Abstract
Under normal physiologic conditions, necrotic cells resulting from tissue injury are rapidly removed from the circulation and tissues by phagocytes, thus preventing the exposure of intracellular antigenic and immunostimulatory molecules that can aid the development of autoimmune disease. Histidine-rich glycoprotein (HRG), a relatively abundant plasma glycoprotein, has a multidomain structure that can interact with many ligands including components of the fibrinolytic and immune systems. Recently, it has been reported that HRG can bind strongly to cytoplasmic ligand(s) exposed in necrotic cells to enhance clearance by phagocytes. Here we describe the molecular mechanisms underpinning this process. A complex consisting of both HRG and immunoglobulin G (IgG) was found as necessary to aid necrotic cell uptake by monocytes, predominantly via an FcγRI-dependent mechanism. The findings in this study also show that HRG can potentially interact with anionic phospholipids exposed in necrotic cells. Furthermore, the enhanced phagocytosis of necrotic cells induced by HRG-IgG complexes triggers phagocytes to release proinflammatory cytokines such as interleukin-8 and tumor necrosis factor. Thus, HRG has the unique property of complexing with IgG and facilitating a proinflammatory innate immune response to promote the clearance of necrotic cells.
Introduction
The immune system plays a vital role in distinguishing foreign pathogens/materials from healthy tissues to prevent the invasion of foreign substances. It is also important for the immune system to discriminate healthy viable cells from dying/dead cells during the course of development, tissue remodeling, and tissue injury to avoid the release of intracellular molecules from dying/dead cells that may damage neighboring cells. Professional phagocytes of the innate immune system use a broad range of germline-encoded pattern recognition receptors and opsonins to specifically detect pathogens/dying/dead cells and aid their removal via the process of phagocytosis.1,2
When viable cells undergo apoptosis in response to either extrinsic or intrinsic mediators, a precise set of morphologic and biochemical changes occurs.3 To achieve the efficient clearance of dying cells, apoptotic cells release “come-get-me” signals (eg, lysophosphatidylcholine) to recruit phagocytes to the site of cell death and expose a specific combination of “eat-me” and “don't-eat-me” signals to trigger phagocytosis.4 Typically, the loss of phospholipid asymmetry by the plasma membrane during the early stages of apoptosis can lead to the exposure of phosphatidylserine (PS), which can function as an “eat-me” signal to aid the uptake of apoptotic cells via a diverse range of phagocytic receptors and opsonins such as Tim-1 and -4, BAI1, and scavenger receptors, as well as the opsonin β2-glycoprotein I (β2GPI).4,5 However, when apoptotic cells persist due to an overload of dying cells and/or an impairment in phagocytosis, early apoptotic cells will progress into late apoptotic and secondary necrotic cells, which possess more permeable cell membranes.6 Similarly, generation of primary necrotic cells by extreme trauma can also result in the permeabilization of the cell membrane.7 The loss of membrane integrity exposes intracellular contents, which may function as additional “eat-me” signals to aid the removal of necrotic cells.8 Most importantly, unlike the recognition of pathogens, apoptotic cell removal is generally considered as nonimmunogenic and induces an anti-inflammatory response if the appropriate clearance mechanisms are present. In contrast, the uptake of necrotic cells is often associated with a proinflammatory response caused by the exposure of immunostimulatory molecules known as “danger” signals.8,9 Therefore, it is critical for the innate immune system to efficiently remove apoptotic and necrotic cells from the circulation and tissues to prevent the exposure of antigenic intracellular molecules as well as “danger” signals that can promote the development of autoimmune diseases such as systemic lupus erythematosus.8,9
While many of the intracellular “eat-me” signals of necrotic cells are not well characterized, a growing number of pattern recognition molecules (PRMs) such as mannose-binding lectin (MBL), Ficolin-2 and -3, C-reactive protein (CRP), serum amyloid protein, and components of the classical complement pathway have been implicated in the clearance of necrotic cells.10–16 In addition to these PRMs, histidine-rich glycoprotein (HRG), a member of the cystatin supergene family, has been demonstrated recently to play an important role in the phagocytosis of late apoptotic17 and necrotic cells.18
HRG is an abundant (∼ 75-kDa) multifunctional protein that is present in the plasma of many vertebrates.19 HRG has a multidomain structure that allows the molecule to interact with multiple ligands including haem, Zn2+, heparin, heparan sulfate (HS), plasminogen, fibrinogen, thrombospondin, immunoglobulin G (IgG), C1q, and Fcγ receptor (FcγR).19 The ability of HRG to interact with various ligands simultaneously has suggested that HRG can act as an adaptor molecule and regulate numerous biologic processes such as immune complex/permeabilized cell/pathogen clearance, angiogenesis, cell adhesion, coagulation, and fibrinolysis.19,20 In this study, the molecular components involved in HRG-mediated necrotic cell uptake have been characterized in detail, with HRG-IgG complex–mediated phagocytosis of necrotic cells shown to result in the release of proinflammatory cytokines by monocytes.
Methods
Reagents
An N-terminal domain–specific anti–human HRG mAb (HRG-4) was provided by AGEN. Mouse anti–human FcγRI mAb (10.1) was from eBioscience. Mouse anti–human FcγRIIA mAb (IV.3) and anti–human FcγRIIA mAb (8.26) were a gift from Dr Bruce Wines and Professor Mark Hogarth (Burnet Institute, Melbourne, Australia). Horseradish peroxidase (HRP)–conjugated sheep anti–mouse Ig, fluorescein isothiocyanate (FITC)–conjugated sheep F(ab′)2 anti–mouse Ig, and phycoerythrin (PE)–conjugated sheep F(ab′)2 anti–mouse Ig Abs were purchased from Chemicon. HS, hemin, ovalbumin (OVA), IgG1κ and IgG2κ myeloma proteins, normal human IgG, cytochalasin-D (Cyto-D), Triton X-100, cardiolipin, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich. DNase I was from Roche Diagnostics. Sulfatide, phosphatidylinositol (PtdIns), and PtdIns(4)P were purchased from Avanti.
Cell lines
Jurkat T, THP-1, and EL4 cell lines were cultured in RPMI-1640 medium (Invitrogen). MS cells were cultured in DMEM medium (Invitrogen) supplemented with 3.2μM methotrexate (Sigma-Aldrich). The glycosaminoglycan (GAG)–deficient Chinese hamster ovary (CHO) cell line (pgsA-745) was cultured in 50% DMEM and 50% Ham nutrient mixture F-12 (Invitrogen). All culture media were supplemented with 10% fetal calf serum, 5mM l-glutamine, and 30 μg/mL penicillin G, 50 μg/mL streptomycin sulfate, and 50 μg/mL neomycin sulfate. Cell lines were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Purification of primary human monocytes
CD14 MicroBeads (Miltenyi Biotec) were used for the positive selection of human monocytes from peripheral blood mononuclear cells according to the manufacturer's instructions.
Protein purification, conjugation, and analysis
Plasma-derived HRG (HRGP) was purified from human plasma according to a previously described method.21,22 EndoTrap red column (Profos AG) was used to remove traces of endotoxin present in HRGP according to the manufacturer's instructions. A HiTrap Protein G column (GE Healthcare) was used to affinity purify human IgG according to the manufacturer's instructions. Purified proteins were biotinylated using EZ-Link Sulfo-NHS-LC-Biotin (Pierce Biotechnology) as recommended by the manufacturer. Purified proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as previously described.21 Commassie Brilliant Blue (Sigma-Aldrich) and silver staining (Invitrogen) of gels were performed according to the manufacturer's instructions.
Quantification of human Ig heavy (H)– and light (L)–chain subclasses
A human IgG subclass profile enzyme-linked immunosorbent assay (ELISA) kit (Zymed Laboratories) was used to estimate the amount of different human Ig H-chain subclasses present in pooled normal human IgG and IgGHRG (the copurified IgG isolated from HRGP) based on instructions from the manufacturer.
The amount of κ and λ L-chains present in pooled normal human IgG and IgGHRG was estimated by densitometry analysis of Western blots, using an anti–human Ig κ-chain mAb (KP-53) and an anti–human Ig λ-chain mAb (HP-6054; Sigma-Aldrich), respectively. Human IgG4κ and IgG4λ myeloma proteins (Calbiochem) were used to construct a standard curve.
ELISA
ELISAs were performed according to a previously described method,21 with the exception that phospholipids were diluted in ethanol and incubated at room temperature for 16 hours to allow evaporation of the ethanol before blocking with PBS/3% BSA.
Lipid-coated membrane strip–based binding assay
HRG binding to PIP Strips and SphingoStrips (Echelon Biosciences) was performed according to the manufacturer's instructions. Membrane-bound HRG was detected by probing the membrane strips with an anti–human HRG mAb (HRG-4), followed by a sheep anti–mouse Ig-HRP Ab. Membrane-bound HRP was detected by chemiluminescence using enhanced chemiluminescence detection reagents (GE Healthcare).
Immunofluorescence flow cytometry
Viable and necrotic cells were analyzed for protein binding by immunofluorescence flow cytometry as previously described.18 Cell-bound HRG and IgG were detected using an anti–human HRG mAb (HRG-4) or rabbit anti–human IgG-PE (Sigma-Aldrich), respectively. Cells were resuspended in PBS/0.1% BSA containing 1 μg/mL Hoechst 33258 (Calbiochem) and immediately analyzed by flow cytometry using a LSR1 Flow Cytometer and CellQuest Pro software (BD Biosciences).
CLSM
Viable and necrotic cells were stained for HRG and IgG as described above for immunofluorescence flow cytometry. Cells were mounted onto glass microscope slides and imaged with confocal laser scanning microscopy (CLSM) using a Nikon Eclipse TE 300 confocal microscope with a Nikon Super High Pressure Mercury Lamp power supply (Nikon) and a Radiance 2000 Laser Scanning System (Bio-Rad).
Phagocytosis assay
Phagocytes (THP-1 cells and primary human monocytes) and Jurkat T cells were labeled with PKH26 (Sigma-Aldrich) and carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes), respectively, according to the manufacturer's instructions. CFSE-labeled Jurkat T cells were induced into necrosis by exposure to hyperthermic conditions (56°C, 30 minutes). The phagocytosis assay was performed immediately under serum-free conditions by incubating PKH26-labeled phagocytes with CFSE-labeled necrotic Jurkat T cells at a cell ratio of approximately 1:10 to 1:20. Samples were then incubated for 60 minutes at 37°C in a humidified atmosphere containing 5% CO2. Samples were immediately placed on ice and analyzed by flow cytometry using an LSR1 Flow Cytometer and CellQuest Pro software (BD Biosciences). Percentage of phagocytosis was determined as the percentage of PKH26-positive phagocytic cells that had ingested CFSE-positive necrotic Jurkat T cells.
Human inflammatory cytokine cytometric bead array (CBA) assay
Cytokine secretion by phagocytes was measured using a BD CBA human inflammation kit (BD Biosciences) according to the manufacturer's instructions. Samples were analyzed by flow cytometry using a LSR1 Flow Cytometer and FCAP Array Software (BD Biosciences).
Results
Human IgG2κ preferentially copurifies with HRGP
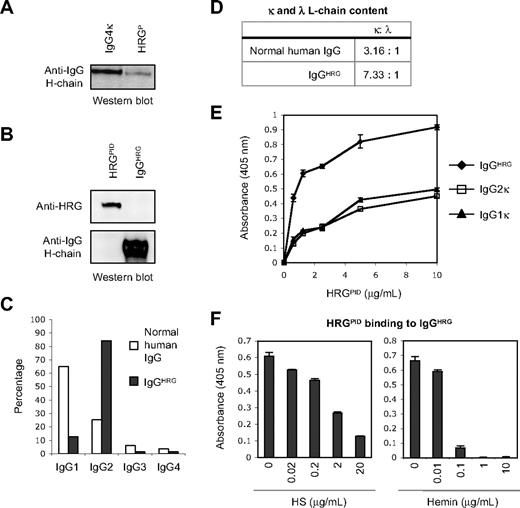
Human HRG has been shown to bind strongly to human IgG23,24 and immune complexes.24–26 Therefore, the purity of HRGP was first examined before experiments involving cells expressing FcγR. It was found by Western blotting that a relatively small amount of IgG was present in plasma-derived HRG (HRGP), which could be removed from HRGP via Protein G column absorption to yield IgG-depleted HRGP (HRGPID) preparations (Figure 1A-B). To further characterize the copurified IgG isolated from HRGP (termed IgGHRG), IgGHRG was eluted from the Protein G column and found to be essentially free of HRG (Figure 1B). The purity of HRGPID and IgGHRG was also analyzed by SDS-PAGE and Coomassie Brilliant Blue or silver staining as well as by Western blotting of biotinylated samples of each preparation (supplemental Figure 1, available online on the Blood website; see the Supplemental Materials link at the top of the online article). The amount of IgG present in HRGP was estimated to be approximately 1% to 4% of HRGP (wt/wt, 3 different preparations of HRGP isolated from different pooled plasma samples), based on densitometry analysis of Western blots for human IgG H-chain content (data not shown).
A specific subclass of IgG copurifies and interacts with human HRGP. (A) Western blot analysis of the IgG content of human HRGP (400 ng), with a human IgG4κ myeloma (20 ng) being included as a positive control. (B) Presence of HRG and IgG in preparations of HRGPID (0.5 μg) and copurified IgG isolated from HRGP (IgGHRG; 0.5 μg) as determined by Western blot analysis. (C) Analysis of the proportion of different IgG subclasses present in IgGHRG and pooled normal human IgG preparations as determined by human IgG subclass specific ELISA. (D) Comparison of the relative amount of κ and λ L-chains present in pooled normal human IgG and IgGHRG as determined by densitometry analysis of κ and λ L-chains on Western blots. (E) Analysis of the ability of HRGPID to bind to different immobilized IgG preparations by ELISA using wells precoated with 2 μg/mL IgGHRG, human IgG2κ or human IgG1κ myeloma proteins. (F) Effect of heparan sulfate (HS) and hemin on HRGPID binding to immobilized IgGHRG, as measured by ELISA, with wells being precoated with 2 μg/mL IgGHRG and then analyzed for HRGPID (2 μg/mL) binding under the different conditions. Error bars in panels E and F represent SEM (n = 3).
A specific subclass of IgG copurifies and interacts with human HRGP. (A) Western blot analysis of the IgG content of human HRGP (400 ng), with a human IgG4κ myeloma (20 ng) being included as a positive control. (B) Presence of HRG and IgG in preparations of HRGPID (0.5 μg) and copurified IgG isolated from HRGP (IgGHRG; 0.5 μg) as determined by Western blot analysis. (C) Analysis of the proportion of different IgG subclasses present in IgGHRG and pooled normal human IgG preparations as determined by human IgG subclass specific ELISA. (D) Comparison of the relative amount of κ and λ L-chains present in pooled normal human IgG and IgGHRG as determined by densitometry analysis of κ and λ L-chains on Western blots. (E) Analysis of the ability of HRGPID to bind to different immobilized IgG preparations by ELISA using wells precoated with 2 μg/mL IgGHRG, human IgG2κ or human IgG1κ myeloma proteins. (F) Effect of heparan sulfate (HS) and hemin on HRGPID binding to immobilized IgGHRG, as measured by ELISA, with wells being precoated with 2 μg/mL IgGHRG and then analyzed for HRGPID (2 μg/mL) binding under the different conditions. Error bars in panels E and F represent SEM (n = 3).
Because HRG was previously reported to bind preferentially to human IgG myeloma proteins containing specific H- and L-chain subclasses,23 the H- and L-chain composition of IgGHRG was further investigated. IgGHRG preparations were found to contain predominantly IgG2 (84.4%) and some IgG1 (12.7%), which is in striking contrast to the proportion of IgG subclasses present in pooled normal human IgG preparations in which IgG1 predominates (Figure 1C). Furthermore, IgGHRG preparations also had a higher proportion of κ L-chains to λ L-chains than normal human IgG (Figure 1D). Collectively, these results indicate that a specific subclass of IgG, namely IgG2κ, copurifies with HRGP, implying that the interaction between HRG and IgGHRG depends on the H- and L-chain composition of the IgG.
To examine further the interaction between HRG and the copurified IgG, direct binding assays were performed by ELISA. Interestingly, HRGPID preferentially bound more to IgGHRG than purified IgG2κ and IgG1κ myeloma proteins (Figure 1E). These results suggest that the interaction between HRGPID and IgGHRG may also depend on other factors such as the antigen binding properties or glycosylation state of IgGHRG, rather than simply the H- and L-chain composition of IgGHRG. Furthermore, the presence of HRG ligands, such as HS and hemin, markedly reduced HRGPID binding to IgGHRG (Figure 1F), suggesting that ligand binding can affect the interaction of HRGPID with IgGHRG.
HRG tethers IgG to necrotic but not viable cells
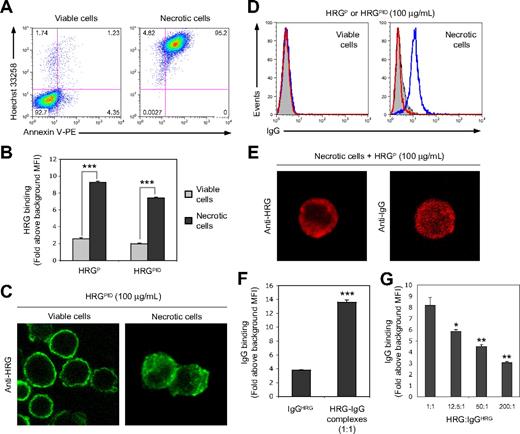
Based on the observation that IgG2κ preferentially copurifies with HRGP, the importance of this copurified IgG in previously reported functions of HRGP was investigated. HRG has been shown recently to bind strongly to permeabilized apoptotic17 and necrotic cells,18 and plays a vital role in dying/dead cell removal by phagocytes. To examine whether the presence of IgG in HRGP preparations can influence the binding of HRG to viable and necrotic cells, Jurkat T cells were induced to be necrotic by exposure to hyperthermic conditions. The necrotic nature of the heat-killed Jurkat T cells was validated by flow cytometry on the basis of increased cell granularity (data not shown), as well as the ability to take up the DNA binding dye Hoechst 33258 and the exposure of PS, detected via annexin V–PE staining (Figure 2A). HRG in both the HRGP and HRGPID preparations (100 μg/mL) bound strongly and to a similar extent to necrotic cells, binding being 3- to 4-fold higher than to viable cells (Figure 2B), suggesting that the presence or absence of the copurified IgG had no major effect on HRG binding to either viable or necrotic cells. Furthermore, CLSM was used to visualize the binding of HRGPID (100 μg/mL) to viable and necrotic cells (Figure 2C). HRGPID binding was localized to the cell surface of viable cells, probably binding to cell surface HS,21 whereas necrotic cells exhibited an intracellular cytoplasmic binding pattern.
HRG mediates the binding of the copurified IgG to necrotic cells but not to viable cells. (A) Analysis of viable and necrotic (56°C for 30 minutes) Jurkat T cells by flow cytometry on the basis of Hoechst 33258 and annexin V–PE staining to determine viable, early apoptotic and necrotic cells. (B) Quantitative comparison of the ability of HRG in HRGP or HRGPID preparations (100 μg/mL) to bind to either viable or necrotic cells, as detected by flow cytometry. (C) Cellular localization of HRGPID (100 μg/mL) bound to viable or necrotic cells as determined by CLSM. (D) Flow cytometric detection of IgG binding to viable and necrotic cells incubated with either HRGP or HRGPID preparations (100 μg/mL) in the absence of any additional IgG. Representative flow cytometric histograms are shown, with filled histograms representing Ab only control and open blue and red histograms representing IgG binding when HRGP and HRGPID preparations, respectively, were used. (E) Cellular localization by CLSM of HRG and IgG bound to necrotic cells after incubation with HRGP (100 μg/mL). (F) Quantitative comparison of IgGHRG (2 μg/mL) binding to necrotic cells either alone or in the presence of an approximately 1:1 molar ratio of HRG, with the HRGP preparation being used as the source of HRG-IgG complexes. (G) Effect of increasing concentrations of HRGPID (as molar ratio of HRG:IgGHRG) on the binding of IgGHRG to necrotic cells. IgG binding in panels F and G was determined by flow cytometry. Data in panels B, F, and G are expressed as fold binding above background mean fluorescence intensity (MFI), with error bars representing SEM (n = 3). *P < .05; **P < .01; ***P < .001.
HRG mediates the binding of the copurified IgG to necrotic cells but not to viable cells. (A) Analysis of viable and necrotic (56°C for 30 minutes) Jurkat T cells by flow cytometry on the basis of Hoechst 33258 and annexin V–PE staining to determine viable, early apoptotic and necrotic cells. (B) Quantitative comparison of the ability of HRG in HRGP or HRGPID preparations (100 μg/mL) to bind to either viable or necrotic cells, as detected by flow cytometry. (C) Cellular localization of HRGPID (100 μg/mL) bound to viable or necrotic cells as determined by CLSM. (D) Flow cytometric detection of IgG binding to viable and necrotic cells incubated with either HRGP or HRGPID preparations (100 μg/mL) in the absence of any additional IgG. Representative flow cytometric histograms are shown, with filled histograms representing Ab only control and open blue and red histograms representing IgG binding when HRGP and HRGPID preparations, respectively, were used. (E) Cellular localization by CLSM of HRG and IgG bound to necrotic cells after incubation with HRGP (100 μg/mL). (F) Quantitative comparison of IgGHRG (2 μg/mL) binding to necrotic cells either alone or in the presence of an approximately 1:1 molar ratio of HRG, with the HRGP preparation being used as the source of HRG-IgG complexes. (G) Effect of increasing concentrations of HRGPID (as molar ratio of HRG:IgGHRG) on the binding of IgGHRG to necrotic cells. IgG binding in panels F and G was determined by flow cytometry. Data in panels B, F, and G are expressed as fold binding above background mean fluorescence intensity (MFI), with error bars representing SEM (n = 3). *P < .05; **P < .01; ***P < .001.
Strikingly, the copurified IgG in the HRGP preparation (100 μg/mL) was tethered specifically to necrotic but not to viable cells. HRGPID (100 μg/mL) was included as a negative control and showed no binding of IgG to either viable or necrotic cells (Figure 2D). Similar to HRGPID, the binding of both HRG and the copurified IgG in the HRGP preparation (100 μg/mL) were localized predominantly to the cytoplasm, and also possibly clustered on the surface, of necrotic cells (Figure 2E). Thus, the ability of the copurified IgG to bind to necrotic cells, either in the presence or absence of HRG, was further investigated. IgGHRG alone exhibited some binding to necrotic cell but this binding was markedly enhanced when a 1:1 molar ratio of HRG was present (Figure 2F). However, the presence of a large molar excess of HRGPID was able to significantly inhibit HRG-IgG complexes binding to necrotic cells, presumably by competing for HRG binding sites on necrotic cells (Figure 2G). Collectively, these results suggest that HRG can mediate the binding of the copurified IgG exclusively to necrotic but not to viable cells.
In addition to binding to heat-induced necrotic Jurkat T cells, HRG and HRG-IgG complexes were also found to bind specifically to heat-killed necrotic cells of other mammalian species (supplemental Figure 2) or permeabilized cells generated via other methods (supplemental Figure 3). These data suggest that the recognition of necrotic cells by HRG is conserved across different cell types and between mammalian species, and the HRG ligand(s) are exposed by multiple cell permeabilization processes.
HRG and the copurified IgG function cooperatively to facilitate phagocytosis of necrotic cells via a FcγR-dependent mechanism
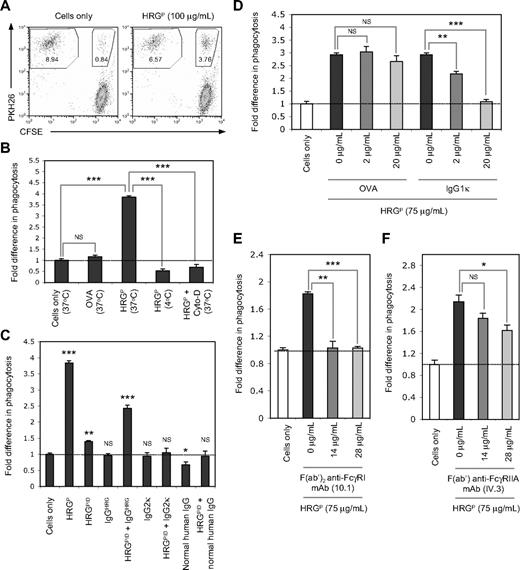
Recently, HRG has been shown to act as an opsonin and specifically aid the phagocytosis of necrotic but not viable or early apoptotic cells.18 Therefore, the ability of HRG to tether the copurified IgG exclusively to necrotic cells can potentially mediate the uptake of necrotic cells by phagocytes. Consistent with our previous work,18 HRGP (100 μg/mL) enhanced the removal of necrotic cells by THP-1 cells (a human macrophage-like monocytic leukemia cell line) at levels 3- to 4-fold higher than the necrotic cells only control or when the phagocytic assay was performed in the presence of the negative control protein OVA (100 μg/mL; Figure 3A-B). The ability of HRGP to enhance necrotic cell uptake was completely abolished by performing the phagocytic assay at 4°C or in the presence of the cytoskeleton-disrupting agent Cyto-D at 37°C (Figure 3B), suggesting that HRGP is not simply enhancing the binding of necrotic cells to the cell surface of phagocytes.
HRG and the copurified IgG function cooperatively to facilitate phagocytosis of necrotic cells via a FcγR-dependent mechanism. Phagocytic assays were performed using the monocytic cell line THP-1 and necrotic Jurkat T cells labeled with PKH26 or CFSE, respectively. THP-1 cells were mixed with necrotic cells at a ratio of 1:10 and incubated at 37°C for 60 minutes before analysis by flow cytometry. (A) Ability of HRGP (100 μg/mL) to enhance the phagocytosis of necrotic cells by THP-1 cells. Representative flow cytometric plots are shown for phagocytic assays performed in the presence or absence of HRGP. Values in each gated area represent percentage of cells in the assay. (B) Effect of temperature (4°C or 37°C) and the cytoskeleton disruptor Cyto-D (25 μM) on the ability of HRGP (100 μg/mL) to mediate the uptake of necrotic cells by THP-1 cells. OVA (100 μg/mL) is included as a negative control protein. (C) Effect of HRGP or HRGPID (100 μg/mL), and IgGHRG (2 μg/mL), a human IgG2κ myeloma (2 μg/mL), or pooled normal human IgG (2 μg/mL) either alone or in the presence of HRGPID (100 μg/mL) on the phagocytosis of necrotic cells by THP-1 cells. (D) Effect of a human IgG1κ myeloma on HRGP (75 μg/mL)–mediated uptake of necrotic cells, with OVA being included as a negative control. Effect of (E) a human FcγRI blocking mAb (mouse F(ab′)2 anti–human FcγRI mAb, clone 10.1) or (F) a human FcγRIIA blocking mAb (mouse F(ab′) anti–human FcγRIIA mAb, clone IV.3) on the ability of HRGP (75 μg/mL) to enhance the phagocytosis of necrotic cells. The level of phagocytosis was determined as the percentage of PKH26-positive THP-1 cells that had ingested CFSE-positive necrotic cells. Data in panels B-F are expressed as fold difference in the level of phagocytosis relative to the necrotic cells only control, with error bars representing SEM (n = 3). NS indicates not significant. *P < .05; **P < .01; ***P < .001.
HRG and the copurified IgG function cooperatively to facilitate phagocytosis of necrotic cells via a FcγR-dependent mechanism. Phagocytic assays were performed using the monocytic cell line THP-1 and necrotic Jurkat T cells labeled with PKH26 or CFSE, respectively. THP-1 cells were mixed with necrotic cells at a ratio of 1:10 and incubated at 37°C for 60 minutes before analysis by flow cytometry. (A) Ability of HRGP (100 μg/mL) to enhance the phagocytosis of necrotic cells by THP-1 cells. Representative flow cytometric plots are shown for phagocytic assays performed in the presence or absence of HRGP. Values in each gated area represent percentage of cells in the assay. (B) Effect of temperature (4°C or 37°C) and the cytoskeleton disruptor Cyto-D (25 μM) on the ability of HRGP (100 μg/mL) to mediate the uptake of necrotic cells by THP-1 cells. OVA (100 μg/mL) is included as a negative control protein. (C) Effect of HRGP or HRGPID (100 μg/mL), and IgGHRG (2 μg/mL), a human IgG2κ myeloma (2 μg/mL), or pooled normal human IgG (2 μg/mL) either alone or in the presence of HRGPID (100 μg/mL) on the phagocytosis of necrotic cells by THP-1 cells. (D) Effect of a human IgG1κ myeloma on HRGP (75 μg/mL)–mediated uptake of necrotic cells, with OVA being included as a negative control. Effect of (E) a human FcγRI blocking mAb (mouse F(ab′)2 anti–human FcγRI mAb, clone 10.1) or (F) a human FcγRIIA blocking mAb (mouse F(ab′) anti–human FcγRIIA mAb, clone IV.3) on the ability of HRGP (75 μg/mL) to enhance the phagocytosis of necrotic cells. The level of phagocytosis was determined as the percentage of PKH26-positive THP-1 cells that had ingested CFSE-positive necrotic cells. Data in panels B-F are expressed as fold difference in the level of phagocytosis relative to the necrotic cells only control, with error bars representing SEM (n = 3). NS indicates not significant. *P < .05; **P < .01; ***P < .001.
To investigate whether the presence of the copurified IgG in HRGP preparations can influence necrotic cell clearance, HRGPID (100 μg/mL) and IgGHRG (2 μg/mL) were either added independently or together to the phagocytic assay. Interestingly, HRGPID alone induced only a small increase in phagocytosis (< 1.5-fold) above the necrotic cells only control, and IgGHRG alone had no opsonic effects (Figure 3C). In contrast, the presence of both HRGPID and IgGHRG markedly enhanced necrotic cell phagocytosis to levels approximately 2.5-fold higher than the necrotic cells only control (Figure 3C). These data suggest that HRGPID and IgGHRG can work cooperatively to aid the removal of necrotic cells. In contrast, the presence of either a human IgG2κ myeloma protein (2 μg/mL) or pooled normal human IgG (2 μg/mL) alone or together with the HRGPID preparation (100 μg/mL) had no apparent enhancing effect on necrotic cell uptake (Figure 3C), implying that a specific population of IgG molecules is needed to function cooperatively with HRG to aid the removal of necrotic cells. Furthermore, consistent with the findings using THP-1 cells, 100 μg/mL HRGP but not HRGPID enhanced the uptake of necrotic Jurkat T cells by primary human monocytes (supplemental Figure 4), indicating that HRGP-aided necrotic cell removal is not limited to phagocytic cell lines such as THP-1.
It has been suggested previously that HRG can directly facilitate the clearance of late apoptotic cells by HMDM via a FcγRI-dependent mechanism.17 Thus, the role of FcγR in HRGP-induced phagocytosis of necrotic cells was investigated. When a human IgG1κ myeloma was added to the phagocytic assay to saturate the IgG binding sites of all FcγR on THP-1 cells, a concentration-dependent and, ultimately, complete inhibition of HRGP-mediated necrotic cell uptake was observed, whereas the control protein OVA had no significant effects on phagocytosis (Figure 3D). To examine whether FcγRI was involved in the enhanced clearance of necrotic cells by HRGP, phagocytic assays were performed in the presence of saturating concentrations of a F(ab′)2 anti-human FcγRI blocking mAb (10.1) and resulted in complete inhibition of HRGP-mediated uptake of necrotic cells (Figure 3E). In addition to the high-affinity IgG receptor, FcγRI, the macrophage-like monocytic cell line THP-1 also expresses the low-affinity IgG receptor, FcγRIIA.27 Thus, the role of FcγRIIA in HRGP-mediated necrotic cell uptake was also investigated. The presence of a F(ab′) anti-human FcγRIIA blocking mAb (IV.3) at saturating concentrations was found to have a small, but statistically significant, inhibitory effect on HRGP-aided uptake of necrotic cells (Figure 3F), suggesting that FcγRIIA may also play a minor role in this process using THP-1 cells. Collectively, these data suggest that FcγR, in particular FcγRI, plays a key role in the HRGP-mediated phagocytosis of necrotic cells by THP-1 cells.
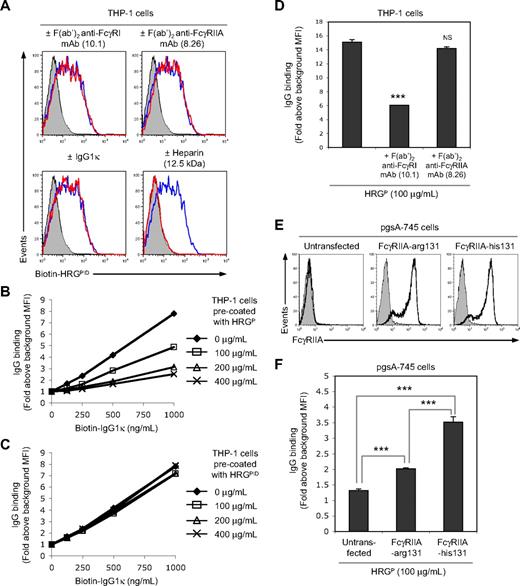
The demonstration that HRG-IgG complexes in human HRGP preparations are responsible for opsonizing necrotic cells casts doubts on a recent study by Gorgani et al,17 where HRG was proposed to function as a bridging molecule that directly interacts with both late apoptotic cells and FcγRI on phagocytes. We have found, however, that this scenario is unlikely as, when depleted of IgG, the binding of HRG to THP-1 cell is not blocked by FcγR-specific mAbs or human IgG1κ myeloma and is totally blocked by heparin (Figure 4A). Furthermore, HRGP blocked the binding of human IgG1κ myeloma to FcγR on THP-1 cells but HRGPID was unable to do so (Figure 4B-C), indicating that any FcγR binding activity in HRGP preparations can be accounted for by the copurified IgG.
HRG does not directly interact with FcγR on THP-1 cells. (A) Effect of precoating THP-1 cells with a human FcγRI-specific blocking F(ab′)2 mAb (10.1, 40 μg/mL), a human FcγRIIA-specific blocking F(ab′)2 mAb (8.26, 40 μg/mL), or a human IgG1κ myeloma (200 μg/mL) on biotinylated HRGPID (100 μg/mL) binding to THP-1 cells. The effect of heparin (12.5 kDa, 50 μg/mL) on biotinylated HRGPID binding to THP-1 cells was also examined. Biotinylated HRGPID binding was detected by flow cytometry using PE-conjugated streptavidin. Representative flow cytometric histograms are shown, with filled histograms representing the PE-conjugated streptavidin only control and open blue and red histograms representing, respectively, biotinylated HRGPID binding in the absence or presence of the indicated treatments. Effect of precoating THP-1 cells with (B) HRGP or (C) HRGPID on the binding of biotinylated human IgG1κ myeloma to THP-1 cells. Biotinylated IgG binding was determined by flow cytometry using PE-conjugated streptavidin, with data being expressed as fold binding above background MFI. (D) Effect of precoating THP-1 cells with a human FcγRI-specific blocking F(ab′)2 mAb (10.1, 40 μg/mL) or a human FcγRIIA-specific blocking F(ab′)2 mAb (8.26, 40 μg/mL) on the binding of the copurified IgG in HRGP (100 μg/mL) to THP-1 cells. (E) Cell-surface expression of human FcγRIIA by the GAG-deficient CHO cell line (pgsA-745) stably transfected with human FcγRIIA containing either an arginine residue or a histidine residue at amino acid position 131 (FcγRIIA-arg131 and FcγRIIA-his131, respectively), with untransfected pgsA-745 cells being included as a negative control. Human FcγRIIA expression was detected by flow cytometry using a mouse F(ab′)2 anti–human FcγRIIA mAb (8.26) and a PE-conjugated sheep F(ab′)2 anti–mouse Ig Ab. Representative flow cytometric histograms are shown, with filled histograms representing a secondary Ab-only control and open black histograms representing cell-surface expression of human FcγRIIA. (F) Binding of the copurified IgG in HRGP (100 μg/mL) to pgsA-745 cells stably transfected with either human FcγRIIA-arg131 or FcγRIIA-his131, with untransfected pgsA-745 cells being included as a negative control. IgG binding in panels D and F was detected by flow cytometry. Data in panels D and F are expressed as fold binding above background MFI, with error bars representing SEM (n = 3). NS indicates not significant. ***P < .001.
HRG does not directly interact with FcγR on THP-1 cells. (A) Effect of precoating THP-1 cells with a human FcγRI-specific blocking F(ab′)2 mAb (10.1, 40 μg/mL), a human FcγRIIA-specific blocking F(ab′)2 mAb (8.26, 40 μg/mL), or a human IgG1κ myeloma (200 μg/mL) on biotinylated HRGPID (100 μg/mL) binding to THP-1 cells. The effect of heparin (12.5 kDa, 50 μg/mL) on biotinylated HRGPID binding to THP-1 cells was also examined. Biotinylated HRGPID binding was detected by flow cytometry using PE-conjugated streptavidin. Representative flow cytometric histograms are shown, with filled histograms representing the PE-conjugated streptavidin only control and open blue and red histograms representing, respectively, biotinylated HRGPID binding in the absence or presence of the indicated treatments. Effect of precoating THP-1 cells with (B) HRGP or (C) HRGPID on the binding of biotinylated human IgG1κ myeloma to THP-1 cells. Biotinylated IgG binding was determined by flow cytometry using PE-conjugated streptavidin, with data being expressed as fold binding above background MFI. (D) Effect of precoating THP-1 cells with a human FcγRI-specific blocking F(ab′)2 mAb (10.1, 40 μg/mL) or a human FcγRIIA-specific blocking F(ab′)2 mAb (8.26, 40 μg/mL) on the binding of the copurified IgG in HRGP (100 μg/mL) to THP-1 cells. (E) Cell-surface expression of human FcγRIIA by the GAG-deficient CHO cell line (pgsA-745) stably transfected with human FcγRIIA containing either an arginine residue or a histidine residue at amino acid position 131 (FcγRIIA-arg131 and FcγRIIA-his131, respectively), with untransfected pgsA-745 cells being included as a negative control. Human FcγRIIA expression was detected by flow cytometry using a mouse F(ab′)2 anti–human FcγRIIA mAb (8.26) and a PE-conjugated sheep F(ab′)2 anti–mouse Ig Ab. Representative flow cytometric histograms are shown, with filled histograms representing a secondary Ab-only control and open black histograms representing cell-surface expression of human FcγRIIA. (F) Binding of the copurified IgG in HRGP (100 μg/mL) to pgsA-745 cells stably transfected with either human FcγRIIA-arg131 or FcγRIIA-his131, with untransfected pgsA-745 cells being included as a negative control. IgG binding in panels D and F was detected by flow cytometry. Data in panels D and F are expressed as fold binding above background MFI, with error bars representing SEM (n = 3). NS indicates not significant. ***P < .001.
To directly examine the interaction between the copurified IgG and THP-1 cells, THP-1 cells were incubated with HRGP (100 μg/mL) and then analyzed for IgG binding. The copurified IgG in the HRGP preparation bound strongly to THP-1 cells, and this interaction was significantly reduced by precoating THP-1 cells with saturating concentrations of a F(ab′)2 anti-human FcγRI blocking mAb (10.1) but not by a F(ab′)2 anti-human FcγRIIA blocking mAb (8.26) before HRGP exposure (Figure 4D). These results suggest that FcγRI is the major FcγR on THP-1 cells involved in binding HRG-IgG complexes, although FcγRIIA may also play a minor role in this process. Thus, the direct binding of the copurified IgG to FcγRIIA was further investigated using a GAG-deficient CHO cell line (pgsA-745) stably transfected with the 2 different allelic forms of human FcγRIIA (Figure 4E). These 2 FcγRIIA contain either a histidine residue or an arginine residue at amino acid position 131 (termed FcγRIIA-his131 or FcγRIIA-arg131, respectively), with the polymorphism altering the binding affinity of FcγRIIA for human IgG2.28 Consistent with previous studies,28 the copurified IgG (containing predominately IgG2) in the HRGP preparation (100 μg/mL) bound significantly stronger to pgsA-745 cells expressing FcγRIIA-his131 than to cells expressing FcγRIIA-arg131 (Figure 4F), indicating that the copurified IgG can also interact with FcγRIIA.
Identifying HRG ligand(s) on necrotic cells
CLSM studies, depicted in Figure 2C and E, indicate that HRG can bind to cytoplasmic ligand(s) exposed in necrotic cells. Thus, we initially attempted to identify cytoplasmic protein ligand(s) that can interact with HRG. This involved preparing detergent lysates of various cell lines, exposing them to Sepharose beads covalently coupled with HRGPID or BSA, and analyzing bead bound material by SDS-PAGE, with bound proteins being detected by Coomassie Brilliant Blue staining. Multiple experiments failed to detect any specific proteins associating with the HRG-coupled beads (data not shown).
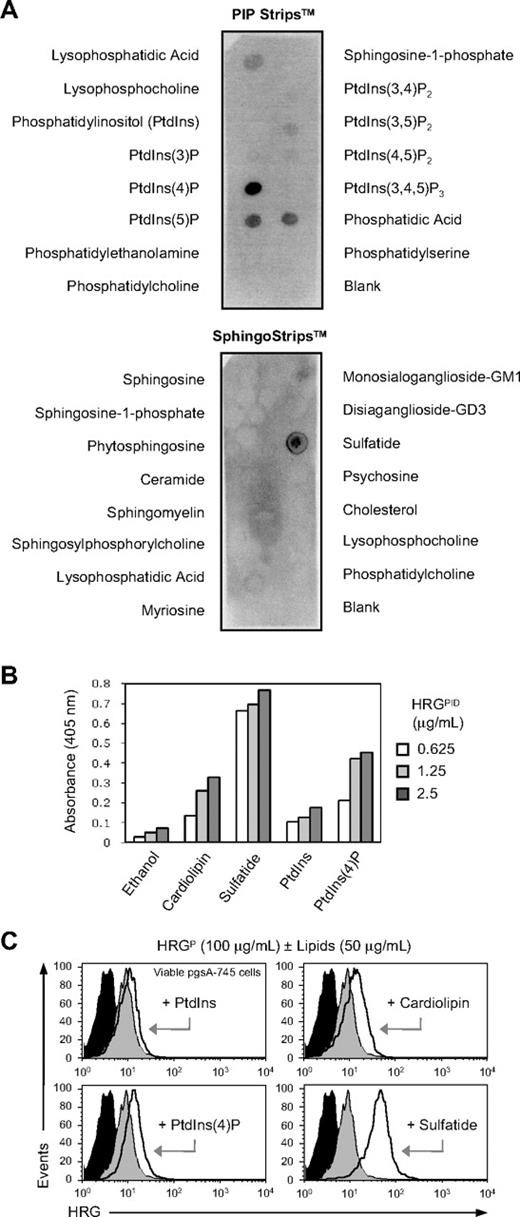
This finding prompted us to search for nonproteinaceous ligand(s) for HRG in necrotic cells. Recent studies by Ball-Rosen et al29 have shown that, like many phospholipid-binding proteins in human plasma, HRG is recognized by autoantibodies from patients with antiphospholipid syndrome (APS). Because APS is a condition often associated with the presence of autoantibodies against phospholipids and phospholipid-binding proteins,30 we hypothesized that HRG may bind to intracellular phospholipids. To examine whether HRG can interact with cellular lipids, lipid binding assays were initially performed using membrane strips (PIP Strips and SphingoStrips) spotted with 100 pmoles of 26 different biologically active lipids. Surprisingly, HRGPID (1 μg/mL) bound strongly to phosphatidic acid, sulfatide, PtdIns(4)P, and PtdIns(5)P; bound moderately to lysophosphatidic acid; and bound weakly to PtdIns(3,5)P2 and PtdIns(4,5)P2 but failed to react with the remaining 19 lipids (Figure 5A). Except for sulfatide, which is a sulfated glycosphingolipid expressed on the cell surface,31 the other lipids that showed interaction with HRGPID are all intracellular lipids located on the inner leaflet of the cell membrane. Thus, these phospholipids could potentially act as endogenous ligand(s) for HRG in permeabilized necrotic and late apoptotic cells. In contrast, HRGPID (1 μg/mL) did not bind to phospholipids that are usually exposed on early apoptotic cells, such as PS and phosphatidylcholine (Figure 5A). These results are consistent with previous studies showing that HRG binds poorly to early apoptotic cells.18 In addition to the membrane strip–based lipid binding assay, the ability of HRG to interact with various phospholipids was examined by ELISA. Similar binding results were observed as with the membrane strip assay (ie, strong binding to sulfatide and PtdIns(4)P but little binding to PtdIns), with HRGPID also showing binding to cardiolipin, a major autoantigen in APS (Figure 5B). To avoid the potential steric constraints of immobilizing small ligands on a nitrocellulose membrane or plastic and the exposure of acyl chains of phospholipids, the interaction between HRG and phospholipids was further investigated using viable pgsA-745 cells precoated with various phospholipids. Viable pgsA-745 cells precoated with PtdIns(4)P, cardiolipin, and sulfatide, but not PtdIns, showed a clear increase in HRGPID (100 μg/mL) binding (Figure 5C), with sulfatide exhibiting the greatest increase, supporting the view that HRG can bind directly to certain phospholipids.
HRG interacts with negatively charged lipids. (A) Analysis of HRGPID (1 μg/mL) binding to an array of different cellular lipids using PIP Strips and SphingoStrips coated with 100 pmoles of the indicated biologically active lipids. (B) Analysis of the ability of HRGPID to bind various phospholipids by ELISA using wells precoated with 50 μg/mL cardiolipin, sulfatide, PtdIns, and PtdIns(4)P, with ethanol coated wells being included as a negative control. (C) Ability of HRG in a HRGP preparation (100 μg/mL) to bind to viable pgsA-745 cells precoated with cardiolipin, sulfatide, PtdIns, and PtdIns(4)P (50 μg/mL, room temperature, 30 minutes). HRG binding was detected by flow cytometry. Representative flow cytometry histograms are shown, with filled black histograms representing Ab-only control and filled gray and open black histograms representing HRG binding to untreated or phospholipid coated cells, respectively.
HRG interacts with negatively charged lipids. (A) Analysis of HRGPID (1 μg/mL) binding to an array of different cellular lipids using PIP Strips and SphingoStrips coated with 100 pmoles of the indicated biologically active lipids. (B) Analysis of the ability of HRGPID to bind various phospholipids by ELISA using wells precoated with 50 μg/mL cardiolipin, sulfatide, PtdIns, and PtdIns(4)P, with ethanol coated wells being included as a negative control. (C) Ability of HRG in a HRGP preparation (100 μg/mL) to bind to viable pgsA-745 cells precoated with cardiolipin, sulfatide, PtdIns, and PtdIns(4)P (50 μg/mL, room temperature, 30 minutes). HRG binding was detected by flow cytometry. Representative flow cytometry histograms are shown, with filled black histograms representing Ab-only control and filled gray and open black histograms representing HRG binding to untreated or phospholipid coated cells, respectively.
A previous study has suggested that HRG may bind to late apoptotic cells via interaction with exposed DNA.17 This conclusion was based on the observation that DNase I treatment of late apoptotic cells results in a partial reduction in HRG binding. We were able to reproduce this finding (supplemental Figure 5) but found that the reduced HRG binding correlated with loss in cytoplasmic contents and, presumably, cytoplasmic HRG ligand(s) after DNase I treatment.
Phagocytosis of necrotic cells via HRGP promotes a proinflammatory response
Necrotic cell removal is often associated with a proinflammatory response, possibly due to the exposure of immunostimmatory molecules known as damage-associated molecular patterns to the immune system when cells become permeabilized.32 Furthermore, the cross-linking of activating FcγR, such as human FcγRI and FcγRIIA, is also known to induce the production and secretion of proinflammatory cytokines such as interleukin-6 (IL-6),33 tumor necrosis factor (TNF),34 and IL-8.35,36 Therefore, the ability of THP-1 cells to release a panel of different proinflammatory cytokines after necrotic cell uptake, either in the presence or absence of endotoxin-free HRGP, was investigated. Necrotic cells alone induced THP-1 cells to produce IL-8 (Figure 6A), which could be the result of the exposure of endogenous damage-associated molecular patterns such as HMGB1 by the necrotic cells.37 In contrast, necrotic cells in the presence of endotoxin-free HRGP (150 μg/mL) resulted in enhanced synthesis of IL-8 and induction of TNF production by THP-1 cells (Figure 6A-B), suggesting that the enhanced necrotic cell disposal induced by HRGP can promote the release of certain proinflammatory cytokines. Furthermore, no apparent induction of IL-10, IL-12p70, IL-1β, or IL-6 was observed under all conditions tested (data not shown), suggesting that necrotic cell clearance, either in the absence or presence of HRGP, does not modulate the production of these cytokines by the monocytic cell line THP-1. Furthermore, when primary human monocytes were used as the phagocytes, the presence of endotoxin-free HRGP-opsonized necrotic cells also stimulated the production of IL-8 and TNF by primary human monocytes (supplemental Figure 6). Collectively, these data suggest that HRGP-mediated necrotic cell uptake can facilitate the induction of a necrotic cell–dependent inflammatory response.
Monocytes release proinflammatory cytokines after HRGP-mediated uptake of necrotic cells. Production of proinflammatory cytokines IL-8 (A) and TNF (B) by THP-1 cells after incubation for 2, 4, 8, and 12 hours at 37°C with necrotic Jurkat T cells (10:1 necrotic Jurkat:THP-1 ratio), either in the presence or absence of endotoxin-free HRGP (150 μg/mL). Cytokine levels were determined by a cytokine bead array (CBA) assay, with THP-1 cells alone or in the presence of endotoxin-free HRGP being included as negative controls. Error bars represent the range of duplicate samples. The dotted line in panel B represents 5 pg/mL, the limit of detection of TNF by the CBA assay.
Monocytes release proinflammatory cytokines after HRGP-mediated uptake of necrotic cells. Production of proinflammatory cytokines IL-8 (A) and TNF (B) by THP-1 cells after incubation for 2, 4, 8, and 12 hours at 37°C with necrotic Jurkat T cells (10:1 necrotic Jurkat:THP-1 ratio), either in the presence or absence of endotoxin-free HRGP (150 μg/mL). Cytokine levels were determined by a cytokine bead array (CBA) assay, with THP-1 cells alone or in the presence of endotoxin-free HRGP being included as negative controls. Error bars represent the range of duplicate samples. The dotted line in panel B represents 5 pg/mL, the limit of detection of TNF by the CBA assay.
Discussion
Despite a growing number of opsonins being identified as key adaptor molecules in aiding apoptotic and necrotic cell clearance, the molecular mechanisms (ie, the phagocytic receptors, dying/dead cell ligands, and immunologic consequences) underpinning these phagocytic pathways are poorly characterized. Here, we describe in detail the molecular components that are involved in HRG-aided necrotic cell removal as summarized in Figure 7. Furthermore, we show that the recognition of necrotic cells via a HRG-mediated pathway may also play an important role in mediating the recruitment of other leukocytes, such as neutrophils and macrophages, to sites of tissue injury.
Proposed molecular mechanism by which HRG enhances the uptake of necrotic cells, but not viable cells, by phagocytes. HRG can simultaneously bind to exposed cellular lipids on necrotic cells and soluble human IgG (in particular IgG2κ) and, consequently, enhance the uptake of necrotic cells by phagocytes via a FcγR-dependent mechanism. This HRGP-mediated uptake of necrotic cells induces the release of proinflammatory cytokines, such as TNF and IL-8, from phagocytes. In contrast, only free HRG, and not HRG-IgG complexes, is able to bind to heparan sulfate proteoglycans (HSPG) on the surface of viable cells, and this prevents HRG-mediated phagocytosis of viable cells.
Proposed molecular mechanism by which HRG enhances the uptake of necrotic cells, but not viable cells, by phagocytes. HRG can simultaneously bind to exposed cellular lipids on necrotic cells and soluble human IgG (in particular IgG2κ) and, consequently, enhance the uptake of necrotic cells by phagocytes via a FcγR-dependent mechanism. This HRGP-mediated uptake of necrotic cells induces the release of proinflammatory cytokines, such as TNF and IL-8, from phagocytes. In contrast, only free HRG, and not HRG-IgG complexes, is able to bind to heparan sulfate proteoglycans (HSPG) on the surface of viable cells, and this prevents HRG-mediated phagocytosis of viable cells.
This study definitively shows that IgG copurifies with human HRGP. These data are consistent with previous studies showing that HRG purified from human plasma may contain trace amounts of various HRG ligands including IgG.38 In contrast, detectable levels of C1q or plasminogen were not found in the HRGP preparations (data not shown). Interestingly, there was a skewed distribution of IgG subclasses (ie IgG2κ) copurifying with HRG compared with healthy human plasma. These data were consistent with our previous studies showing that HRG binds preferentially to human IgG1κ and IgG2κ myeloma proteins.23 It should be noted, however, that HRGPID bound more avidly to IgGHRG than IgG1κ and IgG2κ myeloma proteins, suggesting that the interaction between HRG and the copurified IgG may depend on other factors besides the IgG subclass. Because many plasma proteins, such as C1q, CRP, serum amyloid protein, MBL, and β2GPI, are targeted by autoantibodies in patients with autoimmune diseases such as systemic lupus erythematosus39,40 and APS,41 the copurified IgG in the HRGP preparations may represent high-affinity anti-HRG autoantibodies. Consistent with this view, IgG from APS patients was recently shown to bind to both the major autoantigen β2GPI as well as HRG.29 In addition, anti-β2GPI autoantibodies in APS patients were also found to be significantly skewed toward the IgG2 subclass.42 However, the ability of HRG to bind directly to IgG itself23,24 poses potential technical difficulties in confirming whether the interaction between HRG and the copurified IgG is truly mediated via the antigen binding properties of the copurified IgG. Nevertheless, pooled normal human IgG from 8 to 15 healthy persons (Sigma-Aldrich) was found to bind more avidly to immobilized HRG compared with other human IgG myeloma proteins (data not shown), suggesting that autoantibodies or even “natural” antibodies against HRG may exist in clinically healthy persons.
Although the copurified IgG constituted only approximately 1% to 4% of the HRGP preparations, the copurified IgG was essential for HRGP-mediated disposal of necrotic cells. The ability of HRG to tether the copurified IgG specifically to necrotic but not viable cells provided an important “eat-me” signal on necrotic cells to trigger phagocytic uptake by THP-1 cells as well as primary human monocytes. These results are consistent with previous studies showing that HRGP selectively enhances the clearance of necrotic but not viable or early apoptotic cells.18 It is also important to note that free HRG can bind to cell-surface HS on viable cells, but once coupled with IgG, it is unable to do so (Figure 7). In contrast, HRG-IgG complexes can bind avidly to necrotic cells, possibly via various intracellular phospholipids, such as PtdIns(4)P and cardiolipin, that become exposed when the cell membrane is permeabilized. The binding of these HRG-IgG complexes to necrotic cells then enhances necrotic cell removal by phagocytosis and stimulates the production of proinflammatory cytokines, such as IL-8 and TNF (Figure 7).
To trigger phagocytic uptake, dying cells must expose a specific array of “eat-me” and “don't-eat-me” signals, which can be detected by phagocytes via a range of receptors and opsonins.4 Here, we show that the “eat-me” signal(s) recognized by HRG are present ubiquitously in all permeabilized mammalian cells tested and processes such as apoptosis or heat-induced cell death are not required to generate the ligand(s). Although binding studies with HRGPID coupled beads did not identify any cytoplasmic proteins that specifically interact with HRG, anionic phospholipids were demonstrated as potential necrotic cell ligand(s) of HRG. These molecules can be found in all mammalian cells and are often shielded from the extracellular environment unless the plasma membrane has been permeabilized. Unexpectedly, HRG was found to bind avidly to intracellular phosphoinositide signaling molecules such as PtdIns(4)P, PtdIns(5)P, and PtdIns(4,5)P2 but not PtdIns(3)P, PtdIns(3,4)P2, or PtdIns(3,4,5)P3. The ability of HRG to bind to various phospholipids immobilized on the membrane strip, with such striking specificity, demonstrates that HRG does not simply interact with highly charged anionic molecules but recognizes specific molecular structures. Although the membrane strip-based lipid binding assay is a well-established method used to examine protein-lipid interactions,43 further studies are needed to characterize the binding of HRG to phospholipids in detail using, for example, liposome sedimentation assays or biosensor analysis.
Interestingly, besides intracellular molecules such as γ-adaptin and ceramide-transfer protein,44 serum proteins like rat MBL45 and human HRG are the only 2 extracellular proteins identified to date that can bind specifically to PtdIns(4)P, which is the most abundant form of the monophosphorylated inositol phospholipids in mammalian cells.44 Although the physiologic significance of MBL binding to PtdIns(4)P has not been elucidated, the results presented in this study suggest that intracellular phospholipids like PtdIns(4)P may function as “eat-me” signal by permeabilized cells, which can be recognized by phagocytes via serum opsonins such as HRG and possibly MBL. Indeed, MBL has been shown recently to bind strongly to late apoptotic and necrotic cells but not early apoptotic cells.12 Furthermore, because the binding of β2GPI to apoptotic cells via anionic phospholipids has been proposed to initiate the break of tolerance and induce the generation of anti-β2GPI or antiphospholipid-β2GPI complex autoantibodies,46 the interaction between HRG and phospholipids in necrotic cells may provide a potential mechanism for generating anti-HRG autoantibodies, as suggested by Ball-Rosen et al.29
In addition to regulating coagulation and fibrinolysis, HRG has also been shown to bind to and facilitate the removal of bacteria47,48 and fungi.49 The specific binding of HRG to pathogens47–49 and molecular structures exposed on necrotic cells, as shown in this study, clearly highlights its ability to function as a PRM like C1q, CRP, MBL, and β2GPI. Interestingly, these PRMs, including HRG, are frequently targeted by autoantibodies.29,39–41 Although the presence of PRM-IgG complexes are often considered as pathogenic,50 these immune complexes may actually expand the recognition properties of immunoglobulins through PRM acting as an adaptor molecule and subsequently triggering a cellular immune response through the activation of FcγR on leukocytes at sites of tissue injury.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr. Allison Jones and Dr. Craig Freeman (The John Curtin School of Medical Research, Australian National University) for purified human HRGP. We thank Hannah French (The John Curtin School of Medical Research, Australian National University), Julie White, Katharine Goodall, Gemma Ryan, and Kruti Patel (Department of Biochemistry, La Trobe University) for assistance in the purification of primary human monocytes and silver staining.
This work was funded by Australian National Health and Medical Research Council (NHMRC) research grants 209618, 455395, and 418008.
Authorship
Contribution: I.K.H.P. designed and performed research, analyzed and interpreted data, and wrote the manuscript; and M.D.H. and C.R.P. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Professor Christopher Parish, The John Curtin School of Medical Research, Building 131, Australian National University, Garran Road, Acton, ACT 0200, Australia; e-mail: Christopher.Parish@anu.edu.au; or Dr. Mark Hulett, Department of Biochemistry, La Trobe University, Physical Sciences 4 Building, Kingsbury Drive, Bundoora, Melbourne 3086, Australia; e-mail: m.hulett@latrobe.edu.au.
References
Author notes
M.D.H. and C.R.P. contributed equally to this study.