Abstract
Because the microenvironment that supports hematopoietic stem cell (HSC) proliferation and differentiation is not fully understood, we adapted a heterotopic bone formation model as a new approach for studying the HSC microenvironment in vivo. Endogenous HSCs homed to tissue-engineered ossicles and individually sorted HSCs from ossicles were able to reconstitute lethally irradiated mice. To further explore this model as a system to study the stem cell niche, ossicles were established with or without anabolic parathyroid hormone (PTH) treatment during the 4-week course of bone development. Histology and micro–computed tomography showed higher bone area-to-total area ratios, thicker cortical bone and trabecular bone, significantly higher bone mineral density and bone volume fraction in PTH-treated groups than in controls. By an in vivo competitive long-term reconstitution assay, HSC frequency in the ossicle marrow was 3 times greater in PTH groups than in controls. When whole bone marrow cells were directly injected into the ossicles after lethal irradiation, the PTH-treated groups showed an enhanced reconstitution rate compared with controls. These findings suggest the residence of HSCs in heterotopic bone marrow and support the future use of this ossicle model in elucidating the composition and regulation of the HSC niche.
Introduction
The specialized microenvironment that maintains the hematopoietic stem cell (HSC) state is known as the stem cell niche.1,2 Within this stem cell niche, a number of different supporting cells synthesize growth factors and extracellular cues that maintain stem cell pools by controlling stem cell self-renewal and differentiation. The nature of this niche has been the target of intense investigation because maintaining adequate numbers of stem cells is essential for understanding development, for assuring regeneration of tissues, and for the development of new therapeutic strategies.
HSCs in the bone marrow, the site of hematopoiesis in the adult, have been visualized in close proximity to sinusoids, specialized blood vessels of hematopoietic tissues as well as near to cells lining the endosteum, the interface between bone and bone marrow. With the use of signaling lymphocytic activation molecule family markers for the identification of HSCs in tissue sections, greater than 60% of HSCs were found to be located adjacent to sinusoids in the bone marrow where perivascular reticular cells express stromal cell–derived factor 1 (SDF-1)/CXCL12, a chemokine that regulates the migration and maintenance of HSCs in the bone marrow.3 Perivascular mesenchymal progenitors also reside adjacent to sinusoids and secrete other factors that regulate HSC maintenance, such as angiopoietin,4 a factor that promotes the maintenance of quiescent HSCs.5-7 However, HSCs also may localize at or near the endosteal surfaces of bone,7-10 and osteoblasts and osteoclasts near endosteal surfaces have also been suggested to secrete a variety of factors that regulate HSC maintenance, such as angiopoietin, thrombopoietin, and SDF-1.11,12 It remains to be determined whether one of these cell types or locations is the physiologically important HSC niche or whether multiple cell types contribute to the secretion of factors required for HSC maintenance. The development of a model system in which alterations might be made to an in vivo hematopoietic system in isolation of the skeletal system would clarify our understanding and extend our ability to manipulate the stem cell niche.
A number of different models have been developed in mice for the study of bone formation outside the skeletal system,13-26 and a common observation from these studies is that hematopoietic cells are found inside of the heterotopically formed bones when bone marrow stromal cells (BMSCs) or bone-inductive bone morphogenetic protein or virus were used.27-30 Other studies have shown that heterotopic bone marrow could generate spleen colony-forming units (CFU-Ss) or provide radioprotection in secondarily irradiated mice.31 Although these studies show the possibility of using heterotopic bone to facilitate the engraftment and survival of transplanted blood cells, heterotopic bone models have not been used to study the nature of the HSC niche. To overcome these limitations, we used an in vivo model32 to functionally characterize the HSC niche in vivo and to study how the niche microenvironment supports and maintains stem cell activity. The general method involves harvesting mouse BMSCs, expanding the progenitor cells in vitro, seeding the cells on a gelatin scaffold, and transplanting them into subcutaneous pouches in mice. In this so-called ossicle model, a cortical-like bone capsule develops by 4 weeks and surrounds an area with active hematopoiesis. The bone marrow tissue within the ossicle includes reticular and adipocytic stroma and hematopoietic cells of all lineages and stages of maturation.32,33 This model is much more flexible than conventional, whole animal studies because cells from different genetic backgrounds can be transplanted and compared with wild-type activity in the same animal, and both bone formation and the development of the marrow can be monitored and manipulated over time.
The ability to manipulate this model system allows the study of factors that influence stem cell behavior. One important in vivo manipulation is the response of developing bone to factors that modulate osteoblastic and osteoclastic function such as parathyroid hormone (PTH). PTH is well known for its anabolic and catabolic effects on bone. With an anabolic treatment regimen, PTH increases bone mass by stimulating bone formation and results in a net gain in trabecular bone that is not at the expense of cortical bone. The younger the age at which treatment is started, the more dramatic the effect of PTH.34,35 In the heterotopic bone model, bone formed by transplanted BMSCs is responsive to anabolic dosing of PTH with a marked increase in trabecular bone mass, whereas the number of osteoclasts increases in response to catabolic doses.14 The increase in bone volume in ossicles is significant in the early phase (4 weeks) of bone formation but has only modest effects at later time points (> 7 weeks) compared with control groups.36 An anabolic PTH treatment regimen also increases HSC numbers in the long bone marrow, due at least in part to an increased number of osteoblasts.9
We hypothesized that the highly manipulatable heterotopic bone ossicle model could be used to gain a better understanding of the HSC niche in vivo. Through the use of the model we found that HSCs home to the ossicles through the peripheral circulation, and ossicles can serve as a niche to maintain stem cell activity. The bone–bone marrow environment in tissue-engineered ossicles can be modified by anabolic molecular medicine approaches to enhance the HSC niche.
Methods
Mouse strains
C57BL/6 (CD45.2 or Ly5.1) mice were purchased from Charles River Laboratories Inc. B6.SJL-PtprcaPep3b/BoyJ (CD45.1 or Ly5.2) mice were purchased from The Jackson Laboratory and were bred and maintained under approved protocols of the University of Michigan Unit for Laboratory Animal Medicine.
Culture and transplantation of BMSCs
To isolate BMSCs, marrow was flushed from the femur and tibia of 5-week-old mice with Hanks balanced salt solution (HBSS; Gibco). The marrow was passed through a 26-gauge needle to generate single-cell suspensions. Cells were rinsed twice in HBSS and were plated into T75 flasks with α-MEM (Gibco) supplemented with 10% fetal bovine serum (Gibco) at a density of 1 to 2 × 106 nucleated cells/cm2. Nonadherent cells were removed after 72 hours, and new culture medium was added. The medium was changed every 3 days. When cells reached 80% confluence, the cells were subcultured at the density of 1 to 2 × 104/cm2.
Transplantation procedures were performed as previously described.14 For PTH treatment, 1 day after transplantation, 2 groups of animals (8-10 animals/group) were given daily subcutaneous injections of either recombinant human PTH(1-34) (40 μg/kg; Biochem) or vehicle (0.9% sodium chloride) for 21 days.
Micro-CT and histomorphymetrical analyses
Ossicles were scanned by micro–computed tomography (CT; μCT MS8X-130; EVS Corp). The specimens were fitted in a 10-mm diameter cylindrical sample holder. High-resolution scanning with an in-plane pixel size and slice thickness of 8 μm was preformed. To cover the entire thickness of the ossicles, the number of slices was set at 667. GEMS MicroView software (GE Medical Systems) was used to make a 3-dimentional (3-D) reconstruction from the set of scans.
To obtain 3-D images, a threshold value of 1500 was used. The total volume (in mm3), bone volume (BV; in mm3), and bone mineral density (BMD) were measured directly, and the fractional bone volume (3-D BV/total volume; in %) was calculated from the 3-D dataset. After micro-CT scanning, the ossicles were rinsed in water and then decalcified in 10% formic acid for 5 days.
Immunostaining of VWF, SDF-1, IL-7, and counting of adipocytes and megakaryocytes
Paraffin sections were stained with Gill hematoxylin (Sigma-Aldrich) and aqueous eosin Y solution to visualize tissue morphology or immunostained with antibodies against von Willebrand factor (VWF; factor VIII; DAKO). Adipocytes and megakaryocytes were counted within 10 ×40 fields. In performing immunostaining of VWF, slides were deparaffinized through a descending series of ethyl alcohol and rehydrated in H2O, after which time they were washed for 5 minutes in 3% H2O2 (Sigma-Aldrich) in phosphate-buffered saline (PBS) to quench endogenous peroxidase activity. To improve antigen exposure, the slides were boiled in citrate buffer (Biocare Medical) for 2 minutes at 120°C. The sections were incubated for 10 minutes with terminator blocking solution (Biocare Medical) to prevent nonspecific antibody–antigen interactions. A primary antibody for VWF was diluted 1:1000 in Da Vinci Green antibody diluent (Biocare Medical), added dropwise onto each section, and allowed to incubate overnight at 4°C. The slides were washed for 10 minutes in PBS, dried, and incubated for 20 minutes with a broad-spectrum biotinylated Universal Link (Biocare Medical). The slides were washed again with PBS and incubated for 15 minutes with streptavidin–horseradish peroxidase (Biocare Medical). The horseradish peroxidase reaction was completed with the addition of 3,3′-diaminobenzidine (Zymed) for 30 seconds.
For immunofluorescent staining of SDF-1 and interleukin-7 (IL-7), anti–SDF-1 primary antibody (Abcam) combined with Zenon mouse immunoglobulin G–labeling reagent Alexa Fluor 488 (Invitrogen), or anti–IL-7 combined with Zenon mouse immunoglobulin G–labeling reagent Alexa Fluor 555 were applied. Frozen sections were fixed in acetone at −20°C for 10 minutes and underwent a brief wash with PBS plus 0.2% Triton X-100. Sections were blocked with horse serum solution (Vector Laboratories) for 30 minutes before fluorescence-labeled primary antibodies were applied to the sections at 4°C overnight. The sections were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen) after staining and covered with cover glass. Images were taken with Zeiss LSM 510 confocal microscope. Quantification was performed by Image Pro Plus (Media Cybernetics Inc) on 4 ×200 photos of each sample.
Methylcellulose culture and lineage analysis
Bone marrow from ossicles were harvested by flushing with needles and followed by crushing the ossicles and washing out all the remaining marrow. One thousand nucleated cells from ossicle and femur bone marrow were plated in 96-well plates (Corning) containing 100 μL of 1.0% methylcellulose (StemCell Technologies) containing 50 ng/mL stem cell factor, 10 ng/mL IL-3, 10 ng/mL IL-6, and 3 U/mL erythropoietin. Each sample had 48 repetitions. Cultures were maintained at 37°C in 6.5% CO2 and scored after 14 days. To obtain the percentage of different lineage of blood cells in bone marrow, cells were flushed and filtered to get single-cell suspensions. A cocktail of antibodies (Mac-1APC, Gr-1PE, Ter119FITC, B220FITC, CD3APC, CD4FITC, CD8PE) were used to stain the cells. The percentage of gated cells was obtained by flow cytometry.
Long-term competitive reconstitution assay
Adult recipient mice were lethally irradiated with an Orthovoltage x-ray source delivering approximately 3 Gy (300 rad)/minute. The mice received 2 doses of 5.7 Gy (570 rad), delivered at least 3 hours apart. For transplantation of sorted cell populations, CD45.2 (or CD45.1)–sorted HSCs (CD150+CD48−CD41−Sca-1+c-kit+) or whole bone marrow cells were mixed with a radio protective dose of 200 000 CD45.1 (or CD45.2) whole bone marrow cells in 100 μL of HBSS. The contents of individual wells were drawn into a 500-μL insulin syringe and injected into the retro-orbital venous sinus of lethally irradiated, CD45.1 (or CD45.2) recipient mice. Mice were maintained on water supplemented with antibiotics (1.1 g of neomycin sulfate and 106 U/L polymixin B sulfate; Sigma-Aldrich) and normal diets. Starting at 4 weeks after transplantation and continuing for 16 weeks after transplantation, peripheral blood was obtained from the tail veins of individual recipient mice. Blood samples were subjected to ammonium-chloride potassium for red cell lysis and were stained with antibodies to CD45.2 (clone 104) or CD45.1 (clone A20.1), and B220 (6B2), Mac-1 (M1/70), CD3 (KT31.1), and Gr-1 (8C5). Samples were analyzed on a FACSVantage dual-laser flow cytometer (Becton Dickinson). All the antibodies were obtained from BD PharMingen except those specifically indicated.
Quantification of HSC frequencies in the ossicle and long bone marrow
HSC frequencies in the ossicle and long bone marrow were quantified by limiting-dilution analysis in a competitively repopulated host.37 Lethally irradiated CD45.1 mice received a limiting number of CD45.2 test cells together with a radioprotective dose of 2 × 105 normal bone marrow cells of CD45.1 background by injection into the retro-orbital venous sinus. The proportion of CD45.2+ cells in peripheral blood of different lineages was analyzed by 4-color flow cytometry every 4 weeks after injection. The variation in the proportion of positive animals with the test cell dose was analyzed by Poisson statistics to measure HSC frequency in the ossicle bone marrow or long bone marrow.37
Reconstitution through ossicle and retro-orbital injection
CD45.2 recipient mice with heterotopic ossicles received 2 doses of 5.7 Gy (570 rad) at least 3 hours apart. After anesthesia, the ossicles were surgically exposed. Whole bone marrow (3 × 105 nucleated cells in 100 μL of PBS) from CD45.1 mice was transferred by a thin glass tube. The same number of cells were injected by a retro-orbital injection for the positive control groups. To exclude the possibility of leaking cells entering the peripheral blood, the same number of cells were injected around the ossicles subcutaneously as technical control. Blood was obtained from the tail vein of recipient mice and analyzed by flow cytometry to detect engraftment of donor cells.
Flow cytometric isolation of HSCs and transplantation
Bone marrow cells were flushed from femurs or ossicles with HBSS without calcium or magnesium, supplemented with 2% heat-inactivated calf serum. Cells were gently triturated and filtered through a nylon screen (40 μm; BD Falcon) to obtain single-cell suspensions. Cells were incubated first with an antibody cocktail of anti-CD150PE (clone TC15-12F12.2; BioLegend), CD48FITC (clone BCM-1), and CD41FITC (clone MWReg30), C-kitBIO (clone 2B8), and SCA-1APC (clone E13-161.7) for 20 minutes on ice, then rinsed and stained with antibio micro beads (Miltenyi Biotec) and streptavidin-APC-Cy7–conjugated secondary antibody for another 20 minutes. Cells were enriched for C-kit+ with an AutoMACS (Miltenyi Biotec). The enriched cells were resuspended in 2 mg/mL 7-amino-actinomycin D (eBioscience) to discriminate live from dead cells. Only live cells were included in analyses. HSCs were sorted by gating cells that were CD150+CD48−CD41−SCA-1+C-kit+. For purified HSC injection, 5 HSCs (CD45.2) were mixed with 200 000 radioprotection bone marrow from recipient type of mice (CD45.1) and injected into retro-orbital venous sinus. Blood was obtained from the tail vein of recipient mice and analyzed by flow cytometry to detect engraftment of donor cells.
Immunofluorescence analysis of HSC localization in tissue sections
Freshly dissected undecalcified ossicles were embedded in 8% gelatin in 0.1M phosphate buffer pH 7.4 and snap-frozen in N-methylbutane chilled in a slurry of ethanol and dry ice. Frozen sections were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD48 and anti-CD41 antibodies (each 1/100 dilution) as well as lineage markers, including anti-B220, CD2, CD4, CD8a, Gr-1, and CD11b antibodies and biotinylated pan-endothelial cell antigen (MECA-32). Pan-endothelial cell antigen was visualized with Alexa Fluor 647–conjugated streptavidin (Molecular Probes).3 All antibodies were purchased from Becton Dickinson unless otherwise noted. The nuclear dye DAPI was included in all stains to evaluate nuclear morphology. Microscopy was performed with the use of an Olympus BX51 florescence microscope or an Olympus FV-500 confocal microscope.
Results
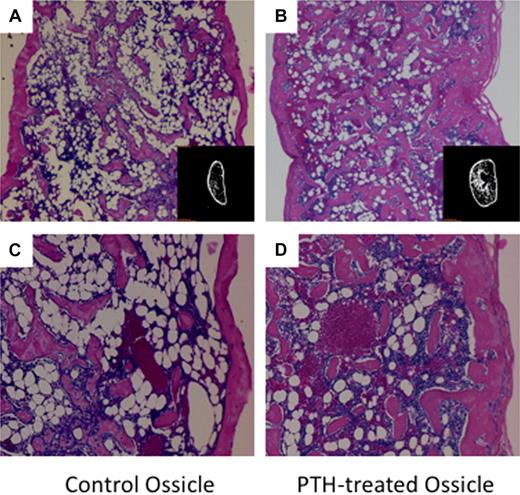
We adapted an in vivo model22,32 to functionally characterize the HSC niche in vivo and to study how the niche microenvironment supports and maintains stem cell activity. Within 4 weeks after implantation of BMSCs within gelatin scaffolds, well-defined vascularized ossicles developed in the subcutaneous spaces (Figure 1A,C). Histologic analysis showed the ossicles formed with intact cortical structures that surrounded trabecular bone and a robust hematopoietic environment. Experimental groups were treated with an anabolic regimen of PTH (40 μg/kg per day for 21 days) immediately after transplantation to manipulate the stem cell niche (Figure 1B,D). After 21 days of daily PTH injections, significant changes in the amount of bone and character of the marrow were observed and quantified.
Low- and high-magnification images of H&E-stained ossicle sections and micro-CT images (inset image). PTH-treated ossicles contained a higher density of trabecular bone and bone marrow cells. (A) Ossicle in control group without PTH treatment (×40). (B) Ossicle in PTH-treated group (×40). (C) Ossicle in control group (×100). (D) Ossicle in PTH-treated group (×100).
Low- and high-magnification images of H&E-stained ossicle sections and micro-CT images (inset image). PTH-treated ossicles contained a higher density of trabecular bone and bone marrow cells. (A) Ossicle in control group without PTH treatment (×40). (B) Ossicle in PTH-treated group (×40). (C) Ossicle in control group (×100). (D) Ossicle in PTH-treated group (×100).
Histomorphometric analyses and micro-CT were performed to evaluate the effects of anabolic PTH on bone and marrow development in the ossicles. Ossicles in the PTH-treated group developed bone with significantly higher bone area-to-total area ratios (BA/TA; P < .05), thicker cortical bone (P < .01), and thicker and more abundant trabeculae (P < .01) than in the control group (Table 1). Micro-CT analysis also showed PTH had a significant effect on several parameters of bone formation. In the PTH-treated group, ossicles had a significantly higher BMD (77.5 ± 3.8 mg/cc [PTH] vs 32.2 ± 5.9 mg/cc [control]; P < .01) and BV fraction (10.0% ± 0.3% [PTH] vs 6.3% ± 2.1% [control]; P < .01). However, there were no significant differences in BMD and BV fraction values between PTH-treated and the control group in the femur (Table 1).
Micro-CT analysis of control and PTH-treated ossicles
| Group (n = 5) . | Bone area/total area, % . | Thickness of cortex, mm . | Thickness of trabeculae, mm . | Bone mineral density, mg/cc . | Bone volume fraction, % . |
|---|---|---|---|---|---|
| Control ossicle | 25.56 ± 3.28 | 5.74 ± 1.50 | 4.08 ± 0.81 | 32.17 ± 5.93 | 6.27 ± 2.11 |
| PTH ossicle | 45.90 ± 9.52* | 9.61 ± 2.27† | 6.22 ± 1.18† | 77.53 ± 3.77† | 10.02 ± 0.29† |
| Group (n = 5) . | Bone area/total area, % . | Thickness of cortex, mm . | Thickness of trabeculae, mm . | Bone mineral density, mg/cc . | Bone volume fraction, % . |
|---|---|---|---|---|---|
| Control ossicle | 25.56 ± 3.28 | 5.74 ± 1.50 | 4.08 ± 0.81 | 32.17 ± 5.93 | 6.27 ± 2.11 |
| PTH ossicle | 45.90 ± 9.52* | 9.61 ± 2.27† | 6.22 ± 1.18† | 77.53 ± 3.77† | 10.02 ± 0.29† |
Data are mean ± SD.
P < .01.
P < .05.
The cellular composition of PTH-treated and control ossicles was evaluated by quantifying the number of blood vessels, adipocytes, and megakaryocytes. Immunostaining of VWF-expressing cells was used to show the density of microvessels in both PTH and control groups (Table 2; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). PTH-treated ossicles exhibited significantly higher numbers of VWF-positive microvessels compared with control groups. In contrast, there was no significant difference in vessel density between PTH-treated and control femurs (Table 2). Adipocyte numbers were significantly lower in PTH-treated ossicles than in the control group, and there were no significant differences in adipocyte numbers in PTH-treated and control femurs (Table 2). There were no significant differences in megakaryocyte numbers between experimental and control groups, although the numbers of megakaryocytes in the femur were higher than those observed in the ossicles (Table 2). These results show that anabolic effects of PTH do not only affect osteoblast numbers and bone density, but they also increase the density of the vasculature in the ossicle bone marrow.
Quantification of microvessel density and adipocyte and megakaryocyte numbers
| Group . | Blood vessel, per ×200 field . | Adipocyte, per ×400 field . | Megakaryocyte, per ×400 field . |
|---|---|---|---|
| Control ossicle | 17.60 ± 8.44 | 119 ± 30.9 | 1 ± 0.9 |
| PTH ossicle | 46.20 ± 17.17* | 61 ± 14.5* | 2 ± 1.3 |
| Control femur | 199.80 ± 41.60 | 4 ± 0.6 | 14 ± 1.2 |
| PTH femur | 182.71 ± 41.01 | 5 ± 2.7 | 12 ± 3.0 |
| Group . | Blood vessel, per ×200 field . | Adipocyte, per ×400 field . | Megakaryocyte, per ×400 field . |
|---|---|---|---|
| Control ossicle | 17.60 ± 8.44 | 119 ± 30.9 | 1 ± 0.9 |
| PTH ossicle | 46.20 ± 17.17* | 61 ± 14.5* | 2 ± 1.3 |
| Control femur | 199.80 ± 41.60 | 4 ± 0.6 | 14 ± 1.2 |
| PTH femur | 182.71 ± 41.01 | 5 ± 2.7 | 12 ± 3.0 |
Data are mean ± SD.
P < .05.
Hematopoietic character of ossicle bone marrow
The bone marrow of ossicles was similar in composition to that of the femur. The percentage of erythroid cells was significantly higher in ossicles than in femurs, but this difference was not as pronounced between PTH-treated ossicles and femurs. There were a higher percentage of T cells in ossicles than in femurs, and the B-cell population exhibited a higher proportion of mature subsets in ossicle bone marrow. Further, IL-7 secretion, which is important for early B-cell development, was not significantly different between ossicle and femur (supplemental Figure 2). In comparing PTH and control groups, the only significant difference between control and PTH femurs was that the control femurs contained a higher percentage of B cells (Table 3). Megakaryocytes were directly counted by microscopy where there were no significant differences observed between PTH and control groups in either ossicles or femurs. However, femurs contained more megakaryocytes than ossicles in both groups (Table 3).
Hematopoietic character of ossicle bone marrow
| Lineage (n = 5) . | Control ossicle . | PTH ossicle . | Control femur . | PTH femur . |
|---|---|---|---|---|
| Mac-1+Gr-1+, myeloid cells | 26.42 ± 5.05 | 33.63 ± 1.93 | 50.85 ± 2.42 | 37.93 ± 4.58 |
| Ter119+, erythroid progenitors | 79.13 ± 7.07* | 68.68 ± 7.29 | 39.47 ± 2.27 | 54.77 ± 4.94 |
| B220+ cells | 8.46 ± 1.39 | 10.95 ± 1.07 | 20.53 ± 2.08† | 17.16 ± 1.84 |
| B220+sIgM−CD43− (pre-B) | 2.09 ± 0.52 | 3.29 ± 0.80 | 9.81 ± 0.23 | 7.39 ± 0.65 |
| B220+sIgM−CD43low (pro-B) | 1.88 ± 0.28 | 1.88 ± 0.31 | 4.32 ± 1.09 | 3.78 ± 0.53 |
| B220+sIgM−CD43+ (pre-pro-B) | 0.40 ± 0.05 | 0.37 ± 0.18 | 1.68 ± 0.52 | 1.43 ± 0.36 |
| B220+ sIgM+ B cells | 3.82 ± 1.32 | 5.13 ± 0.79 | 4.00 ± 0.58 | 3.95 ± 0.48 |
| B220− sIgM− B cells | 7.93 ± 0.84 | 6.83 ± 1.34 | 17.21 ± 1.50 | 13.85 ± 1.59 |
| T cells | 5.71 ± 1.80 | 6.8 ± 0.49 | 2.80 ± 0.64 | 3.14 ± 1.16 |
| Helper T cells | 4.94 ± 1.93 | 5.94 ± 0.73 | 2.56 ± 0.60 | 2.84 ± 1.14 |
| Cytotoxic T cells | 0.11 ± 0.11 | 0.11 ± 0.05 | 0.03 ± 0.01 | 0.05 ± 0.00 |
| Lineage (n = 5) . | Control ossicle . | PTH ossicle . | Control femur . | PTH femur . |
|---|---|---|---|---|
| Mac-1+Gr-1+, myeloid cells | 26.42 ± 5.05 | 33.63 ± 1.93 | 50.85 ± 2.42 | 37.93 ± 4.58 |
| Ter119+, erythroid progenitors | 79.13 ± 7.07* | 68.68 ± 7.29 | 39.47 ± 2.27 | 54.77 ± 4.94 |
| B220+ cells | 8.46 ± 1.39 | 10.95 ± 1.07 | 20.53 ± 2.08† | 17.16 ± 1.84 |
| B220+sIgM−CD43− (pre-B) | 2.09 ± 0.52 | 3.29 ± 0.80 | 9.81 ± 0.23 | 7.39 ± 0.65 |
| B220+sIgM−CD43low (pro-B) | 1.88 ± 0.28 | 1.88 ± 0.31 | 4.32 ± 1.09 | 3.78 ± 0.53 |
| B220+sIgM−CD43+ (pre-pro-B) | 0.40 ± 0.05 | 0.37 ± 0.18 | 1.68 ± 0.52 | 1.43 ± 0.36 |
| B220+ sIgM+ B cells | 3.82 ± 1.32 | 5.13 ± 0.79 | 4.00 ± 0.58 | 3.95 ± 0.48 |
| B220− sIgM− B cells | 7.93 ± 0.84 | 6.83 ± 1.34 | 17.21 ± 1.50 | 13.85 ± 1.59 |
| T cells | 5.71 ± 1.80 | 6.8 ± 0.49 | 2.80 ± 0.64 | 3.14 ± 1.16 |
| Helper T cells | 4.94 ± 1.93 | 5.94 ± 0.73 | 2.56 ± 0.60 | 2.84 ± 1.14 |
| Cytotoxic T cells | 0.11 ± 0.11 | 0.11 ± 0.05 | 0.03 ± 0.01 | 0.05 ± 0.00 |
Data are mean ± SD.
Femur versus ossicle (P < .01).
PTH versus control ossicle or femur (P < .01).
Methylcellulose cultures to identify hematopoietic progenitor cells showed that bone marrow from PTH-treated ossicles contained fewer progenitor cells than those of control groups. PTH-treated femurs also contained fewer hematopoietic progenitor cells than control femurs; however, the difference was not statistically significant. When directly compared with femur marrow, the marrow of ossicles contained fewer progenitor cells (P < .01; Table 4).
Hematopoietic progenitor cells in ossicle bone marrow
| Group (n = 5) . | CFU-G/M/GM . | CFU-GEMM . | CFU-megakaryocyte . | BFU-erythroid . | Total/100 000 MNCs . |
|---|---|---|---|---|---|
| Control ossicle | 47 ± 37.5 | 1 ± 1.2 | 0 | 24 ± 29.9 | 35 ± 32.4 |
| PTH ossicle | 17 ± 9.7 | 0 | 0 | 14 ± 1.5 | 12 ± 8.3 |
| Control femur | 196 ± 22.0 | 4 ± 5.5 | 5 ± 4.3 | 67 ± 10.3 | 271 ± 31.5* |
| PTH femur | 138 ± 41.6 | 10 ± 5.0 | 2 ± 6.5 | 57 ± 7.0 | 208 ± 50.1* |
| Group (n = 5) . | CFU-G/M/GM . | CFU-GEMM . | CFU-megakaryocyte . | BFU-erythroid . | Total/100 000 MNCs . |
|---|---|---|---|---|---|
| Control ossicle | 47 ± 37.5 | 1 ± 1.2 | 0 | 24 ± 29.9 | 35 ± 32.4 |
| PTH ossicle | 17 ± 9.7 | 0 | 0 | 14 ± 1.5 | 12 ± 8.3 |
| Control femur | 196 ± 22.0 | 4 ± 5.5 | 5 ± 4.3 | 67 ± 10.3 | 271 ± 31.5* |
| PTH femur | 138 ± 41.6 | 10 ± 5.0 | 2 ± 6.5 | 57 ± 7.0 | 208 ± 50.1* |
Data are mean ± SD.
CFU-G/M/GM indicates granulocyte/macrophage/granulocyte-macrophage colony forming unit; granulocyte, erythroid, macrophage, megakaryocyte colony-forming unit; BFU, burst-forming unit; and MNCs, mononuclear cells.
Femur versus ossicle (P < .01).
HSCs colonize ossicles
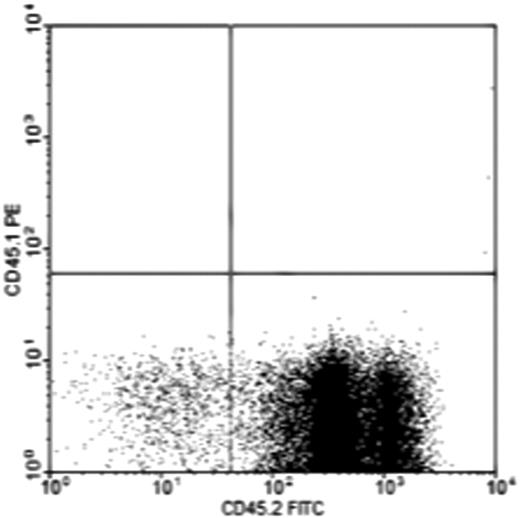
Subcutaneous ossicles were generated in CD45.2 mice with BMSCs harvested from CD45.1 mice. These mice share an identical genetic background except for the CD45.1/45.2 locus and thus allows the fate of cells to be followed after transplantation without confounding histocompatibility barriers. Peripheral blood and bone marrow were harvested 16 weeks after transplantation. No CD45.1 donor type blood cells were found in either the peripheral blood or the bone marrow derived from the ossicles (n = 5). Instead, all of the marrow cells flushed from ossicles were host derived (CD45.2+; Figure 2). This suggested that the hematopoietic cells inside the ossicles were not generated from the implanted donor cells, consistent with prior studies showing that bone marrow stroma and hematopoietic cells arise from developmentally distinct lineages of cells.32,38
Flow cytometric analysis of peripheral blood and bone marrow of ossicles shows the origin of the ossicle bone marrow. Ossicles were made subcutaneously in CD45.2 mice with BMSCs harvested from CD45.1 mice. No CD45.1 donor type hematopoietic cells were found in either the peripheral blood or ossicle bone marrows during the 16 weeks after the implantation period (n = 5). The chart is a representative analysis of ossicle bone marrow.
Flow cytometric analysis of peripheral blood and bone marrow of ossicles shows the origin of the ossicle bone marrow. Ossicles were made subcutaneously in CD45.2 mice with BMSCs harvested from CD45.1 mice. No CD45.1 donor type hematopoietic cells were found in either the peripheral blood or ossicle bone marrows during the 16 weeks after the implantation period (n = 5). The chart is a representative analysis of ossicle bone marrow.
HSC frequency in ossicle bone marrow
Limiting-dilution analysis in competitively repopulated irradiated recipients was performed to quantify the HSC frequency in ossicle bone marrow and to determine whether HSC numbers could be modified by anabolic PTH treatment. We found that the HSC frequency in the ossicle bone marrow of PTH-treated groups was approximately 3-fold higher than in the control group (Figure 3). Marrow harvested from the long bones of the PTH-treated groups had a slightly higher HSC frequency than the control group, although this was not statistically significant. The stem cell frequency in ossicle bone marrow was approximately 6 times lower than in femur bone marrow in the control group and 2 times lower in the PTH-treated group (Figure 3).
HSC frequency in ossicle and femur bone marrow measured by in vivo long-term competitive reconstitution assays (n = 3). Bone marrow from PTH-treated ossicles had nearly a 3-fold higher stem cell frequency than the control group (P < .01). The femur bone marrow in the PTH-treated group also displayed a slightly higher of stem cell frequency than the control group. *P < .01. Error bars represent SD.
HSC frequency in ossicle and femur bone marrow measured by in vivo long-term competitive reconstitution assays (n = 3). Bone marrow from PTH-treated ossicles had nearly a 3-fold higher stem cell frequency than the control group (P < .01). The femur bone marrow in the PTH-treated group also displayed a slightly higher of stem cell frequency than the control group. *P < .01. Error bars represent SD.
Reconstitution of C57BL/6 mice by ossicle injection
We sought to determine whether our model system could be used for direct injection of purified cells. Ossicles were established subcutaneously in CD45.2 mice with BMSCs from CD45.2 mice. After lethal irradiation, 3 × 105 of donor whole bone marrow from CD45.1 long bones was directly injected into the ossicles under a dissecting microscope. The peripheral blood of recipient mice was analyzed over a 16-week period by flow cytometry to identify donor cell-type reconstitution. In addition, to determine whether only the cells directly injected into ossicles could reconstitute mice, 3 × 105 of donor whole bone marrow from CD45.1 long bones were also injected subcutaneously near ossicles in another 5 lethally irradiated CD45.2 mice and served as controls.
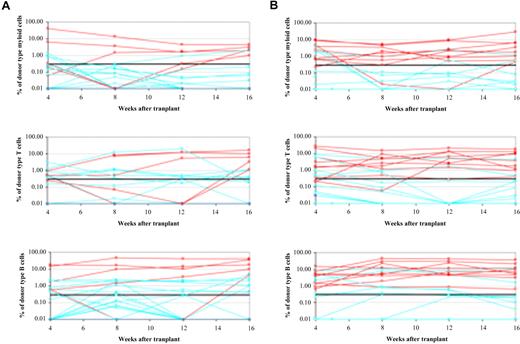
The subcutaneous injection of whole bone marrow cells near the ossicle did not reconstitute the mice, which excluded the possibility of reconstitution by the cells leaking out of ossicles. In the control group, 19% of mice exhibited long-term reconstitution, whereas 48% of mice in the PTH-treated group were reconstituted (P < .05; Figure 4). Each experiment was repeated 3 times. There was no statistically significant difference in the level of chimerism in donor type–positive reconstituted mice between PTH-treated and control groups (data not shown). These data show that mice can be long-term multilineage reconstituted by HSCs injected directly into subcutaneous ossicles.
Long-term reconstitution through direct ossicle injection or retro-orbital injection of HSCs. (A) Direct injection of whole bone marrow into ossicles resulted in long-term reconstitution in 5 of 25 irradiated mice. Each line represents the frequency of donor-derived myeloid, B, or T cells in a single mouse at 4, 8, 12, and 16 weeks after transplantation. The red lines represent mice that were long-term multilineage reconstituted, blue lines represent nonreconstituted mice. (B) Directly injecting whole bone marrow into PTH-treated ossicles reconstituted 10 of 20 irradiated mice.
Long-term reconstitution through direct ossicle injection or retro-orbital injection of HSCs. (A) Direct injection of whole bone marrow into ossicles resulted in long-term reconstitution in 5 of 25 irradiated mice. Each line represents the frequency of donor-derived myeloid, B, or T cells in a single mouse at 4, 8, 12, and 16 weeks after transplantation. The red lines represent mice that were long-term multilineage reconstituted, blue lines represent nonreconstituted mice. (B) Directly injecting whole bone marrow into PTH-treated ossicles reconstituted 10 of 20 irradiated mice.
After 16 weeks, ossicles were harvested from those mice showing positive CD45.1 cell-type reconstitution. Bone marrow was flushed, and 106 mononuclear cells were injected into secondary lethally irradiated CD45.2 mice. Peripheral blood was analyzed for another 16 weeks to determine whether CD45.1 reconstitution persisted. All 9 mice showed CD45.1 type of reconstitution, suggesting that the HSCs injected into the ossicles remained in the ossicles, were in a microenvironment that maintained the stem cell phenotype, and were capable of reconstituting the secondary recipient mice (data not shown).
To determine whether the tissue-engineered ossicles could provide a functional niche for circulating HSCs, ossicles were developed subcutaneously in CD45.2 mice with BMSCs from mice of the same strain. After lethal irradiation, 2 × 105 whole bone marrow cells from the long bones of CD45.1 mice were transplanted by retro-orbital injection into recipient mice containing the tissue-engineered ossicles. All 7 injected mice were reconstituted with long-term multilineage CD45.1-type cells found either in peripheral blood or in the ossicles during the 16 weeks after transplantation. Next, the ossicle bone marrow was harvested, and 1 × 106 nucleated cells were injected into multiple lethally irradiated CD45.2 mice. Peripheral blood analysis was performed for 16 weeks to determine the percentage of mice with positive reconstitution from CD45.1 HSCs. The CD45.1 HSCs from ossicles reconstituted 48% of the mice when PTH treated, whereas 19% of mice were reconstituted in the control group (Table 5.). This showed that HSCs engrafted the ossicles, maintained stem cell activity, and could subsequently reconstitute irradiated recipient mice possible because of a higher frequency of HSCs in PTH-treated ossicle bone marrow. Immunofluorescent staining of SDF-1, a key stem cell niche component, showed increased SDF-1 secretion in both ossicles and femurs of PTH-treated groups (supplemental Figure 3).
Reconstitution rates of irradiated mice with HSCs from ossicles
| Group . | Engrafted mice with long-term multilineage reconstitution . |
|---|---|
| Control ossicle | |
| Experiment 1, % (n of n) | 30 (3 of 10) |
| Experiment 2, % (n of n) | 12.5 (1 of 8) |
| Experiment 3, % (n of n) | 14 (1 of 7) |
| Mean ± SD, % | 19 ± 10 |
| PTH ossicle | |
| Experiment 1, % (n of n) | 40 (2 of 5) |
| Experiment 2, % (n of n) | 50 (4 of 8) |
| Experiment, % (n of n) | 57 (4 of 7) |
| Mean ± SD, % | 48 ± 9* |
| Group . | Engrafted mice with long-term multilineage reconstitution . |
|---|---|
| Control ossicle | |
| Experiment 1, % (n of n) | 30 (3 of 10) |
| Experiment 2, % (n of n) | 12.5 (1 of 8) |
| Experiment 3, % (n of n) | 14 (1 of 7) |
| Mean ± SD, % | 19 ± 10 |
| PTH ossicle | |
| Experiment 1, % (n of n) | 40 (2 of 5) |
| Experiment 2, % (n of n) | 50 (4 of 8) |
| Experiment, % (n of n) | 57 (4 of 7) |
| Mean ± SD, % | 48 ± 9* |
P < .05.
HSCs capable of long-term multilineage reconstitution reside within ossicle bone marrow
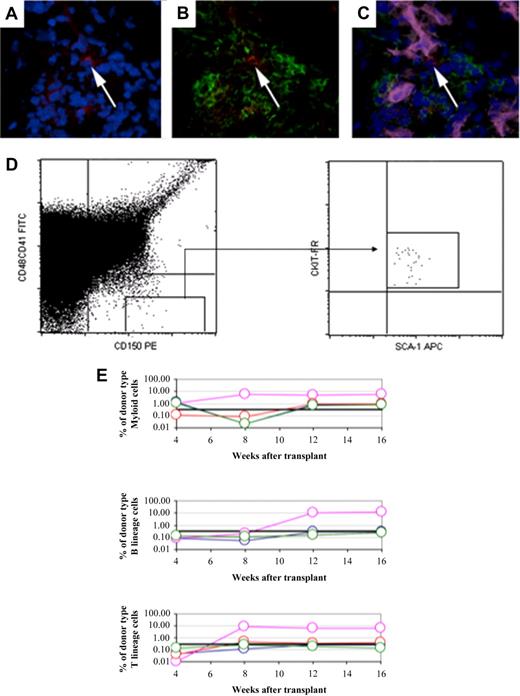
Individual HSCs (CD150+CD48−CD41−Lineage− cells) were identified within the bone marrow of ossicles (Figure 5A-C), and, as another test to determine the value of tissue-engineered ossicles as a functional HSC niche, HSCs were directly harvested from the ossicles and tested for their ability to rescue lethally irradiated mice. HSCs were sorted from ossicles by flow cytometry as CD150+CD48−CD41−SCA-1+ c-Kit+ cells. Five purified, ossicle-derived HSCs were then transplanted by retro-orbital injection into lethally irradiated mice with a radioprotective dose of whole bone marrow. Two of 4 mice showed long-term reconstitution with all lineages of blood cells (Figure 5D-E). Four of 4 mice were positive for myeloid cells, and 2 of 4 mice were positive for B- and T-cell lineage at 16 weeks.
Long constitution capacity of purified HSCs from ossicle bone marrow. HSCs were identified in ossicle bone marrow by immunofluorescent staining with a combination of CD150, CD48, CD41, MECA32 antibodies. The white arrow depicts a HSC that is CD150+ (red; A) but negative for CD48, CD41, Lin (green; B), and MECA32 (pink; C). The nuclei are stained with DAPI (blue). (D) HSCs from ossicles were isolated from the combined gate of CD150+CD41−CD48− and were confirmed to be positive for Sca-1 and c-kit. (E) Five HSCs harvested from ossicles supported long-term multilineage reconstitution in 2 of 4 lethally irradiated mice. Each line represents the frequency of donor-derived myeloid, B, or T cells in a single mouse at 4, 8, 12, and 16 weeks after transplantation. The black line at 0.3% represents the background threshold, meaning that reconstitution is not considered in mice falling below this line.
Long constitution capacity of purified HSCs from ossicle bone marrow. HSCs were identified in ossicle bone marrow by immunofluorescent staining with a combination of CD150, CD48, CD41, MECA32 antibodies. The white arrow depicts a HSC that is CD150+ (red; A) but negative for CD48, CD41, Lin (green; B), and MECA32 (pink; C). The nuclei are stained with DAPI (blue). (D) HSCs from ossicles were isolated from the combined gate of CD150+CD41−CD48− and were confirmed to be positive for Sca-1 and c-kit. (E) Five HSCs harvested from ossicles supported long-term multilineage reconstitution in 2 of 4 lethally irradiated mice. Each line represents the frequency of donor-derived myeloid, B, or T cells in a single mouse at 4, 8, 12, and 16 weeks after transplantation. The black line at 0.3% represents the background threshold, meaning that reconstitution is not considered in mice falling below this line.
Discussion
HSCs are arguably the most well-characterized stem cells, yet there remains much to be learned about their behavior and the influence of their niche microenvironment. It is thought that several cell types play a role in regulating the microenvironment and provide the signals for stem cell proliferation and differentiation.3 4,39 To facilitate a better understanding of the HSC niche in vivo, we characterized and manipulated a heterotopic model of bone formation in mice.
In this model system, heterotopic ossicles were developed from transplanted BMSCs. We demonstrated that hematopoietic cells in the ossicle bone marrow were derived from the host, which means that the hematopoietic components of ossicles are not derived from residual blood cells that may have been transplanted with the BMSCs. These data are consistent with previous reports that show the hematopoietic components and endothelial cells of the ossicle are from the recipient, whereas osteoblasts and reticular cells are of donor origin,4,15,32,40 comparing the bone marrow content of ossicles and femurs, heterotopic ossicles contained fewer HSCs and progenitor cells except for erythroid cells and had a lower density of blood vessels and megakaryocytes. PTH treatment resulted in marrow that more closely resembles long bone marrow. The factors that led to this difference are not clear. However, the differences may be due to a more limited blood supply in the subcutaneous site or lack of mechanical stimulation to which the skeleton is normally exposed.
Our experiments used the in vivo long-term competitive reconstitution assay and confirmed the existence of HSCs inside the ossicles, although the frequency of HSCs was lower than that observed in the long bone marrow. A few studies have sought to determine whether HSCs reside and function within heterotopic ossicle marrow. It has been established that heterotopically induced ossicles are repopulated with bone marrow after irradiation, and these repopulated cells have the same proliferative potential of CFU-Ss in the skeletal bone marrow.21,41 Now it is recognized that CFU-Ss are also derived from progenitor cells. Currently, the competitive long-term reconstitution assay is the most rigorous way to prove HSC activity. In our study, HSCs were also phenotypically sorted from ossicle bone marrow by signaling lymphocytic activation molecule family markers (CD105 and CD48) and injected into irradiated mice. The marked HSCs showed similar reconstitution activity to HSCs from long bone marrow. Thus, we demonstrated HSCs reside in heterotopic bone marrow by both functional assays and phenotypical sorting. These findings suggest that the microenvironment of tissue-engineered ossicle functions as a stem cell niche that can sustain HSCs.
In addition to finding that HSCs in ossicles were recruited from recipient mice long bone marrow, we also found that HSCs injected into the peripheral circulation homed to ossicles after whole body lethal irradiation. Furthermore, injection of whole bone marrow directly into ossicles resulted in donor type reconstitution in lethally irradiated mice. These data suggest that HSCs can migrate in and out of ossicles similar to long bone. The mechanism of trafficking and lodging of HSCs into the skeletal bones has been extensively studied,42 and such mechanisms may also apply to the HSCs in the ossicles.
Interestingly, we found that the frequency of HSCs in the ossicles increased after anabolic PTH treatment and to a lesser extent in long bone marrow. One potential reason for the greater influence of PTH on HSC numbers in the ossicles relative to femurs is that the transplanted BMSCs were actively differentiating into bone as the anabolic dose of PTH was administered, whereas the femurs were already fully developed in these mature animals. This explanation is consistent with studies that showed the anabolic effects of PTH on bone are greater in developing versus mature bone.36 Another study has shown that PTH treatment increases HSC frequency in long bone marrow.10 The difference between this and the current study could be due to the methods used to identify the cells. Calvi et al10 counted Lin−Sca-1+ C-kit+ cells by flow cytometry and used an in vitro long-term culture-initiating cell assay to quantify the number of HSCs, whereas we used an in vivo competitive long-term reconstitution assay. Our results did demonstrate a trend toward an increase of HSCs in PTH-treated femurs which would be consistent with the previous report,10 but did not reach statistical significance in our experiments.
In addition to increasing HSC frequency, a higher reconstitution rate was observed in mice with PTH-treated ossicles after direct ossicle injection. A more suitable niche environment may be present in PTH-treated ossicles. It is suggested that PTH may increase HSC numbers by regulating the expression of Jagged-1 on osteoblasts which in turn bind Notch receptors on linked HSCs,9,10,43 although deletion of Jagged-1 or Notch 1 from mice does not affect HSC maintenance. There is also compelling evidence to suggest that bone marrow endothelium may be a target tissue for PTH and PTH-related protein. PTH may exert its effect on vascular endothelial cells via the PTH/PTH related protein receptor44 or indirectly through insulin-like growth factor receptor.45 Bone-derived endothelial cells respond to PTH by production of cyclic adenosine monophosphate46 or through proteinase K pathway.47 In our study, we also found that the number of microvessels in ossicles increased significantly after PTH treatment. Our findings also suggest that PTH modulates SDF-1 expression in both ossicles and long bones. As a key regulator of the HSC niche, the increase of SDF-1 in PTH-treated groups supports the observation that PTH can enhance the stem cell niche in bone.
The tissue-engineered, heterotopic model system provides an innovative platform to understand the mechanisms used by HSCs, endothelial cells, and BMSCs to regulate each other's function. Advantages of this model include the potential to manipulate the stromal cells that form the ossicle and the ability to augment the niche with molecular medicine approaches within the context of normal host hematopoiesis. Thus, this model may serve as a model to dissect the stem cell niche in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Army Research Laboratory and the United States Army Research Office (grant DAAD 190310168) and the National Institutes of Health (grant DK082481).
National Institutes of Health
Authorship
Contribution: J.S. performed experiments, analyzed data, and wrote the manuscript; M.J.K. and P.H.K. performed experiments, analyzed data, and contributed to the writing; Z.W. and J.W. performed experiments and analyzed data; and R.S.T. and S.J.M. helped design the study, analyzed data, and contributed to the writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul H. Krebsbach, Department of Biologic and Materials Sciences, School of Dentistry, K1030, 1011 N University Ave, University of Michigan, Ann Arbor, MI 48109-1078; e-mail: paulk@umich.edu.