.G.).
Authorship
Contribution: S.V.S. evaluated electron microscopy; C.-W.F and P.R. conducted experiments; M.M. provided the mouse aPL antibodies and contributed to manuscript editing; N.M. provided the low TF mice and contributed to discussions; and G.G. conceptually designed, executed, and interpreted experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guillermina Girardi, Hospital for Special Surgery, 535 East 70th St, New York, NY 10021; e-mail: girardig@hss.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal