Tissue-type plasminogen activator (tPA) is an extracellular protease that converts plasminogen into plasmin. For tPA to generate plasmin under biologic conditions, a cofactor must first bring tPA and plasminogen into physical proximity. Fibrin provides this cofactor for tPA-mediated plasmin generation in blood. Despite being naturally devoid of fibrin(ogen), tPA-mediated plasmin formation also occurs in the brain. The fibrin-like cofactor(s) that facilitates plasmin formation in the injured brain has remained unknown. Here we show that protein aggregates formed during neuronal injury provide a macromolecular, nonfibrin cofactor that promotes tPA-mediated plasmin formation and subsequent cell breakdown. The binding of plasminogen and tPA to these protein aggregates occurs via distinct mechanisms. Importantly, nonneuronal cell types also exhibit this cofactor effect upon injury, indicating a general phenomenon. This novel cofactor identified in nonviable cells has ramifications for ischemic stroke where tPA is used clinically and where plasmin activity within the injured brain is unwanted. A means of selectively inhibiting the binding of tPA to nonviable cells while preserving its association with fibrin may be of benefit for the treatment of ischemic stroke.
Introduction
Tissue-type plasminogen activator (tPA) is a multidomain 70-kDa extracellular glycoprotein that converts inactive plasminogen into the broad-acting protease plasmin.1,2 For tPA to generate plasmin optimally in the blood, both tPA and plasminogen must first bind to fibrin.2,3 The physical colocalization of tPA and plasminogen on the fibrin surface significantly facilitates tPA-mediated plasmin generation and subsequent fibrinolysis.2,4 Hence, fibrin acts as both a cofactor for tPA-mediated plasmin formation and as a substrate for plasmin.3
In contrast to the blood, the brain is devoid of fibrin.5,–7 Nevertheless, tPA-mediated plasmin generation is also important for brain function and dysfunction.8,9 Indeed, tPA-mediated plasmin formation in the brain has been shown to be neurotoxic.10,,–13 For instance, it was shown that hippocampal neurons of tPA−/− and plasminogen−/− mice were resistant to excitotoxic cell death, whereas administration of exogenous tPA restored sensitivity to excitotoxic injury in tPA−/− but not plasminogen−/− mice.14,–16 Furthermore, fibrinogen−/− mice were found to be as vulnerable to intrahippocampal excitotoxicity as wild-type mice, although genetic ablation of plasminogen on either background conferred equal protection from excitotoxic injury.15 Hence, fibrin-independent tPA-mediated plasmin generation sensitizes neurons to excitotoxicity—an insult that contributes to infarction during stroke,17 traumatic brain injury,18 and numerous other neurodegenerative paradigms. Whereas β-amyloid and NG2 are likely cofactors for plasmin generation during Alzheimer disease19 and spinal cord injury,20 respectively, the “fibrin-like” cofactor that promotes tPA-mediated plasmin formation within the brain during stroke and other acute cerebral injuries remains entirely unknown.
To address this, we devised experiments to assess both the binding of tPA and plasminogen and the generation of plasmin on injured neurons in vitro and in vivo following a variety of insults including excitotoxicity and experimental stroke. We observed that tPA binds strongly, specifically, and with high capacity to protein aggregates that are formed by nonviable cells under these conditions. We also show that plasminogen strongly associates with nonviable cells, but in contrast to tPA, this binding is completely dependent upon kringle domain–mediated lysine binding. Most importantly, the assembly of tPA and plasminogen on nonviable cells results in the potent propagation of plasmin generation. Therefore, protein aggregates formed in nonviable cells act as a nonfibrin, macromolecular cofactor for tPA-mediated plasminogen activation. This cofactor activity was observed in numerous nonviable cells, irrespective of species, cell lineage, or type of injury. The ability of nonviable cells to accelerate tPA-mediated plasmin formation also leads to their own proteolytic degradation. We therefore propose that the cofactor activity of nonviable cells is a general physiological mechanism that contributes to the efficient removal of cellular debris. Inadvertently, however, the sudden generation of dead cells following acute brain injury creates an abundant source of protein aggregates that may facilitate excessive and deleterious plasmin formation. The cofactor activity of nonviable cells is of particular relevance to ischemic stroke where promotion of intravascular plasmin generation by tPA is beneficial, but where excessive plasmin generation within the injured brain parenchyma is deleterious. Our findings also have ramifications for other disease states where fibrin-independent tPA-mediated plasmin formation is of importance.
Methods
Animals
Male C57Black/6 mice (8-12 weeks) and Sprague-Dawley rats (12-14 weeks) were used. Experiments adhered to the National Health and Medical Research Council of Australia guidelines for live animal use and were approved by the Institutional Animal Ethics Committee of Monash University (no. E/2004/0321/M).
Materials
All reagents were from Invitrogen unless indicated otherwise. Recombinant human tPA used was Actilyse (Boehringer Ingelheim). Urokinase (uPA) was from Medac GmbH. Human plasminogen, human fibrinogen, and bovine thrombin were from Calbiochem. Human plasmin was from Hematologic Technologies Inc. Staurosporine was from Alexis Biochemicals. Congo red, avertin, aprotinin, 2-deoxy-d-glucose, thiazine red R (TRR), glutamate, N-methyl-d-aspartate (NMDA), and mannose were from Sigma. Tranexamic acid (TXA) was from Pfizer. S2251 was from Abacus Diagnostics. Reteplase was from Roche Products.
Dialysis and AlexaFluor633 conjugation
Actilyse and uPA were dialyzed (3.5 kDa cutoff Slide-A-Lyser; ThermoScientific) overnight against 3 changes of 0.35 M Hepes-NaOH (pH 7.4) at 4°C. To prepare tPA633, Actilyse was dialyzed overnight against 3 changes of phosphate-buffered saline (PBS) with 30% glycerol, adjusted to 2 g/L, and conjugated using the AlexaFluor633-conjugation kit according to the manufacturer's instructions. tPA633 was separated from free AlexaFluor633 by overnight dialysis against 3 changes of PBS with 30% glycerol. As a control, the buffer after dialysis was perfused over neuronal cultures; however, no binding of AlexaFluor633 to nonviable cells was seen (not shown). All material was 0.2 μm-filtered after dialysis and tested by Spectrofluor tPA hydrolysis (American Diagnostica) and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)/Coomassie staining (not shown). These tests show that dialysis and AlexaFluor633 conjugation caused no discernible change to proteolytic activity or electrophoretic mobility of tPA.
Neuronal culture
Neuronal cultures were prepared from E15-6 mice as described.21 Injury was inflicted upon cultures that had been maintained for 5 to 9 days in vitro. To elicit injury, media were replaced with Neurobasal media containing antioxidant-free B27, 0.5 mM l-glutamine, 50 U/mL penicillin/streptomycin. Staurosporine or glutamate was then added to a final concentration of 200 nM or 200 μM, respectively. To elicit oxygen-glucose deprivation (OGD), 2-deoxy-d-glucose was added to the media (10 mM) and cultures were placed in a sealed bag containing a wet Anaerocult A (Merck) sachet to deplete O2 to less than 1%.
THP-1 cell culture
Cells were maintained in RPMI media containing 10% heat-inactivated fetal calf serum, 10 mM Hepes-KOH (pH 7.4), 2 mM l-glutamine, 50 U/mL penicillin/streptomycin, 1 mM sodium pyruvate, and 55 μM 2-mercaptoethanol under humidified 5% CO2. Cultures were maintained at less than 106 cells/mL. To inflict injury, cells were centrifuged (900g, 5 minutes), washed in serum-free RPMI, and then incubated at 56°C for 30 minutes. Cells were again centrifuged and resuspended in serum-free RPMI at 106 cells/mL. Treatment at 56°C caused complete necrosis as determined by 7AAD uptake and by flow cytometric shifts in forward and side scatter (not shown).
In vivo excitotoxicity
Mice were anesthetized by 0.5-mL intraperitoneal injection of 1.875% avertin, and then placed in a stereotaxic frame. The left striatum was injected with 1 μL of 50 mM NMDA (coordinates: bregma +0.5 mm, mediolateral +2.0 mm, and dorsoventral −3.0 mm) at 0.2 μL/min, after which the needle was left for an additional 2 minutes to minimize fluid reflux. Twenty-four hours after injection, brains were removed and cut into 1-mm coronal slices using a Mouse Brain Blocker (Kopf). Slices were maintained overnight in serum-containing culture media with or without 100 to 400 nM tPA under humidified 5% CO2 conditions. The next day, slices were washed in serum-free media to remove loosely bound material and then fixed in 4% paraformaldehyde for several hours. Sections were equilibrated in 30% sucrose then frozen in isopentane. Coronal sections (25 μm each) were mounted onto slides and then subjected to immunofluorescence.
Immunofluorescence
Neuronal cultures on coverslips were submerged in 4% paraformaldehyde for 15 minutes. Cultures were then washed twice in Tris-balanced saline (TBS) and incubated in TBS plus 10% horse serum plus 0.1% Tx-100 for 1 hour. Cells were washed twice in TBS and incubated overnight in primary antibody (diluted 1:100 in TBS plus 4% horse serum plus 0.1% Tx-100). Cells were washed twice in TBS and incubated for 3 hours in the appropriate AlexaFluor-conjugated secondary antibody (diluted 1:1000 in TBS with 4% horse serum plus 0.1% Tx-100). Cells were washed twice in TBS and incubated for 20 minutes in 20 μg/mL 7AAD in TBS plus 0.1% Tx-100. Lastly, cells were washed twice in TBS, mounted in Prolong Gold medium, and imaged via confocal Z-stack analysis (microscope: Leica DM-IRBE; objective: PL APO, 63× magnification, 1.20 numeric aperture, water immersion; excitation: argon and HeNe lasers; detector: TCS SP photomultiplier tube; acquisition software: TCS Confocal Software Version 2.61; Image Processing Software: ImageJ Version 1.42q [National Institutes of Health]. Note that the micrographs in Figures 1, 3, and 5 of nonfixed cultures submerged in Hanks balanced salt solution were also obtained using this platform). The same immunofluorescence protocol was used for staining fixed tissue sections, with the exception that DAPI was used as a nuclear counterstain and sections were imaged on a different platform (microscope: Leica DM-RB; objective: Leica PL FLUOTAR, 40× magnification, 0.70 numeric aperture, air; excitation: Leica mercury burner; detector: Leica DC 300 digital camera; acquisition software: Leica IM50 Version 4.0; Image Processing Software: ImageJ Version 1.42q).
Immunoblotting
Samples were boiled in SDS-loading buffer with dithiothreitol, subjected to SDS-PAGE, and transferred onto polyvinylidene fluoride membranes. Membranes were probed with primary antibodies (goat anti-tPA [Santa Cruz Biotechnology], rabbit anti–poly ADP-ribose polymerase [PARP; Santa Cruz Biotechnology], sheep anti–plasminogen [Serotec], mouse anti–β tubulin [Sigma]) followed by the appropriate HRP-conjugated secondary antibody. Signals were revealed by chemiluminescence (ThermoScientific).
S2251 assay in neuronal cultures
Media of neuronal cultures were replaced with phenol red–free Neurobasal media containing l-glutamine and antibiotics. S2251 and tPA were then added to the media to a final concentration of 0.36 mM and 0 to 5 nM, respectively. Cultures were then incubated for 15 minutes at 37°C under humidified 5% CO2. Plasminogen was then added to a final concentration of 90 nM. At 15, 30, 45, 60, and 75 minutes after plasminogen addition, 50 μL aliquots of media were transferred to a 96-well plate containing 50 μL of 3 μM aprotinin in each well. Absorbance at λ = 405 nm was then measured using a fluorescence plate reader (BMG Fluostar). As previously described,22 second-order polynomial equation regression was applied to each “absorbance at λ = 405 nm versus time” curve using GraphPad Prism Version 4.03 software. The coefficient of each best-fit polynomial equation was taken as the initial rate of plasminogen activation.
S2251 assay in the presence of fibrin
Plasminogen activation assays on fibrin clots were performed as described.23 In brief, a 96-well plate was blocked with 0.1% Tween-20, 40 mM Tris-HCl, pH 7.4, and 75 mM NaCl for 2 hours at 37°C. Blocking solution was replaced with a mixture of fibrinogen and plasminogen (± TXA). Thrombin was added and the fibrin clots were polymerized for 1 hour in a humidified 37°C chamber (final concentrations: 2.65 g/L fibrinogen, 90 nM plasminogen, ± 1 mM TXA, 1 U/mL thrombin). Forty-microliter aliquots of 0 to 5 nM tPA and 0.36 mM S2251 were pipetted on top of the fibrin clots. Mineral oil was then placed on top of each well and changes in absorbance at λ = 405 nm over time were measured by a fluorescence plate reader. The initial rates of plasminogen activation were determined as described.22
Cell degradation assay
Injured (heat-treated) cells were incubated at room temperature for 15 minutes with the stipulated proteases and protease inhibitors. Cells were then washed twice, resuspended at 106 cells/mL in serum-free RPMI-1640 media, and incubated at room temperature. At increasing time points thereafter, THP-1 cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson) using forward scatter as a measure of cell size. Ten thousand events per sample per time point were assessed and presented as a percentage of mean cell size (relative to cell size at t = 0 hours).
Results
tPA binds to injured neurons in vitro
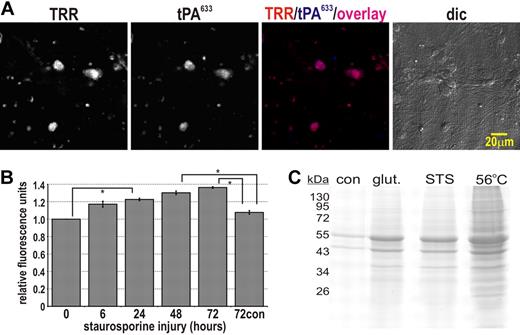
To determine whether new binding sites for tPA were created by neurons upon injury, we subjected neuronal cultures to oxygen glucose deprivation (OGD) or treated them with glutamate or staurosporine. Following injury, cultures were perfused with Alexa Fluor633-conjugated tPA (tPA633) and tPA633 binding was visualized by confocal microscopy (Figure 1A). The cell-impermeable DNA dye, 7AAD, was coperfused over these cultures to demarcate nonviable cells. As expected, exposure to OGD, glutamate, or staurosporine for 72 hours resulted in a marked increase in the number of 7AAD-positive nonviable cells (Figure 1A). These nonviable cells invariably bound tPA633, whereas the viable cells in cultures failed to bind tPA633 to any significant extent. Indicative of a loss of plasma and nuclear membrane integrity, tPA633 bound diffusely to both cytosolic and nuclear epitopes within nonviable cells. In addition, there appeared to be no dramatic differences in the binding of tPA633 to cells that had died from either glutamate-induced necrosis or staurosporine-induced apoptosis (Figure 1A). To confirm the binding of tPA to nonviable cells, we labeled all viable cells in neuronal culture with Oregon green 488 BAPTA-1AM. This experiment showed that the binding of tPA633 to nonviable 7AAD-positive cells was orders of magnitude above the binding of tPA633 to viable Oregon green–positive cells (not shown).
tPA binds to injured neurons in vitro. (A) Neuronal cultures were exposed to OGD, glutamate, or staurosporine for 72 hours. Cultures were then perfused with 10 μg/mL 7AAD and 10 nM tPA633 for 5 minutes and then imaged sequentially by confocal microscopy. Micrographs in the top row represent an overlay of 7AAD fluorescence (red) and tPA633 fluorescence (white). The bottom row of micrographs shows the corresponding bright-field images. Arrowheads indicate examples of necrotic cells. Arrows indicate examples of apoptotic cells. Appropriate “bleed-through” controls were conducted to ensure specific signal detection (not shown). (B-E) Neuronal cultures were treated with staurosporine (B,D) or glutamate (C,E). At increasing time intervals, cultures were incubated with 400 nM tPA for 15 minutes and washed, and then cellular proteins harvested and subjected to immunoblot analysis. (B-C) Representative immunoblots for tPA, full-length PARP, cleaved PARP (cl PARP), and GAPDH. A Coomassie stain of each membrane is shown as a loading control. “72con” refers to lysates from uninjured cultures at 72 hours. Note, endogenous tPA expression is low in our neuronal cultures and could not be detected by immunoblot analysis (not shown). (D-E) Densitometric quantitations of tPA-binding from 3 independent experiments (normalized for GAPDH levels). Data represent average ± SEM. *P < .05 by 1-way ANOVA with Neumann-Keuls correction.
tPA binds to injured neurons in vitro. (A) Neuronal cultures were exposed to OGD, glutamate, or staurosporine for 72 hours. Cultures were then perfused with 10 μg/mL 7AAD and 10 nM tPA633 for 5 minutes and then imaged sequentially by confocal microscopy. Micrographs in the top row represent an overlay of 7AAD fluorescence (red) and tPA633 fluorescence (white). The bottom row of micrographs shows the corresponding bright-field images. Arrowheads indicate examples of necrotic cells. Arrows indicate examples of apoptotic cells. Appropriate “bleed-through” controls were conducted to ensure specific signal detection (not shown). (B-E) Neuronal cultures were treated with staurosporine (B,D) or glutamate (C,E). At increasing time intervals, cultures were incubated with 400 nM tPA for 15 minutes and washed, and then cellular proteins harvested and subjected to immunoblot analysis. (B-C) Representative immunoblots for tPA, full-length PARP, cleaved PARP (cl PARP), and GAPDH. A Coomassie stain of each membrane is shown as a loading control. “72con” refers to lysates from uninjured cultures at 72 hours. Note, endogenous tPA expression is low in our neuronal cultures and could not be detected by immunoblot analysis (not shown). (D-E) Densitometric quantitations of tPA-binding from 3 independent experiments (normalized for GAPDH levels). Data represent average ± SEM. *P < .05 by 1-way ANOVA with Neumann-Keuls correction.
To further assess the binding of tPA to nonviable cells, neuronal cultures were again challenged with staurosporine or glutamate. At various time points after injury, cultures were then incubated with 400 nM tPA for 15 minutes and washed, and protein lysates were prepared and subjected to immunoblot analysis. Treatment of cultures with staurosporine caused immediate cleavage of PARP (generation of the 89-kDa PARP fragment indicates caspase-mediated apoptosis24 ). Continued exposure to staurosporine caused a steady increase in the abundance of the 89-kDa PARP fragment, a reciprocal decrease in the abundance of full-length PARP (Figure 1B-E), and a 30% reduction in culture viability (as measured by MTS conversion at 24 hours; not shown). By comparison, glutamate treatment caused an initial degree of PARP cleavage and a 45% reduction in culture viability (as measured by MTS conversion at 24 hours), but did not result in the appearance of the 89-kDa PARP fragment or a decrease in the abundance of full-length PARP. Hence, whereas staurosporine treatment elicited an apoptotic injury,25 glutamate exposure triggered a nonapoptotic, presumably necrotic injury in neuronal culture.26 Despite eliciting different types of injury, both staurosporine and glutamate treatment caused an immediate increase in tPA-binding, in line with our initial observation that tPA binds to nonviable neurons in culture (Figure 1C,E). In the case of staurosporine treatment, an approximately 4-fold rise in tPA-binding was evident after 6 hours of injury, with tPA-binding increasing to approximately 27-fold after 72 hours of injury (Figure 1C). These observations demonstrate that within hours of injury, cells acquire the capacity to bind tPA. Furthermore, tPA-binding increases with both the severity and duration of injury. Lastly, faster progressing injuries such as excitotoxicity or heat treatment (supplemental Figures 1,4, available on the Blood website; see the Supplemental Materials link at the top of the online article) lead to a more rapid onset of tPA-binding.
We also observed tPA-binding to nonviable cells in other primary cultures (rat hippocampal neurons and human brain endothelial cells) and in other human secondary cultures: SVG astrocytes, Raji B cells (supplemental Figure 1); Jurkat T cells (supplemental Figure 4); and HEK293 embryonic kidney cells (not shown). Therefore, tPA binds not only to injured neurons, but to various nonviable cell types regardless of species or lineage.
tPA binds to injured neurons ex vivo
To determine whether tPA binds to injured cells within intact brain tissue we injected the glutamate analog, NMDA, into the striatum of wild-type mice. Such excitotoxic challenge promotes fibrin-independent tPA-mediated plasmin formation that significantly contributes to infarction.10,15,16 Twenty-four hours after NMDA injection, coronal slices of the brain were prepared and incubated overnight in culture media containing 100 to 400 nM tPA. Slices were then washed, fixed, and subjected to anti-tPA immunofluorescence. As shown in Figure 2A-D, tPA bound abundantly to many cells within and around the injection site, but failed to bind to cells within the uninjured contralateral striatum. When assessing the DAPI counterstain of these sections, the foremost binding targets for tPA within the lesioned area were injured cells. These tPA-binding cells displayed a lack of nuclear material or had obvious signs of nuclear deterioration (Figure 2E-G; compare arrows and arrowheads), consistent with our in vitro observations that tPA binds to nonviable cells. Also in keeping with our in vitro results, tPA was found to bind to both cytosolic and nuclear epitopes within injured cells ex vivo (Figure 2E-G). Similarly, exogenous tPA was shown to bind to injured cells within the ischemic cortex of rats (supplemental Figure 2) and mice (supplemental Figure 3) following middle cerebral artery occlusion. Therefore, tPA binds to injured cells that arise both in culture and within intact tissues.
tPA binds to injured neurons ex vivo. NMDA was injected into the mouse striatum. Twenty-four hours later 1-mm coronal slices were prepared and incubated in 100 to 400 nM tPA overnight. Sections were then washed, fixed, and subjected to anti-tPA immunofluorescence (B,D,F) with a DAPI counterstain (A,C,E,G). A 40-μm scale bar is shown for panels A through D and a 20-μm scale bar, for panels E through G. (A,C) DAPI staining indicates a reduction in density of DAPI-positive cells following NMDA injection (compare ipsilateral [ipsi] with contralateral [contra] regions). (B,D) The contralateral striatum is devoid of tPA-binding cells, whereas numerous tPA-binding cells are present within the injured ipsilateral striatum. Panels E through G are the same section stained for tPA (F) and DAPI (E,G). Arrowheads point to healthy cells that do not bind tPA within the ipsilateral striatum. High- and low-exposure DAPI counterstaining reveals that these non–tPA-binding cells possess distinct brightly stained, regular-shaped nuclei and are therefore deemed viable. In contrast, arrows point to several tPA-binding cells that possess shrunken or irregular-shaped nuclei and are therefore considered injured. The binding of tPA to injured cells within the lesion was observed in experiments conducted on 3 separate mice. No comparable tPA-binding cells were found in sections that were incubated in the absence of recombinant tPA (not shown).
tPA binds to injured neurons ex vivo. NMDA was injected into the mouse striatum. Twenty-four hours later 1-mm coronal slices were prepared and incubated in 100 to 400 nM tPA overnight. Sections were then washed, fixed, and subjected to anti-tPA immunofluorescence (B,D,F) with a DAPI counterstain (A,C,E,G). A 40-μm scale bar is shown for panels A through D and a 20-μm scale bar, for panels E through G. (A,C) DAPI staining indicates a reduction in density of DAPI-positive cells following NMDA injection (compare ipsilateral [ipsi] with contralateral [contra] regions). (B,D) The contralateral striatum is devoid of tPA-binding cells, whereas numerous tPA-binding cells are present within the injured ipsilateral striatum. Panels E through G are the same section stained for tPA (F) and DAPI (E,G). Arrowheads point to healthy cells that do not bind tPA within the ipsilateral striatum. High- and low-exposure DAPI counterstaining reveals that these non–tPA-binding cells possess distinct brightly stained, regular-shaped nuclei and are therefore deemed viable. In contrast, arrows point to several tPA-binding cells that possess shrunken or irregular-shaped nuclei and are therefore considered injured. The binding of tPA to injured cells within the lesion was observed in experiments conducted on 3 separate mice. No comparable tPA-binding cells were found in sections that were incubated in the absence of recombinant tPA (not shown).
tPA binds to protein aggregates exposed by nonviable neurons
The binding of tPA to viable cells is usually via a low-density lipoprotein receptor or the mannose receptor,27 however, neither 500 nM RAP (a low-density lipoprotein receptor antagonist) nor 10 mM mannose (a mannose receptor competitor) inhibited the binding of tPA to injured neurons (not shown). Annexin A2, another prominent cellular binding partner for tPA, was excluded on the basis that tPA bound to nonviable annexin A2–deficient HEK293 cells2 (not shown). Thus, the binding of tPA to nonviable cells appeared not to involve a recognized cellular receptor. We then formed the alternate hypothesis that tPA was binding to intracellular protein aggregates that were formed and exposed during the cell death process. This hypothesis was based on 2 lines of evidence: first, cell death promotes the aggregation of intracellular proteins,28 and second, tPA can bind specifically to purified protein aggregates such as β-amyloid in vitro19,29,30 with a Kd of 0.5 to 9 nM.19 To test this theory, we perfused neuronal cultures with a mixture of tPA633 and thiazine red R (TRR)—a dye that binds specifically to protein aggregates.31 As shown in Figure 3A, near complete colocalization between the binding of TRR and tPA633 to nonviable cells was observed. Hence, tPA-binding spatially correlates with protein aggregates in culture.
tPA binds to aggregates proteins formed by injured neurons. (A) Neuronal cultures were perfused with 40 nM tPA633 and 0.01 g/L TRR and sequential confocal images taken. This experiment was reproduced across 3 independent cultures. (B) Staurosporine (200 nM final) was added to neuronal cultures at various time points after which the conditioned media were replaced with PBS containing 20 μM Congo red and fluorescence was determined by plate reader (λexcitation = 544, λemission = 590). Graph depicts the relative change in TRR fluorescence across 3 independent experiments. Data represent average ± SEM. *P < .05 by 1-way ANOVA with Neumann-Keuls correction. (C) Neuronal cultures were treated with 200 μM glutamate or 200 nM staurosporine for 24 hours (or incubated at 56°C for 0.5 hours as a positive control for cell death and protein aggregation). Cultures were then lysed in PBS containing 1% Triton-X 100. Aggregated proteins are well known to be detergent insoluble.28 The detergent-insoluble fraction was isolated from these lysates by centrifugation (16 000g, 20 minutes), solubilized in SDS-loading buffer with dithiothreitol, and subjected to 10% SDS-PAGE/Coomassie staining. Densitometry of the Coomassie-stained 40- to 80-kDa proteins demonstrates that aggregated proteins increase following injury in neuronal culture: 270% ± 10% for glutamate injury, 250% ± 10% for staurosporine injury, and 320% ± 20% for heat injury relative to uninjured “con” cultures. Data represent average ± SEM from 2 independent experiments performed in duplicate.
tPA binds to aggregates proteins formed by injured neurons. (A) Neuronal cultures were perfused with 40 nM tPA633 and 0.01 g/L TRR and sequential confocal images taken. This experiment was reproduced across 3 independent cultures. (B) Staurosporine (200 nM final) was added to neuronal cultures at various time points after which the conditioned media were replaced with PBS containing 20 μM Congo red and fluorescence was determined by plate reader (λexcitation = 544, λemission = 590). Graph depicts the relative change in TRR fluorescence across 3 independent experiments. Data represent average ± SEM. *P < .05 by 1-way ANOVA with Neumann-Keuls correction. (C) Neuronal cultures were treated with 200 μM glutamate or 200 nM staurosporine for 24 hours (or incubated at 56°C for 0.5 hours as a positive control for cell death and protein aggregation). Cultures were then lysed in PBS containing 1% Triton-X 100. Aggregated proteins are well known to be detergent insoluble.28 The detergent-insoluble fraction was isolated from these lysates by centrifugation (16 000g, 20 minutes), solubilized in SDS-loading buffer with dithiothreitol, and subjected to 10% SDS-PAGE/Coomassie staining. Densitometry of the Coomassie-stained 40- to 80-kDa proteins demonstrates that aggregated proteins increase following injury in neuronal culture: 270% ± 10% for glutamate injury, 250% ± 10% for staurosporine injury, and 320% ± 20% for heat injury relative to uninjured “con” cultures. Data represent average ± SEM from 2 independent experiments performed in duplicate.
Like TRR, Congo red is a dye that selectively binds to misfolded protein.19 An useful property of Congo red is that its fluorescence intensity increases when it binds to aggregated proteins.19 Accordingly, we performed a set of experiments whereby cultures were injured with staurosporine. After injury, Congo red was added to the cultures and global changes in fluorescence were quantitated. As shown in Figure 3B, Congo red fluorescence increased throughout the injury time course. Similarly, significant global changes in the fluorescence of TRR were also observed when bath-applied to neuronal cultures that had been injured with glutamate or staurosporine for 6 to 72 hours (n = 3; not shown).
To further support that protein aggregates were being formed under injury conditions, we again injured neuronal cultures with either glutamate or staurosporine for 24 hours. Cultures were then lysed in PBS containing 1% Triton-X 100 and the detergent-insoluble fractions were isolated and subjected to SDS-PAGE and Coomassie staining. As shown in Figure 3C, the abundance of detergent-insoluble protein aggregates in neuronal culture increased approximately 2.5-fold following a 24-hour injury with either glutamate or staurosporine. Therefore, protein aggregates do indeed form during staurosporine- or glutamate-induced injury. Moreover, the formation of these protein aggregates during injury progression correlated temporally with the enhancement of tPA-binding. Collectively, the spatial (Figure 3A) and temporal (Figures 3B and 1B) correlations between tPA-binding and TRR/Congo red fluorescence strongly suggest that tPA binds to intracellular protein aggregates that are formed and exposed during the cell death process.
tPA and plasminogen bind to nonviable cells via distinct mechanisms
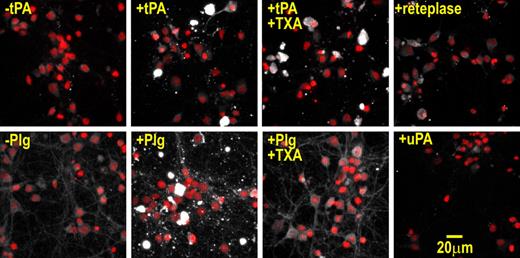
Previous studies have shown that plasminogen associates with injured cells both in vivo and in vitro.32,,–35 Consistent with these studies, we found that plasminogen bound to nonviable neurons (Figure 4). In contrast, immunofluorescence experiments revealed that urokinase (uPA) only very weakly associated with nonviable cells (Figure 4), which is in keeping with the inability of protein aggregates to facilitate uPA-mediated plasminogen activation.29,36 Hence, both tPA and plasminogen, but not uPA, bind to nonviable cells.
tPA and plasminogen bind to injured neurons via different mechanisms. Neuronal cultures were incubated in B27 protein–containing media supplemented with 400 nM tPA, 100 nM plasminogen, 10 mM TXA, or 400 nM reteplase or 800 nM uPA for 15 minutes. Cultures were then washed, fixed, and subjected to anti-tPA/anti-plasminogen/anti-uPA immunofluorescence. Samples were imaged by confocal Z-stack analysis. Shown are maximum projection micrographs where immunopositive signals (white) and nuclear counterstain signals (red) are overlaid. These results were reproduced across at least 3 independent experiments. Note, the immunopositive signals of nonviable cells are so intense that the nuclear counterstain of these cells is not seen in the overlay. In addition, no comparable immunopositive cells were found in sections that were incubated in the absence of tPA, plasminogen, or reteplase. Immunoblot analysis demonstrated that the anti-tPA antibody binds tPA and reteplase with comparable affinities (not shown). Binding to nonviable cells was still apparent when the concentration of tPA was lowered to 50 nM (not shown).
tPA and plasminogen bind to injured neurons via different mechanisms. Neuronal cultures were incubated in B27 protein–containing media supplemented with 400 nM tPA, 100 nM plasminogen, 10 mM TXA, or 400 nM reteplase or 800 nM uPA for 15 minutes. Cultures were then washed, fixed, and subjected to anti-tPA/anti-plasminogen/anti-uPA immunofluorescence. Samples were imaged by confocal Z-stack analysis. Shown are maximum projection micrographs where immunopositive signals (white) and nuclear counterstain signals (red) are overlaid. These results were reproduced across at least 3 independent experiments. Note, the immunopositive signals of nonviable cells are so intense that the nuclear counterstain of these cells is not seen in the overlay. In addition, no comparable immunopositive cells were found in sections that were incubated in the absence of tPA, plasminogen, or reteplase. Immunoblot analysis demonstrated that the anti-tPA antibody binds tPA and reteplase with comparable affinities (not shown). Binding to nonviable cells was still apparent when the concentration of tPA was lowered to 50 nM (not shown).
tPA consists of a finger domain (F), an epidermal growth factor–like domain (E), a non–lysine-binding kringle domain (K1), a lysine-binding kringle domain (K2), and a protease domain, whereas plasminogen is composed of 5 kringle domains and a protease domain. As both tPA and plasminogen harbor lysine-binding kringle domains, we determined whether tPA/plasminogen interact with nonviable cells via lysine residues. To test this, we used tranexamic acid (TXA), a free-lysine analog that competitively binds to kringle domains. Whereas TXA completely blocked the binding of plasminogen to dead cells, TXA only weakly interfered with tPA-binding (Figure 4). Thus, plasminogen binds to nonviable cells in a lysine-dependent manner, whereas tPA-binding is only weakly lysine-dependent. To extend these results we used the tPA variant, reteplase: a truncated tPA molecule that is composed of only the lysine-binding K2 domain and the protease domain. As shown in Figure 4, reteplase poorly bound to injured neurons, consistent with our earlier observation that TXA weakly inhibited the binding of tPA to nonviable cells. The reduced binding of reteplase suggests that the F, E, or K1 domains are largely responsible for the binding of tPA to nonviable cells.
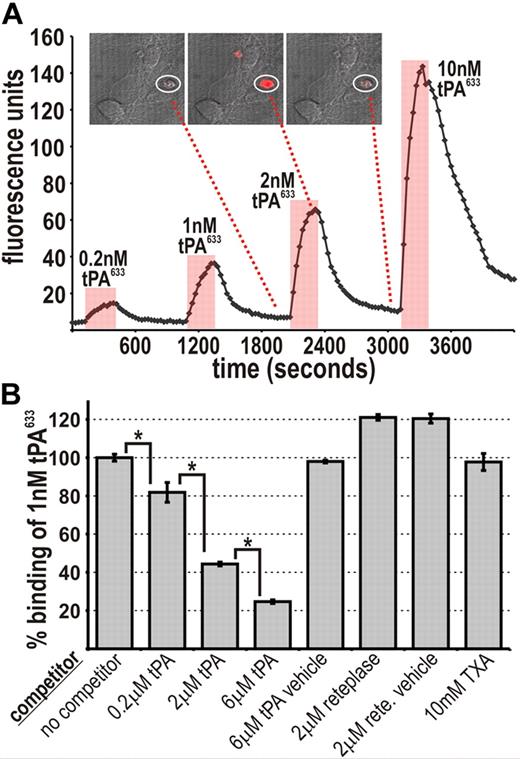
To further assess the mechanism of tPA-binding, tPA633 was transiently perfused at various concentrations over neuronal cultures and its binding to individual nonviable cells was visualized by confocal microscopy. tPA633-binding to injured neurons was dose dependent and slowly reversible and occurred at low-nanomolar concentrations (Figure 5A). Competition experiments were next performed by coperfusing tPA633 together with an excess of unlabeled tPA. As shown in Figure 5B, a 2000-fold molar excess of unlabeled tPA was required to reduce the binding of tPA633 by approximately 60%. Such competition at high molar excess suggests that the binding target for tPA633 is in tremendous abundance (supplemental Figure 4), consistent with the notion of macromolecular protein aggregates being present. A 2000-fold molar excess of reteplase failed to reduce tPA633-binding, further demonstrating that the binding of tPA to nonviable cells is specific. Similarly, a high concentration of TXA (10 mM) failed to inhibit the binding of tPA633 to nonviable cells (Figure 5B). These results support the conclusion that the binding of tPA to nonviable cells is specific, lysine-independent, and mediated by the F, E, or K1 domains.
tPA-binding to injured neurons is specific and occurs via the F, E, or K1 domains. (A) Increasing amounts of tPA633 were transiently perfused over neuronal cultures. Plotted is the binding of tPA633 to a single dead neuron over time (circled in the micrograph where tPA633 fluorescence is depicted in red). (B) tPA633 (1 nM) was perfused over neuronal cultures either alone (“no competitor”) or with various competitors. For a single experiment, the average relative amount of tPA633 binding was determined for all nonviable cells in a field. Shown are collated data from n = 3 to 7 independent experiments. Data represent average ± SEM. *P < .05 by 1-way ANOVA with Neumann-Keuls correction.
tPA-binding to injured neurons is specific and occurs via the F, E, or K1 domains. (A) Increasing amounts of tPA633 were transiently perfused over neuronal cultures. Plotted is the binding of tPA633 to a single dead neuron over time (circled in the micrograph where tPA633 fluorescence is depicted in red). (B) tPA633 (1 nM) was perfused over neuronal cultures either alone (“no competitor”) or with various competitors. For a single experiment, the average relative amount of tPA633 binding was determined for all nonviable cells in a field. Shown are collated data from n = 3 to 7 independent experiments. Data represent average ± SEM. *P < .05 by 1-way ANOVA with Neumann-Keuls correction.
Nonviable cells promote tPA-mediated plasmin generation
The observation that both tPA and plasminogen bind to exposed intracellular protein aggregates led us to hypothesize that nonviable cells represent a nonfibrin cofactor for tPA-mediated plasmin generation. Accordingly, we subjected neuronal cultures to OGD, after which exogenous tPA and/or plasminogen were added to the cultures and rates of plasmin formation were measured (Figure 6A). Application of exogenous plasminogen alone onto uninjured cultures resulted in a low rate of plasmin generation. By comparison, application of plasminogen alone onto injured cultures resulted in a 3.7-fold lower rate of plasmin generation, presumably because OGD injury caused a decrease in the levels of endogenous plasminogen activators.
Injured neurons act as a fibrin-like plasminogen-activating cofactor. (A) Neuronal cultures were subjected to OGD for 72 hours. After this, tPA, plasminogen, and S2251 (a plasmin-sensitive substrate) were added to the cultures and S2251 hydrolysis was monitored over time. The reaction velocities from 4 independent experiments were collated. Data represent average ± SEM. *P < .05 by Student t test. Note, no S2251 hydrolysis occurred when only exogenous tPA was bath-applied, suggesting a lack of endogenous plasminogen expression in neuronal culture. Also note, a limited amount of S2251 hydrolysis occurred when plasminogen was bath-applied alone, suggesting a low level of endogenous tPA expression in neuronal culture (which is significantly decreased by OGD injury). The ability of uninjured cultures to drive high rates of plasminogen activation upon bath application of both exogenous tPA and plasminogen reflects the existence of cofactor molecules (eg, annexin II37 ) on the surface of viable neurons and the unavoidable presence of a low number of nonviable cells. As OGD dramatically increased the number of nonviable cells, bath application of both tPA and plasminogen to injured cultures achieved even higher rates of plasmin generation. (B) Using the same concentrations of exogenous tPA, plasminogen, and S2251, the rates of plasmin generation in the presence of a preformed fibrin clot were determined. Inclusion of 1 mM TXA in the reaction inhibited the cofactor activity of fibrin and thereby significantly attenuated the rate of plasminogen activation. The reaction velocities from 3 independent experiments were collated. Data represent average ± SEM. *P < .05 by Student t test.
Injured neurons act as a fibrin-like plasminogen-activating cofactor. (A) Neuronal cultures were subjected to OGD for 72 hours. After this, tPA, plasminogen, and S2251 (a plasmin-sensitive substrate) were added to the cultures and S2251 hydrolysis was monitored over time. The reaction velocities from 4 independent experiments were collated. Data represent average ± SEM. *P < .05 by Student t test. Note, no S2251 hydrolysis occurred when only exogenous tPA was bath-applied, suggesting a lack of endogenous plasminogen expression in neuronal culture. Also note, a limited amount of S2251 hydrolysis occurred when plasminogen was bath-applied alone, suggesting a low level of endogenous tPA expression in neuronal culture (which is significantly decreased by OGD injury). The ability of uninjured cultures to drive high rates of plasminogen activation upon bath application of both exogenous tPA and plasminogen reflects the existence of cofactor molecules (eg, annexin II37 ) on the surface of viable neurons and the unavoidable presence of a low number of nonviable cells. As OGD dramatically increased the number of nonviable cells, bath application of both tPA and plasminogen to injured cultures achieved even higher rates of plasmin generation. (B) Using the same concentrations of exogenous tPA, plasminogen, and S2251, the rates of plasmin generation in the presence of a preformed fibrin clot were determined. Inclusion of 1 mM TXA in the reaction inhibited the cofactor activity of fibrin and thereby significantly attenuated the rate of plasminogen activation. The reaction velocities from 3 independent experiments were collated. Data represent average ± SEM. *P < .05 by Student t test.
As expected, coapplication of 1 to 5 nM tPA and plasminogen onto uninjured cultures produced significantly higher rates of plasmin generation that were constant across all tested t-PA concentrations, suggesting that 1 nM tPA was sufficient to saturate the lower number of cofactor sites in uninjured cultures. The coapplication of 1 to 5 nM tPA and plasminogen onto OGD-injured cultures, however, caused even higher rates of plasmin generation that increased dramatically as the concentration of exogenous tPA was increased. Thus, rates of plasminogen activation can be enhanced by increasing the number of dead cells and cofactor sites in neuronal cultures. These observations suggest that nonviable cells bring tPA and plasminogen into close physical proximity and thereby facilitate tPA-mediated plasmin generation in a manner akin to fibrin.
Consistent with this concept, the rates of plasminogen activation on fibrin (Figure 6B) were found to be comparable with those measured on neuronal cultures (Figure 6A). In addition, the classical antifibrinolytic agent, TXA, significantly lowered the rate of plasminogen activation in the presence of fibrin. Hence, rates of plasminogen activation can be either enhanced by increasing the number of cofactor sites (ie, increasing the number of dead cells; Figure 6A) or decreased by competitively inhibiting existing cofactor sites (ie, coincubation of TXA with fibrin; Figure 6B). Collectively, our findings intimate that injured neurons represent a biologically relevant “fibrin-like” cofactor that promotes tPA-mediated plasminogen activation.
Plasmin formation on nonviable cells leads to their proteolytic degradation
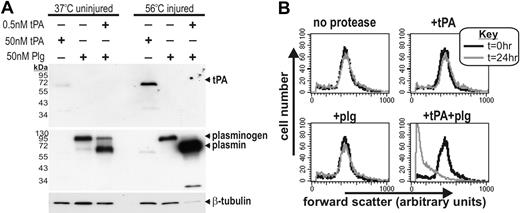
The cofactor ability of fibrin focuses plasmin generation to the microenvironment of thrombi and consequently promotes targeted fibrin degradation. By analogy, we postulated that the cofactor ability of nonviable cells may trigger their own proteolytic degradation. To test this, we used heat-injured THP-1 cells because (1) changes in the size of injured THP-1 cells could be easily and accurately monitored by flow cytometry, (2) like injured neurons, heat-treated THP-1 cells act as a cofactor for tPA-mediated plasmin generation (Figure 7A) and undergo rapid necrosis that coincides with the formation of protein aggregates (not shown). After heat treatment, nonviable THP-1 cells were incubated with tPA and/or plasminogen and cell size was monitored over time by flow cytometry. As shown in Figure 7B, nonviable THP-1 cells that were incubated with tPA plus plasminogen, but not with tPA or plasminogen alone, underwent a significant reduction in cell size. Comparable results were obtained when heat-treated THP-1 cells were incubated with recombinant plasmin (supplemental Figure 5A). Furthermore, either aprotinin (a plasmin inhibitor) or TXA fully ablated the ability of plasmin to degrade nonviable THP-1 cells (supplemental Figure 5B). Hence, the cofactor activity of nonviable cells promotes their plasmin-mediated proteolytic degradation.
The cofactor activity of injured cells leads to their proteolytic degradation. (A) tPA (50 nM), plasminogen (50 nM), or tPA (0.5 nM) + plasminogen (50 nM) were added to uninjured (37°C incubated) or injured (56°C incubated) THP-1 cells for 15 minutes. Cells were then washed and cellular proteins harvested and subjected to immunoblot analysis for tPA, plasmin(ogen), and β-tubulin. Although viable THP-1 cells are known to possess cell-surface plasminogen-activating cofactors,38 injuring these cells substantially increases both tPA-binding and the rate of plasminogen generation. This result corroborates the notion that dead cells are a potent plasminogen-activating cofactor. The increased binding of plasmin to nonviable cells is presumably due to a positive feedback loop where plasmin-mediated proteolysis of nonviable cells reveals more lysine residues (a phenomenon seen also on fibrin). In addition, plasmin activity leads to a loss of β-tubulin signal only in nonviable cells, presumably because the intact plasma membrane of viable cells prevents plasmin-mediated degradation of intracellular tubulin. (B) THP-1 cells treated at 56°C were incubated with 100 nM tPA, 100 nM plasminogen, or 1 nM tPA + 100 nM plasminogen for 15 minutes. Cells were then washed and incubated at room temperature. Cells were then assessed by flow cytometry 24 hours later, with forward scatter taken as a measure of cell size. Histograms show that only tPA + plasminogen–treated cells become significantly smaller, presumably as a result of proteolytic degradation (supplemental Figure 5).
The cofactor activity of injured cells leads to their proteolytic degradation. (A) tPA (50 nM), plasminogen (50 nM), or tPA (0.5 nM) + plasminogen (50 nM) were added to uninjured (37°C incubated) or injured (56°C incubated) THP-1 cells for 15 minutes. Cells were then washed and cellular proteins harvested and subjected to immunoblot analysis for tPA, plasmin(ogen), and β-tubulin. Although viable THP-1 cells are known to possess cell-surface plasminogen-activating cofactors,38 injuring these cells substantially increases both tPA-binding and the rate of plasminogen generation. This result corroborates the notion that dead cells are a potent plasminogen-activating cofactor. The increased binding of plasmin to nonviable cells is presumably due to a positive feedback loop where plasmin-mediated proteolysis of nonviable cells reveals more lysine residues (a phenomenon seen also on fibrin). In addition, plasmin activity leads to a loss of β-tubulin signal only in nonviable cells, presumably because the intact plasma membrane of viable cells prevents plasmin-mediated degradation of intracellular tubulin. (B) THP-1 cells treated at 56°C were incubated with 100 nM tPA, 100 nM plasminogen, or 1 nM tPA + 100 nM plasminogen for 15 minutes. Cells were then washed and incubated at room temperature. Cells were then assessed by flow cytometry 24 hours later, with forward scatter taken as a measure of cell size. Histograms show that only tPA + plasminogen–treated cells become significantly smaller, presumably as a result of proteolytic degradation (supplemental Figure 5).
Discussion
The identification of cofactors that facilitate tPA-mediated plasmin formation within the brain has been a long-standing question. In the uninjured brain, tPA-mediated plasmin formation occurs physiologically and participates in numerous synaptic plasticity paradigms.9,39,40 These physiological roles are presumably fibrin-independent as the uninjured brain is devoid of fibrin. In pathological states, tPA-dependent plasmin generation plays a major role in excitotoxicity.8 This pathological role for plasmin is also fibrin-independent as fibrinogen−/− mice and wild-type mice are equally susceptible to intrahippocampal excitotoxicity and because ablation of the plasminogen gene confers equal protection against excitotoxicity to fibrinogen−/− mice and wild-type mice.15 Hence, efficient tPA-mediated generation of plasmin during excitotoxicity occurs in the absence of fibrin. We therefore propose a novel model of fibrin-independent tPA-mediated plasminogen activation that occurs in the injured brain. This model is reliant on the formation of intracellular protein aggregates upon cellular injury.41 When exposed to the extracellular environment, these aggregates effectively substitute for fibrin by providing a macromolecular template for both tPA and plasminogen and facilitating plasmin formation. Our data also suggest that the aggregated proteins in nonviable cells become a substrate for plasmin. Although this process would provide a means for dead cell removal, excessive plasmin generation within the brain parenchyma is likely to contribute to neurotoxic cascades (supplemental Figure 6).
Previously only purified denatured proteins have been shown to act as a cofactor for tPA-mediated plasmin formation in vitro.29,30 Our study is the first to show that protein aggregates formed during the cell death process provide an endogenous cofactor for tPA-mediated plasminogen activation. It seems likely that the aggregates formed during cellular injury primarily contain misfolded, abundant, and ubiquitous cytoskeletal proteins (eg, actin) given that (1) tPA binds to all injured cell types, (2) tPA binds to both cytosolic and nuclear epitopes, and (3) it has been shown that actin readily aggregates during the cell death process.28
Irrespective of their composition, several observations strongly suggest that protein aggregates formed by nonviable cells represent a biologically relevant plasminogen activating cofactor. First, our in vivo experiments demonstrate that nonviable cells represent the foremost binding target for tPA within the injured brain. Second, the rates of tPA-mediated plasminogen activation achieved on injured neuronal cultures and fibrin clots are comparable (Figure 6). Third, prior studies have reported abundant and specific binding of endogenous plasminogen to nonviable human tissue. For instance, plasminogen is known to bind nonviable cells in amniochorionic membranes.32 Similarly, plasminogen binds to the necrotic core of colorectal cancers.33 Fourth, the cofactor ability of nonviable cells reconciles the seemingly contradictory observation that although no dramatic changes in tPA expression arise in the brain after stroke,42,,–45 tPA-mediated plasminogen activation increases markedly within the ischemic infarct.46,,–49
Our data showing that nonviable cells promote their own proteolytic degradation suggest that the plasminogen activator system participates in dead cell removal. Interestingly, molecular systems that promote the removal of debris from nonviable cells are known to mitigate autoimmunity. For instance, DNase1 mediates the removal of chromatin from nonviable cells and helps prevent the onset of systemic lupus erythematosus.50 Might the cofactor effect of nonviable cells promote the degradation of autoantigens and protect against autoimmunity? Already published data intimate that plasmin and DNase1 cooperatively participate in dead cell removal in vitro.51,–53 The existence of anti-tPA and antiplasminogen antibodies and altered fibrinolytic states in many autoimmune patients further connote that the cofactor ability of exposed protein aggregates mediates nonviable cell clearance and autoimmunity.54,,–57 It would be interesting to determine whether tPA−/− and plasminogen−/− mice are predisposed to autoimmune diseases.
Importantly, although our studies demonstrate the binding of tPA to nonviable cells during either in vivo or in vitro injury, tPA-binding was always more prominent in our cell culture experiments. This point presumably reflects the fact that phagocytes are able to remove nonviable cells in vivo, whereas the removal of nonviable cells in neuronal culture cannot occur. Future studies should monitor phagocytic clearance of protein aggregates within nonviable cells and how plasmin generation affects this process.
With respect to the mechanism of tPA-binding, our tPA633-competition experiments (Figure 5) performed at low concentrations of tPA indicated that the binding to nonviable cells was primarily lysine-independent and mediated by the F, E, or K1 domains. Curiously, immunoblot studies (not shown) performed using much higher concentrations of tPA showed that the binding of tPA to nonviable cells could be partially blocked by TXA, suggesting a lysine dependency under these conditions. The reason for these discrepancies is not clear, but likely reflects differences in experimental design (high vs low concentrations of tPA, protein-free vs protein-containing buffers, static application vs perfusion). A similar binding mechanism exists between tPA and fibrin, where it has been proposed that the F domain is more important at lower concentrations of tPA, and lysine binding is more important at higher concentration of tPA.58 Notably, the binding of tPA to purified nonfibrin protein aggregates also occurs via both lysine-dependent and lysine-independent mechanisms.19 It remains to be determined whether the binding of tPA to fibrin and to protein aggregates in nonviable cells occurs via a similar mechanism.
Our findings suggest that the presence of nonviable cells may confound past studies that assessed the binding of tPA and plasminogen to cells in culture. Our findings also imply that fibrin and protein aggregates formed in dead cells are competing plasminogen-activating cofactors in vivo. A means of specifically inhibiting the binding of tPA to nonviable cells, whilst leaving its association with fibrin untouched, is an intriguing and novel therapeutic possibility. Such an approach would be of particular benefit to ischemic stroke, where intravascular thrombolysis is beneficial and cerebral plasmin activity is harmful. Alternatively, molecules that inhibit protein aggregation (eg, curcumin) could be tested in stroke and other neurodegenerative scenarios where plasmin activity within the brain is influential. These inhibitors could potentially minimize protein aggregation during the cell death process, which in turn, may attenuate tPA-mediated plasmin generation within the injured brain.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Karl-Uwe Petersen (PAION GmbH) for providing reteplase, A/Prof Andrew Spencer for anti-PARP antibodies, Prof Dudley Strickland for providing RAP, and A/Prof David Howells for assistance with the rat stroke model.
This study was funded by grants awarded to R.L.M. from the National Health and Medical Research Council of Australia.
Authorship
Contribution: A.L.S. conceived and performed experiments, analyzed data, and wrote the paper; R.J.B. and B.N. undertook experiments and analyzed data; C.H.Y.W. and T.Y. undertook experiments; P.J.C. analyzed data; and R.L.M. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Medcalf, Australian Centre for Blood Diseases, Monash University, Level 6, Burnet Bldg, 89 Commercial Rd, Melbourne 3004, Victoria, Australia; e-mail: robert.medcalf@med.monash.edu.au.


![Figure 2. tPA binds to injured neurons ex vivo. NMDA was injected into the mouse striatum. Twenty-four hours later 1-mm coronal slices were prepared and incubated in 100 to 400 nM tPA overnight. Sections were then washed, fixed, and subjected to anti-tPA immunofluorescence (B,D,F) with a DAPI counterstain (A,C,E,G). A 40-μm scale bar is shown for panels A through D and a 20-μm scale bar, for panels E through G. (A,C) DAPI staining indicates a reduction in density of DAPI-positive cells following NMDA injection (compare ipsilateral [ipsi] with contralateral [contra] regions). (B,D) The contralateral striatum is devoid of tPA-binding cells, whereas numerous tPA-binding cells are present within the injured ipsilateral striatum. Panels E through G are the same section stained for tPA (F) and DAPI (E,G). Arrowheads point to healthy cells that do not bind tPA within the ipsilateral striatum. High- and low-exposure DAPI counterstaining reveals that these non–tPA-binding cells possess distinct brightly stained, regular-shaped nuclei and are therefore deemed viable. In contrast, arrows point to several tPA-binding cells that possess shrunken or irregular-shaped nuclei and are therefore considered injured. The binding of tPA to injured cells within the lesion was observed in experiments conducted on 3 separate mice. No comparable tPA-binding cells were found in sections that were incubated in the absence of recombinant tPA (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/9/10.1182_blood-2009-02-203448/5/m_zh89990941190002.jpeg?Expires=1767698330&Signature=ozag29dUFM29e1M4F8DSXohFBj4Cc6FMlu9s0OtFeK8uclX9UAeCbpdysgJB6vOw3nCitRBwwhcsVE8jIsYIYRgpnBodQmxdPoaWmjtxTcmiE9~YxuCt3IKYjllvfF6E3w7ew-8EloQOkMI408up9nXfHXLXBuy-46diYNCT8VA49CmR4JIUbCu1Q13s0blgW1hlevp2-yZ6sm50mGwxfeap6UcZ-GcncBQ5kmDgKuT44fbz~RGPa5C3a3Dn-nKN0haeyvdjKYoum1z1CkgxkoRGesfQvrpK800ZZ6d~XFdqV1Z-GErXn3jZG0An9pPa8aE3oa9faGwgv1rKEMh7Gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)