To the editor:
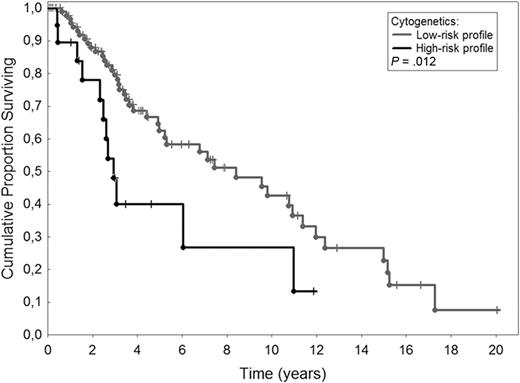
Primary myelofibrosis (PMF) is a Philadelphia chromosome–negative myeloproliferative neoplasm whose diagnostic criteria have been recently updated.1 Roughly one-third of the patients have an abnormal karyotype for the most part corresponding to deletions of chromosome 13, of chromosome 20q and to partial duplication of chromosome 1q.2 Current prognostication in PMF is based on the International Prognostic Scoring System (IPSS).3 Different studies demonstrated that individual cytogenetic abnormalities may affect survival of patients with PMF.4-6 Hussein et al7 have just developed a cytogenetic-based prognostic model useful to predict survival in patients with PMF by grouping cytogenetics as favorable (sole +9, sole 20q−, sole 13q−), normal, unfavorable (complex karyotype or sole +8), and other abnormalities. A significant impact on survival was also obtained by merging cytogenetics into larger categories such as favorable/normal and unfavorable/other abnormalities. To validate this prognostic stratification, we evaluated 114 patients with PMF whose cytogenetics was available at diagnosis. Approval was obtained from the Pavia Institutional Review Board. Informed consent was provided in accordance with the Declaration of Helsinki. Patients have been regularly followed at the Division of Hematology of Pavia between 1975 and 2009. Quinicrine banding (1975-1989) and Giemsa banding (1990-2009) analyses were performed on 24- and 48-hour bone marrow cultures or peripheral blood.8 Median age was 59 years (range, 18-84 years) with a male/female ratio of 70/44. According to IPSS, 34 (30%) patients had low-risk PMF, 29 (25%) intermediate-1, 29 (25%) intermediate-2, and 22 (20%) high-risk. Among 52 patients evaluated for JAK2 status, 28 (54%) were JAK2V617F-positive. Median follow-up was 3.1 years (range, 0.6-20 years). Although missing abnormalities could not be ruled out in patients evaluated many years ago, karyotype was favorable in 2 patients (sole 20q−, sole +9), normal in 92, unfavorable in 8 (sole +8 in 5, complex karyotype in 3), and included other abnormalities in 12 patients. We did not find 13q deletions. The low number of patients belonging to the favorable category does not allow a meaningful comparison among groups. So, we carried out survival analysis by stratifying patients into larger categories: favorable/normal (n = 94) and unfavorable/other abnormalities (n = 20). We found that patients with unfavorable/other cytogenetic profile had a significant shorter survival than patients with favorable/normal cytogenetic profile (P = .012). Figure 1 shows Kaplan-Meier estimate of survival in the 2 groups: median survival was 2.9 years in the group with low-risk profile and 7.8 years in that with high-risk profile. After adjusting for IPSS in a multivariable Cox proportional hazard regression, the cytogenetic-based prognostic stratification retained its significant impact on survival with a hazard ratio of 2.19 (95% confidence interval 1.13-4.26; P = .021). This means that patients with high-risk profile have a 2.19-fold higher risk of death than those with low-risk profile. To rule out the effect of the JAK2V617F mutation, we performed a multivariable analysis with cytogenetic-based risk categories, JAK2 mutation status, and IPSS groups as covariates in 52 patients with known JAK2 status. Cytogenetics remained an independent predictor of survival (P = .027).
Kaplan-Meier estimate of survival in 114 patients with primary myelofibrosis according to cytogenetic profile at diagnosis. Low-risk profile included 20 patients with sole +9, sole 20q−, and normal karyotype. High-risk profile included 94 patients with complex karyotype, sole +8, or other abnormalities. The 2 survival curves were significantly different (P = .012).
Kaplan-Meier estimate of survival in 114 patients with primary myelofibrosis according to cytogenetic profile at diagnosis. Low-risk profile included 20 patients with sole +9, sole 20q−, and normal karyotype. High-risk profile included 94 patients with complex karyotype, sole +8, or other abnormalities. The 2 survival curves were significantly different (P = .012).
In conclusion, this study confirms that having a complex karyotype or abnormalities other than sole 20q− or sole +9 implies a shorter survival in PMF.
Authorship
Acknowledgments: This work has been supported in part by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro), Fondazione Cariplo, and Alleanza Contro il Cancro to M.C.
Contribution: E.R. and F.P. conceived the study, collected, analyzed, interpreted data, and wrote the paper; P.B. performed cytogenetics; L.A. and C.E. collected clinical data; D.P. performed JAK2 mutation analysis; E.B. performed bone marrow evaluation; C.P. did statistical analysis; and M.C. and M.L. analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisa Rumi, MD, Division of Hematology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: elisarumi@hotmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal