Abstract
The Children's Cancer Group 1952 (CCG-1952) clinical trial studied the substitution of oral 6-thioguanine (TG) for 6-mercaptopurine (MP) and triple intrathecal therapy (ITT) for intrathecal methotrexate (IT-MTX) in the treatment of standard-risk acute lymphoblastic leukemia. After remission induction, 2027 patients were randomized to receive MP (n = 1010) or TG (n = 1017) and IT-MTX (n = 1018) or ITT (n = 1009). The results of the thiopurine comparison are as follows. The estimated 7-year event-free survival (EFS) for subjects randomized to TG was 84.1% (± 1.8%) and to MP was 79.0% (± 2.1%; P = .004 log rank), although overall survival was 91.9% (± 1.4%) and 91.2% (± 1.5%), respectively (P = .6 log rank). The TG starting dose was reduced from 60 to 50 mg/m2 per day after recognition of hepatic veno-occlusive disease (VOD). A total of 257 patients on TG (25%) developed VOD or disproportionate thrombocytopenia and switched to MP. Once portal hypertension occurred, all subjects on TG were changed to MP. The benefit of randomization to TG over MP, as measured by EFS, was evident primarily in boys who began TG at 60 mg/m2 (relative hazard rate [RHR] 0.65, P = .002). The toxicities of TG preclude its protracted use as given in this study. This study is registered at http://clinicaltrials.gov as NCT00002744.
Introduction
When the Children's Cancer Group 1952 (CCG-1952) study was designed, the long-term event-free survival (EFS) rate for patients with standard-risk acute lymphoblastic leukemia (SR-ALL) approached 80%.1-3 Nonetheless, relapses within this favorable group represented nearly half of all relapses in childhood ALL. We hypothesized that a significant decrease in the relapse rate could be achieved with more effective use of conventional chemotherapeutic agents. With a 2 × 2 factorial design, we compared substitution of intrathecal triple therapy (ITT: methotrexate, cytarabine, and hydrocortisone sodium succinate) for intrathecal methotrexate (IT-MTX) and oral 6-thioguanine (TG) for oral 6-mercaptopurine (MP) after remission induction. Results of the intrathecal comparison were previously reported.4 The current report focuses on the thiopurine comparison.
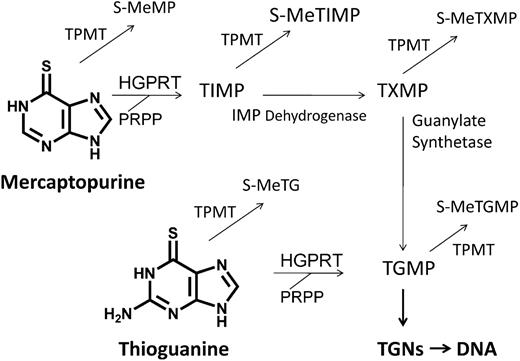
Oral MP has been a mainstay of ALL maintenance for more than 40 years. MP and TG are structural analogs of hypoxanthine and guanine, 2 naturally occurring purines.5 Both compounds are prodrugs and exert their primary cytotoxicity after enzymatic conversion to thioguanylate (TGMP) and incorporation as thioguanine nucleotides (TGNs) into DNA and RNA as false purine bases (Figure 1).6 Thiopurines and their metabolites also inhibit de novo purine synthesis.7
Thiopurine conversion to active nucleotides and methylated compounds. MP is converted to thioguanine nucleotides (TGNs) via 3 enzymatic steps and TG via 1 step. Both MP and TG undergo S-methylation by thiopurine methyltransferase (TPMT) to methyl-mercaptopurine (meMP) and methyl-thioguanine (meTG), respectively. TPMT competes with hypoxanthine-guanine phosphoribosyltransferase (HGPRT), the first enzymatic step in conversion of thiopurines to TGNs. Thioinosine monophosphate (TIMP), thioxanthene monophosphate (TXMP), and thioguanylate (TGMP) are also S-methylated by TPMT. Methyl-6-thioinosine monophosphate (meTIMP), a product of MP but not TG metabolism, is a potent inhibitor of de novo purine synthesis. meTXMP indicates methyl-thioxanthene monophosphate; IMP, inosine monophosphate; and meTGMP, methyl-thioguanylate.
Thiopurine conversion to active nucleotides and methylated compounds. MP is converted to thioguanine nucleotides (TGNs) via 3 enzymatic steps and TG via 1 step. Both MP and TG undergo S-methylation by thiopurine methyltransferase (TPMT) to methyl-mercaptopurine (meMP) and methyl-thioguanine (meTG), respectively. TPMT competes with hypoxanthine-guanine phosphoribosyltransferase (HGPRT), the first enzymatic step in conversion of thiopurines to TGNs. Thioinosine monophosphate (TIMP), thioxanthene monophosphate (TXMP), and thioguanylate (TGMP) are also S-methylated by TPMT. Methyl-6-thioinosine monophosphate (meTIMP), a product of MP but not TG metabolism, is a potent inhibitor of de novo purine synthesis. meTXMP indicates methyl-thioxanthene monophosphate; IMP, inosine monophosphate; and meTGMP, methyl-thioguanylate.
In vitro evidence suggested a potential therapeutic advantage of TG over MP: TG is converted to active TGMP and TGNs with fewer steps than MP (Figure 1), TG has cytotoxicity against ALL cell lines and leukemic blasts at concentrations 10-fold less than MP, and TG requires briefer exposure than MP to induce cytotoxicity.8 Other data also suggested an advantage of TG over MP: TG produced 4-fold higher concentrations of red blood cell (RBC) TGNs than isotoxic MP, lower concentrations of RBC TGNs correlated with relapse in patients on MP, and clinically achievable central nervous system (CNS) levels of TG were cytotoxic in vitro.8-11 Taken together, these data fueled the hypothesis that substitution of TG for MP may improve the outcome in SR-ALL.
Methods
Patients
Subjects were enrolled in CCG-1952 between May 1, 1996, and February 1, 2000. Eligible patients met criteria for precursor-B or T-cell ALL and the National Cancer Institute definition of SR-ALL (1 to < 10 years of age with presenting white blood cell count [WBC] < 50 × 109/L),12 with inclusion of subjects with T-cell ALL who met this National Cancer Institute definition. Patients treated with systemic corticosteroids for more than 48 hours during the preceding month were ineligible.
Treatment protocol
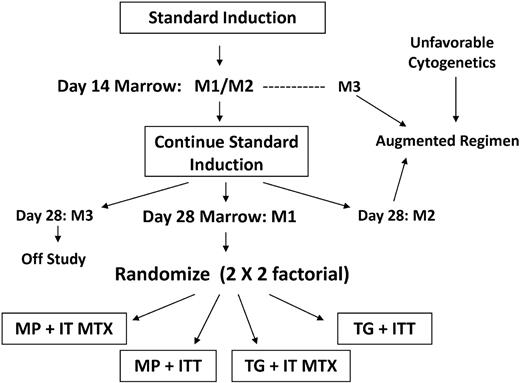
The protocol was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from parents or guardians according to guidelines of the National Institutes of Health. A schematic diagram of the study design is shown in Figure 2. Patients eligible for postinduction randomization could not have unfavorable blast cytogenetics (t(4;11), t(9;22), or < 45 hypodiploidy) or M3 (> 25% blasts) marrow status at induction day 14, and they had to achieve morphologic remission (M1: < 5% blasts) by day 28. SR-ALL patients with overt CNS or testicular disease at diagnosis were included. Randomization to 1 of 4 treatment regimens (MP/IT-MTX; MP/ITT; TG/IT-MTX; or TG/ITT) was carried out by the CCG statistical office according to a 2 × 2 factorial design. Details of therapy are shown in Table 1. Girls were treated for 2 years and boys for 3 years from the start of the first interim maintenance (IM) phase. The backbone chemotherapy was based on results of CCG-1891, wherein 2 delayed intensification (DI) phases appeared to improve the outcome of children with intermediate-risk ALL.2 Prednisone was the steroid used in induction and maintenance; dexamethasone was given in both DI phases. The doses of thiopurines and oral MTX were adjusted during maintenance to maintain an absolute neutrophil count (WBC × %[segmented + band neutrophils]) between 1 ×109/L (1000/μL) and 2 ×109/L (2000/μL) and platelet count 100 × 109/L (100 000/μL) or higher.
Study schema for CCG-1952. Randomization was performed at the completion of standard induction. Patients with M1 marrow status at day 7 did not require a day-14 sample.
Study schema for CCG-1952. Randomization was performed at the completion of standard induction. Patients with M1 marrow status at day 7 did not require a day-14 sample.
Treatment
| Phase/drug . | Dose . | Schedule . |
|---|---|---|
| Induction (4 wk) | ||
| IT cytarabine | Age-adjusted§ | Day 0 |
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 7, 14, 21 |
| Asparaginase† IM | 6000 U/m2 | M, W, F ×9 doses |
| Prednisone | 40 mg/m2 per day | Days 0-27 |
| IT-MTX | Age-adjusted‖ | Days 7, 28¶ |
| Consolidation* (4 wk) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Day 0 |
| Prednisone | Taper | Days 0-10 |
| Mercaptopurine or thioguanine | 75 mg/m2 per day or 60-50 mg/m2 per day# | Days 1-27 |
| IT-MTX or ITT | Age-adjusted‖ | Days 7, 14, 21** |
| Interim maintenance 1 (8 wk) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 28 |
| Prednisone | 40 mg/m2 per day | Days 0-4, 28-32 |
| Mercaptopurine or thioguanine | 75 mg/m2 per day or 60-50 mg/m2 per day# | Days 0-49 |
| Methotrexate | 20 mg/m2 per day PO | Weekly ×8 doses |
| Delayed intensification 1 (8 wk) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 7, 14 |
| Asparaginase† | 6000 U/m2 per dose | M, W, F ×6 doses |
| Dexamethasone PO | 10 mg/m2 per day | Days 0-6, 14-20 |
| Doxorubicin | 25 mg/m2 per day | Days 0, 7, 14 |
| Cytarabine IV or SQ | 75 mg/m2 per day | Days 28-31, 35-38 |
| Cyclophosphamide | 1000 mg/m2 | Day 28 |
| Thioguanine | 60 mg/m2 per day | Days 28-41 |
| IT-MTX or ITT | Age-adjusted‖ | Days 0, 28, 35 |
| Maintenance‡ (12-wk cycles) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 28, 56 |
| Prednisone | 40 mg/m2 per day | Days 0-4, 28-32, 56-60 |
| Mercaptopurine or thioguanine | 75 mg/m2 per day or 60-50 mg/m2 per day# | Daily |
| Methotrexate PO | 20 mg/m2 per dose | Weekly |
| IT-MTX or ITT | Age-adjusted‖ | Day 0 |
| Phase/drug . | Dose . | Schedule . |
|---|---|---|
| Induction (4 wk) | ||
| IT cytarabine | Age-adjusted§ | Day 0 |
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 7, 14, 21 |
| Asparaginase† IM | 6000 U/m2 | M, W, F ×9 doses |
| Prednisone | 40 mg/m2 per day | Days 0-27 |
| IT-MTX | Age-adjusted‖ | Days 7, 28¶ |
| Consolidation* (4 wk) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Day 0 |
| Prednisone | Taper | Days 0-10 |
| Mercaptopurine or thioguanine | 75 mg/m2 per day or 60-50 mg/m2 per day# | Days 1-27 |
| IT-MTX or ITT | Age-adjusted‖ | Days 7, 14, 21** |
| Interim maintenance 1 (8 wk) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 28 |
| Prednisone | 40 mg/m2 per day | Days 0-4, 28-32 |
| Mercaptopurine or thioguanine | 75 mg/m2 per day or 60-50 mg/m2 per day# | Days 0-49 |
| Methotrexate | 20 mg/m2 per day PO | Weekly ×8 doses |
| Delayed intensification 1 (8 wk) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 7, 14 |
| Asparaginase† | 6000 U/m2 per dose | M, W, F ×6 doses |
| Dexamethasone PO | 10 mg/m2 per day | Days 0-6, 14-20 |
| Doxorubicin | 25 mg/m2 per day | Days 0, 7, 14 |
| Cytarabine IV or SQ | 75 mg/m2 per day | Days 28-31, 35-38 |
| Cyclophosphamide | 1000 mg/m2 | Day 28 |
| Thioguanine | 60 mg/m2 per day | Days 28-41 |
| IT-MTX or ITT | Age-adjusted‖ | Days 0, 28, 35 |
| Maintenance‡ (12-wk cycles) | ||
| Vincristine | 1.5 mg/m2 (2 mg max) | Days 0, 28, 56 |
| Prednisone | 40 mg/m2 per day | Days 0-4, 28-32, 56-60 |
| Mercaptopurine or thioguanine | 75 mg/m2 per day or 60-50 mg/m2 per day# | Daily |
| Methotrexate PO | 20 mg/m2 per dose | Weekly |
| IT-MTX or ITT | Age-adjusted‖ | Day 0 |
For interim maintenance 2 (8 wk) see interim maintenance 1; for delayed intensification 2 (8 wk) see delayed intensification 1.
IT indicates intrathecal; PO, by mouth; IM, intramuscular; IV, intravenous; SQ, subcutaneous; M, Monday; W, Wednesday; and F, Friday.
In 12 fractions, 2400 cGy to cranium and in 3 fractions, 600 cGy to spine for CNS-3; 2400 cGy in 8 fractions to both testes in consolidation for testicular disease at diagnosis.
Escherichia coli preparation, or Erwinia asparaginase for patients with allergic reactions.
Boys receive ∼ 4 more cycles than girls.
Age: 1-1.99 y, 30 mg; age 2-2.99 y, 50 mg; and age ≥ 3 y, 70 mg.
IT-MTX: age 1-1.99 y, 8 mg; age 2-2.99 y, 10 mg; and age ≥ 3 y, 12 mg; ITT: MTX the same as IT-MTX until age ≥ 9 y, 15 mg; hydrocortisone: same as age-adjusted MTX doses; cytarabine: 1-1.99 y, 16 mg; 2-2.99 y, 20 mg; 3-8.99 y, 24 mg; and ≥ 9 y, 30 mg.
Patients with CNS disease at diagnosis dosed on days 7, 14, 21, and 28.
Dose reduced from 60 to 50 mg/m2 per day in February 1998.
Patients with CNS disease at diagnosis on day 7 only.
Protocol modifications
Four episodes of reversible congestive hepatopathy and consumptive thrombocytopenia, consistent with TG-induced hepatic veno-occlusive disease (VOD), or sinusoidal occlusion syndrome (SOS),13 were reported during the first year of patient accrual, prompting the addition of a data capture form designed to assess additional cases. The protocol was amended with guidelines for early detection and supportive care management of VOD. With further reports of VOD on the TG regimen, the target dose of TG was reduced in January 1998 from 60 to 50 mg/m2.14 In early 2001, portal hypertension was recognized as a late complication of TG. This unexpected toxicity prompted the COG Data Monitoring Committee in April 2001 to require that all patients on TG switch to MP.
Bone marrow evaluations
Cytogenetic studies were performed on marrow samples at local institutions using standard techniques and nomenclature.15 The CCG Cytogenetics Committee centrally reviewed each karyotype. ETV6-RUNX1 (formerly TEL-AML1) expression was analyzed by reverse-transcriptase–polymerase chain reaction in the CCG ALL Reference Laboratory on blasts from the first 1000 subjects, using the conditions and primers reported by Shurtleff et al.16 Local institutions determined marrow blast percentage by morphology on aspirates obtained on induction days 7, 14, and 28.17
Statistical methods
A detailed description of the power calculations, tests for statistical interaction between the intrathecal and thiopurine regimens, and interim analyses for CCG-1952 were previously published.4 Outcome comparisons used an intent-to-treat approach. EFS and OS comparisons began at the time of randomization with a data cutoff time point of spring 2006. Analysis of isolated CNS and bone marrow (BM) relapse rates was performed using a cumulative incidence function.18 EFS and OS life table estimates were determined by the Kaplan-Meier method,19 with standard error calculated using the Peto variance formula.20 Events after remission induction were defined as first relapse at any site, death in first remission, and second malignant neoplasm. Patients lost to follow-up were censored at the date of last contact. Relative hazard rates (RHRs) were estimated by the log-rank method of observed divided by expected events.21 The cumulative incidence of isolated CNS (iCNS) relapse, EFS, and OS are presented as percentage (± standard error). Outcome analyses initially compared the entire TG versus MP cohorts. Subsequently, subjects were divided into 2 subgroups: those enrolled before and those enrolled after December 26, 1997, reflecting the date of enrollment in the study after which subjects randomized to TG began consolidation at the reduced dose of 50 mg/m2. Chi-square tests for homogeneity of distributions, 2-tailed Fisher exact test, and Cox proportional hazards model were used in some analyses.22
Relapse definitions
Isolated CNS (iCNS) relapse was defined as the presence of WBC .005×109/L (5/μL) or higher in cerebrospinal fluid (CSF) with morphologically identifiable blasts on cytospin, and with no evidence of leukemia elsewhere. Diagnosis of testicular relapse required tissue confirmation. Bone marrow relapse was defined as M3 status (> 25% blasts) after first complete remission with or without relapse at another site.
Results
Patients
Of 2175 patients enrolled on CCG-1952, 2030 subjects were randomized.4 Three randomized subjects were deemed ineligible because of improper consent, lack of day-14 marrow sample, or M3 marrow status at day 14. A total of 2027 eligible patients are included in the statistical comparisons of the randomized treatment regimens: 1017 randomized to TG (TG/IT-MTX: 509; TG/ITT: 508) and 1010 randomized to MP (MP/IT-MTX: 509; MP/ITT: 501). As shown in Table 2, presenting features were statistically similar between the 2 thiopurine cohorts, except for palpable hepatomegaly and CNS-2 status (CSF WBC < .005×109/L [5/μL] with blasts on cytocentrifuge preparation). Hepatomegaly was present significantly more often in the TG group (P = .01). CNS-2 disease was more prevalent among subjects on MP than TG (P = .07). Sixty-eight subjects on the TG regimen and 80 on the MP regimen have been lost to follow-up.
Presenting features
| Characteristic . | Randomized treatment, no. (%) . | P* . | ||
|---|---|---|---|---|
| Total . | MP . | TG . | ||
| Age, y | .49 | |||
| 1 to 1.99 | 165 (8.1) | 79 (7.8) | 86 (8.5) | |
| 2 to 5.99 | 1402 (69.2) | 711 (70.4) | 691 (67.9) | |
| 6 to 9.99 | 460 (22.7) | 220 (21.8) | 240 (23.6) | |
| Sex | .37 | |||
| Boys | 1129 (55.7) | 552 (54.7) | 577 (56.7) | |
| Girls | 898 (44.3) | 458 (45.3) | 440 (43.3) | |
| Race/ethnicity† | .27 | |||
| White | 1376 (68.2) | 677 (67.2) | 699 (69.1) | |
| Black | 72 (3.6) | 35 (3.5) | 37 (3.7) | |
| Hispanic | 444 (22.0) | 221 (22.0) | 223 (22.1) | |
| Other | 125 (6.2) | 73 (7.3) | 52 (5.1) | |
| Down syndrome | .98 | |||
| Yes | 57 (2.8) | 28 (2.8) | 29 (2.9) | |
| CNS status† | .16 | |||
| CNS-1 | 1831 (92.8) | 902 (91.7) | 929 (93.8) | |
| CNS-2 | 113 (5.7) | 66 (6.7) | 47 (4.8) | .07¶ |
| CNS-3 | 30 (1.5) | 16 (1.6) | 14 (1.4) | |
| WBC, ×109/L | .52 | |||
| Lower than 20 | 1660 (81.9) | 821 (81.3) | 839 (82.5) | |
| 20 or higher | 367 (18.1) | 189 (18.7) | 178 (17.5) | |
| Hepatomegaly | .01 | |||
| Yes | 999 (49.6) | 466 (46.4) | 533 (52.8) | |
| Immunophenotype† | .60 | |||
| B lineage | 1802 (93.3) | 681 (94.1) | 689 (93.5) | |
| T lineage | 130 (6.7) | 43 (5.9) | 48 (6.5) | |
| Trisomies 4, 10, and 17‡ | .96 | |||
| Yes | 121 (13.7) | 60 (13.9) | 61 (13.6) | |
| ETV6-RUNX1§ | .93 | |||
| Yes | 225 (23.9) | 115 (24.2) | 110 (23.7) | |
| Day-14 marrow status† | .86 | |||
| M1‖ | 1795 (91.1) | 890 (90.9) | 905 (91.2) | |
| M2 | 176 (8.9) | 89 (9.1) | 87 (8.8) | |
| Characteristic . | Randomized treatment, no. (%) . | P* . | ||
|---|---|---|---|---|
| Total . | MP . | TG . | ||
| Age, y | .49 | |||
| 1 to 1.99 | 165 (8.1) | 79 (7.8) | 86 (8.5) | |
| 2 to 5.99 | 1402 (69.2) | 711 (70.4) | 691 (67.9) | |
| 6 to 9.99 | 460 (22.7) | 220 (21.8) | 240 (23.6) | |
| Sex | .37 | |||
| Boys | 1129 (55.7) | 552 (54.7) | 577 (56.7) | |
| Girls | 898 (44.3) | 458 (45.3) | 440 (43.3) | |
| Race/ethnicity† | .27 | |||
| White | 1376 (68.2) | 677 (67.2) | 699 (69.1) | |
| Black | 72 (3.6) | 35 (3.5) | 37 (3.7) | |
| Hispanic | 444 (22.0) | 221 (22.0) | 223 (22.1) | |
| Other | 125 (6.2) | 73 (7.3) | 52 (5.1) | |
| Down syndrome | .98 | |||
| Yes | 57 (2.8) | 28 (2.8) | 29 (2.9) | |
| CNS status† | .16 | |||
| CNS-1 | 1831 (92.8) | 902 (91.7) | 929 (93.8) | |
| CNS-2 | 113 (5.7) | 66 (6.7) | 47 (4.8) | .07¶ |
| CNS-3 | 30 (1.5) | 16 (1.6) | 14 (1.4) | |
| WBC, ×109/L | .52 | |||
| Lower than 20 | 1660 (81.9) | 821 (81.3) | 839 (82.5) | |
| 20 or higher | 367 (18.1) | 189 (18.7) | 178 (17.5) | |
| Hepatomegaly | .01 | |||
| Yes | 999 (49.6) | 466 (46.4) | 533 (52.8) | |
| Immunophenotype† | .60 | |||
| B lineage | 1802 (93.3) | 681 (94.1) | 689 (93.5) | |
| T lineage | 130 (6.7) | 43 (5.9) | 48 (6.5) | |
| Trisomies 4, 10, and 17‡ | .96 | |||
| Yes | 121 (13.7) | 60 (13.9) | 61 (13.6) | |
| ETV6-RUNX1§ | .93 | |||
| Yes | 225 (23.9) | 115 (24.2) | 110 (23.7) | |
| Day-14 marrow status† | .86 | |||
| M1‖ | 1795 (91.1) | 890 (90.9) | 905 (91.2) | |
| M2 | 176 (8.9) | 89 (9.1) | 87 (8.8) | |
χ2, 2-sided P value; P values reflect the comparison between TG and MP cohorts with respect to the categories within each characteristic.
Fewer than 2027 cases reflect missing data.
Among 882 cases with centrally approved cytogenetics.
Among 940 cases analyzed by reverse-transcriptase–polymerase chain reaction for ETV6-RUNX1.
Includes patients with M1 marrow on day 7.
P value reflects CNS-2 vs CNS-1.
TG-induced toxicity
VOD.
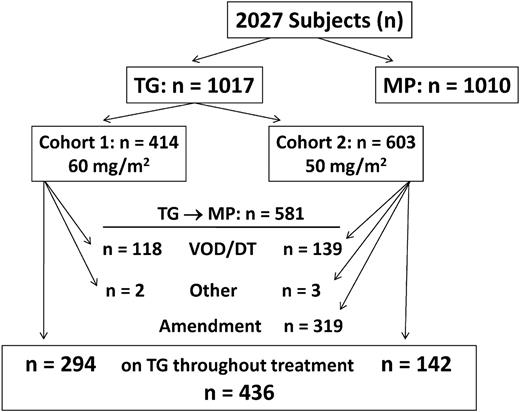
As determined by analysis of the first 30 episodes of VOD in this study, a diagnosis of VOD required at least 2 of 3 conditions: acute onset of palpable hepatomegaly, acute thrombocytopenia, and ascites on ultrasound (USG).14 Elevated bilirubin and reversal of hepatic blood flow by Doppler USG were not required. During the entire study period, 206 (20%) patients randomized to TG developed reversible VOD and switched to MP upon clinical recovery (Table 3). Among subjects on TG, 414 (40.7%) began consolidation at the daily TG dose of 60 mg/m2, and 603 (59.3%) began at the amended dose of 50 mg/m2. In addition, 3 patients randomized to the MP regimens developed VOD after completing 14 days of TG in DI. No VOD occurred while patients received MP. Among the 8 patients who had liver biopsies, 6 had pathologic evidence of VOD.
Episodes of TG-induced toxicities requiring switch to MP
| . | C/IM 1* . | DI 1* . | IM 2* . | DI 2* . | Maint 1-4 . | Maint > 4* . | Total (%†) . |
|---|---|---|---|---|---|---|---|
| VOD | 16 | 13 | 23 | 11 | 119 | 24 | 206 (80) |
| DT | 0 | 0 | 0 | 0 | 28 | 23 | 51 (20) |
| Total (%†) | 16 (6.2) | 13 (5.0) | 23 (8.9) | 11 (4.3) | 147 (57.3) | 47 (18.3) | 257 (100) |
| . | C/IM 1* . | DI 1* . | IM 2* . | DI 2* . | Maint 1-4 . | Maint > 4* . | Total (%†) . |
|---|---|---|---|---|---|---|---|
| VOD | 16 | 13 | 23 | 11 | 119 | 24 | 206 (80) |
| DT | 0 | 0 | 0 | 0 | 28 | 23 | 51 (20) |
| Total (%†) | 16 (6.2) | 13 (5.0) | 23 (8.9) | 11 (4.3) | 147 (57.3) | 47 (18.3) | 257 (100) |
Data represent number and percentage of episodes of VOD or DT by treatment phase.
C/IM indicates consolidation/interim maintenance; DI, delayed intensification (TG 60 mg/m2 per day for 14 days); and maint, maintenance.
Primarily boys, treated 1 year longer than girls.
Reflects percentage of total episodes.
Clinical parameters of all 206 cases of VOD were captured in detail. Although there was no central review, the study chair reviewed each case. The liver was palpably enlarged in 91% of cases, extending below the umbilicus in 32%. Ascites was documented in 42% (n = 70) of 168 patients who underwent Doppler USG and 11 of the 168 had reversal of flow. Median platelet count and hemoglobin at onset were 46 × 109/L (46 000/μL) and 91 g/L (9.1 g/dL), respectively. Fever was reported in 43% of cases; right upper quadrant tenderness, in 41%; splenomegaly, in 40%; and significant weight gain, in 13%. The median (range) peak total bilirubin and alanine aminotransferase during an episode was 18.8144 μM (1.1 mg/dL; range, 5.1312-384.84μM [0.3-22.5 mg/dL]) and 100 (13-3488) IU/L, respectively. The majority of patients required hospitalization for supportive care; 46% received platelet transfusions and 54%, packed RBC transfusions. Three patients developed acute hepatic failure during or after VOD, of whom 1 required liver transplantation, and 1 was hospitalized in the intensive care unit for 3 weeks, but no episode of VOD was fatal. After resolution of VOD, MP was tolerated for the remainder of treatment.
Disproportionate thrombocytopenia.
Fifty-one patients (5%) on TG were recognized to have ongoing thrombocytopenia (platelets < 75 × 109/L [75 000/μL]) over a minimum of 2 months, out of proportion to the degree of neutropenia or anemia, and without signs of acute VOD (Table 3). Some cases had mild hepatomegaly or splenomegaly. The thrombocytopenia necessitated numerous dose reductions of TG and oral MTX, and some patients received platelet transfusions. This toxicity, coined disproportionate thrombocytopenia (DT), was presumed to reflect subclinical VOD or sinusoidal obstruction syndrome (SOS), caused by repeated TG-induced sinusoidal injury but without congestive hepatopathy.13 All 51 cases of DT were identified in maintenance and all patients switched to MP. DT initially resolved in all cases, but splenomegaly and mild thrombocytopenia persisted or developed in a subset. In addition, 6 patients who continued TG throughout treatment were determined retrospectively to have developed DT during the second year of maintenance.
In summary, VOD or DT developed in 28.5% (n = 118/414) and 23% (n = 139/603) of patients who began TG at the 60 mg/m2 and 50 mg/m2 doses, respectively (P = .056, Fisher exact test). Comparison by sex found boys more likely than girls to develop these toxicities by the end of maintenance no. 4, when girls were still in treatment: 17.7% (n = 78/440) of girls and 22.9% (n = 132/577) of boys (P = .05, Fisher exact test). Incidence of VOD did not differ by age or WBC at diagnosis or by intrathecal regimen. An additional 5 patients were removed from the TG regimen, 2 because of severe myelosuppression with homozygous thiopurine methyltransferase (TPMT) deficiency and 3 for unknown reasons.23 Thus, 262 patients (26%) randomized to TG switched to MP because of toxicity. In April 2001, 2 patients randomized to TG were reported to have bleeding varices from portal hypertension. TG was then discontinued in 221 boys and 98 girls who remained on treatment. As detailed in Figure 3, a total of 581 cases (57%) among 1017 randomized to TG switched to MP before completion of therapy, although only 10% did so before starting maintenance.
Switch from TG to MP. A total of 2027 eligible children were randomized to TG (1017) or MP (1010) and are included in this intent-to-treat analysis. The first 414 subjects randomized to TG began consolidation with TG at 60 mg/m2; the last 603 began at 50 mg/m2. During the course of treatment, 581 patients switched from TG to MP. Of the 1017 subjects, 436 (43%) received TG throughout treatment: 294 in cohort 1 and 142 in cohort 2.
Switch from TG to MP. A total of 2027 eligible children were randomized to TG (1017) or MP (1010) and are included in this intent-to-treat analysis. The first 414 subjects randomized to TG began consolidation with TG at 60 mg/m2; the last 603 began at 50 mg/m2. During the course of treatment, 581 patients switched from TG to MP. Of the 1017 subjects, 436 (43%) received TG throughout treatment: 294 in cohort 1 and 142 in cohort 2.
Outcome of treatment
EFS and OS estimates at 7 years for the 2027 randomized patients were 81.5% (± 1.4%) and 91.5% (± 1.0%), respectively.
Outcome according to thiopurine regimen.
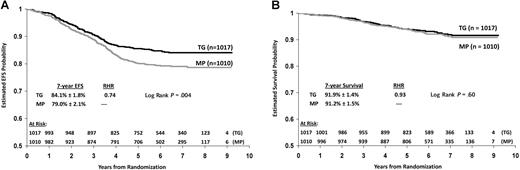
Patients randomized to TG had significantly better 7-year EFS than those randomized to MP, despite the crossover of 581 patients to the MP treatment regimen: 84.1% (± 1.8%) versus 79% (± 2.1%), respectively (P = .004 log rank; Figure 4A). However, there was no difference in the 7-year estimates of OS between TG and MP: 91.9% (± 1.4%) and 91.2% (± 1.5%), respectively (P = .6 log rank; Figure 4B). With a median follow-up of 7 years, 156 events (relapses, remission deaths, or second malignant neoplasms) occurred among 1017 patients on TG, and 206 events occurred in 1010 patients on MP. Compared with patients randomized to TG, patients on MP experienced a higher rate of iCNS relapse (56 vs 33; 7-year cumulative incidence = 5.8% vs 3.4%, P = .01 log rank). The latter difference is largely explained by the higher rate of iCNS relapse among patients with CNS-2 disease on MP than on TG (MP iCNS relapse: CNS-2 = 20%, CNS-1 = 4.7%, RHR = 5.06, P < .001; TG iCNS relapse: CNS-2 = 7%, CNS-1 = 3.4%, RHR = 2.05, P = .37). Patients on MP had a higher incidence of bone marrow relapse than those on TG (114 vs 84; 7-year cumulative incidence = 12.9% vs 9.2%, P = .018 log rank). Both thiopurine groups had similar testicular or other extramedullary site relapses (22 vs 25), remission deaths (9 vs 10), and second malignant neoplasms (5 vs 4).
Outcome according to randomized thiopurine regimen, TG versus MP. (A) EFS and (B) OS by intent-to-treat analysis.
Outcome according to randomized thiopurine regimen, TG versus MP. (A) EFS and (B) OS by intent-to-treat analysis.
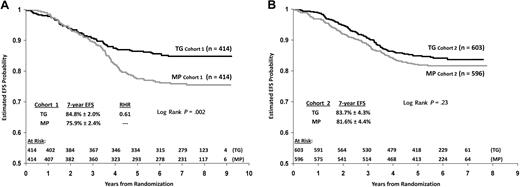
To determine the difference in outcome between subjects who began TG at 60 mg/m2 compared with 50 mg/m2, patients randomized to TG were divided into 2 cohorts: cohort 1: 828 patients enrolled between May 1, 1996, and December 26, 1997 (60 mg/m2); cohort 2: 1199 patients enrolled after December 26, 1997 (50 mg/m2). Seven-year EFS estimates for cohort 1 showed significant benefit of TG over MP (84.8% ± 2.0% vs 75.9% ± 2.4%; RHR = 0.61, P = .002 log rank), with median follow-up of more than 7 years (Figure 5A). In cohort 1, 118 subjects switched from TG to MP because of toxicity (Table 3 and Figure 3). In cohort 2, estimated 7-year EFS for TG and MP were similar (83.7% ± 4.3% vs 81.6% ± 4.4%; RHR = 0.84, P = .23 log rank), with a median follow-up in excess of 6 years (Figure 5B). In cohort 2, 139 subjects switched from TG to MP because of toxicity (Table 3 and Figure 3). In addition, 53% of patients in cohort 2 substituted MP for TG in maintenance as required in April 2001. Notably, the estimated 7-year EFS for patients on MP in cohort 2 trended more favorably than for those on MP in cohort 1 (81.6% ± 4.4% vs 75.9% ± 2.4%, P = .09 log rank). In contrast, EFS was similar in both TG cohorts. There was no survival advantage for TG over MP in cohort 1 or cohort 2, when comparing all randomized subjects or subdividing by sex (P = .51-.95, log rank).
EFS comparison of TG versus MP by cohort. (A) Cohort 1 and (B) cohort 2.
Impact of VOD or DT on outcome
EFS was similar among patients randomized to TG whether or not they experienced TG-induced toxicities. Seven-year estimated EFS for those with VOD or DT was 85.4% (± 3.4%) compared with 83.6% (± 2.1%) for those without VOD or DT (P = .36 log rank).
Impact of sex on outcome
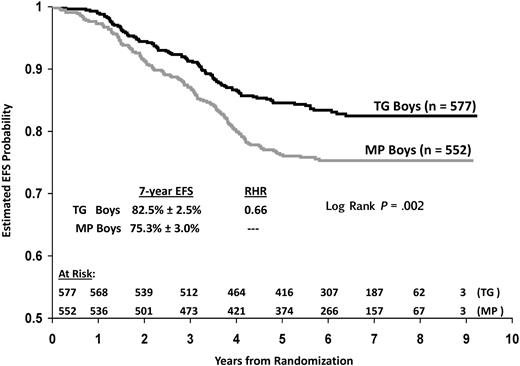
Subset analyses suggest that substitution of TG for MP provided a greater EFS advantage for boys than girls in this study. Boys on TG had significantly higher estimated 7-year EFS than boys on MP: 82.5% (±2.5%) versus 75.3% (±3.0%), respectively (RHR = 0.66, P = .002 log rank; Figure 6). Superior EFS for boys on TG was clearly evident in cohort 1 but less apparent in cohort 2. TG versus MP 7-year EFS estimates for boys in cohort 1 were 82.8% (± 2.8%) versus 72.3% (± 3.4%; RHR 0.58, P = .008 log rank) and 82.3% (± 5.8%) versus 77.8% (± 6.6%; RHR = 0.73, P = .08 log rank) for boys in cohort 2. Again, the higher EFS observed on MP in cohort 2 compared with cohort 1 impacts these statistical results. In contrast to boys, the difference in 7-year estimated EFS for girls on TG versus MP was not statistically significant in either cohort (cohort 1: 87.4% ± 2.8% vs 81.1% ± 3.4%, RHR = 0.66, P = .13 log rank; cohort 2: 85.4% ± 6.2% vs 85.8% ± 5.5, RHR 1.04, P = .89 log rank).
The 7-year cumulative incidence of iCNS relapse was significantly higher for boys than girls on MP (8.9% ± 2.2% vs 2.0% ± 1.2%, RHR = 3.87, P < .001 log rank) but not statistically different for boys than girls on TG (4.0% ± 1.4% vs 2.6% ± 1.3%, P = .27 log rank). The cumulative incidence of marrow relapse was sex equivalent for TG (9.2% ± 2.0% vs 9.3% ± 2.2%, P = .71 log rank) and MP (12.8% ± 2.5% vs 13.0% ± 2.7%, P = .95 log rank). Despite 1 more year of maintenance therapy for boys than girls, 7-year estimated EFS was significantly worse for males overall: 78.9% (± 1.9%) versus 84.9% (± 1.9%; RHR = 1.5, P = .002 log rank), although OS was similar (Table 4).
Prognostic factors significant for EFS or OS
| Prognostic factor . | Total randomized . | TG regimens . | MP regimens . | |||
|---|---|---|---|---|---|---|
| Relapse . | Death . | Relapse . | Death . | Relapse . | Death . | |
| Age, y | 1.53 | 1.58 | 1.64 | 1.86 | 1.44 | 1.33 |
| Younger than 2, older than 5 vs 2-5 | P = .001 | P = .03 | P = .02 | P = .04 | P = .02 | P = .44 |
| Sex | 1.5 | 1.15 | 1.2 | 1.09 | 1.4 | 1.2 |
| Male vs female | P = .002 | P = .4 | P = .25 | P = .69 | P = .002 | P = .43 |
| CNS status | 1.94 | 1.39 | 1.49 | 0.86 | 2.25 | 1.81 |
| CNS-2 vs CNS-1 | P = .001 | P = .5 | P = .46 | P = .57 | P = .001 | P = .07 |
| WBC, ×109/L | 1.6 | 1.85 | 1.72 | 1.77 | 1.6 | 1.91 |
| 20 or higher vs lower than 20 | P < .001 | P = .001 | P = .003 | P = .03 | P = .003 | P = .008 |
| Phenotype | 1.35 | 1.3 | 1.13 | 1.45 | 1.54 | 1.25 |
| T vs pre-B | P = .2 | P = .43 | P = .75 | P = .42 | P = .14 | P = .76 |
| Day-14 BM | 2.2 | 1.9 | 2.68 | 2.3 | 1.9 | 1.56 |
| M2 vs M1 | P < .001 | P = .005 | P < .001 | P = .006 | P = .001 | P = .19 |
| Prognostic factor . | Total randomized . | TG regimens . | MP regimens . | |||
|---|---|---|---|---|---|---|
| Relapse . | Death . | Relapse . | Death . | Relapse . | Death . | |
| Age, y | 1.53 | 1.58 | 1.64 | 1.86 | 1.44 | 1.33 |
| Younger than 2, older than 5 vs 2-5 | P = .001 | P = .03 | P = .02 | P = .04 | P = .02 | P = .44 |
| Sex | 1.5 | 1.15 | 1.2 | 1.09 | 1.4 | 1.2 |
| Male vs female | P = .002 | P = .4 | P = .25 | P = .69 | P = .002 | P = .43 |
| CNS status | 1.94 | 1.39 | 1.49 | 0.86 | 2.25 | 1.81 |
| CNS-2 vs CNS-1 | P = .001 | P = .5 | P = .46 | P = .57 | P = .001 | P = .07 |
| WBC, ×109/L | 1.6 | 1.85 | 1.72 | 1.77 | 1.6 | 1.91 |
| 20 or higher vs lower than 20 | P < .001 | P = .001 | P = .003 | P = .03 | P = .003 | P = .008 |
| Phenotype | 1.35 | 1.3 | 1.13 | 1.45 | 1.54 | 1.25 |
| T vs pre-B | P = .2 | P = .43 | P = .75 | P = .42 | P = .14 | P = .76 |
| Day-14 BM | 2.2 | 1.9 | 2.68 | 2.3 | 1.9 | 1.56 |
| M2 vs M1 | P < .001 | P = .005 | P < .001 | P = .006 | P = .001 | P = .19 |
Data are relative hazard rates (RHR); P value log rank.
Prognostic factors
Table 4 details the prognostic significance by univariate analysis of presenting features for all randomized patients and a comparison between MP and TG. Age (< 2, > 5 vs 2-5 years), sex, CNS status (CNS-1 vs CNS-2), WBC (< or ≥ 20 × 109/L), and day-14 induction BM response (M1 vs M2) had prognostic significance for EFS among all patients, whereas blast immunophenotype (T vs precursor-B) did not. These same factors predicted relapse in subjects randomized to MP. In contrast, CNS-2 status and male sex lost their poor prognostic significance in patients randomized to TG, consistent with the log-rank EFS and iCNS comparisons. Age, WBC, and day-14 BM status were also significant predictors of OS for all patients and for those on TG, whereas age and day-14 BM were not predictive of OS in the MP group. Risk of death among CNS-2 patients compared with CNS-1 treated with MP trended toward significance (RHR = 1.81, P = .07 log rank; Table 4). Different ethnicities as captured in this study (white, black, Hispanic, Asian, other) did not predict EFS or OS.
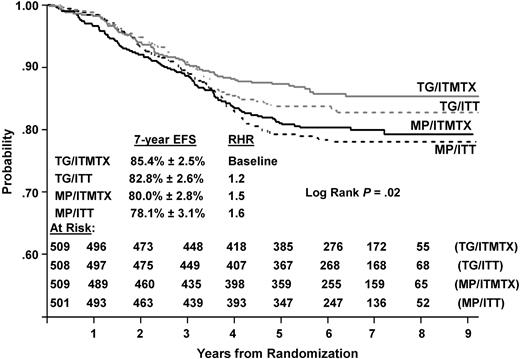
Outcome comparison of all 4 regimens
As shown in Figure 7, EFS among the 4 randomized regimens on CCG-1952 was significantly different (P = .02 log rank), with best outcome for patients randomized to TG/IT-MTX (85.4% ± 0.2.5%) and worst outcome for those randomized to MP/ITT (78.1% ± 3.1%). OS comparison trended toward significance (P = .06 log rank, data not shown). The intrathecal comparison on CCG-1952 was previously reported by Matloub et al: ITT regimens produced lower rates of iCNS relapses but higher rates of BM and testicular relapses compared with IT-MTX, resulting in similar 6-year EFS estimates of 80.7% (± 1.9%) and 82.5% (± 1.8%), respectively, (P = .3).4
Discussion
The goal of this intent-to-treat outcome comparison of TG versus MP was compromised by the variability in TG dosing that occurred during this study. In an attempt to reduce the incidence of TG-induced VOD, the starting dose of TG was decreased from 60 to 50 mg/m2 per day at 1½ years into accrual. In addition, 26% of subjects randomized to TG switched to MP because of toxicity. Moreover, many of the latter patients underwent repeated TG dose reductions because of thrombocytopenia before recognition of VOD or DT. Finally, 31% of subjects randomized to TG were ultimately required by protocol amendment to discontinue TG and substitute MP. Thus, rather than remaining a constant variable like MP, TG became a time-dependent, changing variable. Some of the statistical analyses performed for this study attempted to account for the changes in TG administration over time. However, these analyses were unanticipated and not part of the initial statistical design and power calculations.
Overall, TG provided significantly better estimated 7-year EFS and freedom from iCNS relapse than MP. Retrospective subset analyses indicate that EFS was significantly higher with TG than MP in cohort 1, when TG was initiated at 60 mg/m2 per day. However, EFS was similar between TG and MP in cohort 2, when TG began at 50 mg/m2 per day. Further examination of these cohort data shows that EFS with TG was similar in both cohorts, regardless of the starting dose of TG or the fact that many subjects switched to MP on cohort 2. In contrast, EFS with MP was divergent between cohorts, with 7-year EFS of 76% in cohort 1 and 82% in cohort 2. No differences in presenting features between the MP cohorts were identified to explain these results. Although CNS-2 status trended higher in the entire group of MP than TG subjects, incidence of CNS-2 was similar in the 2 MP cohorts. Thus, this disparity in outcome between the MP cohorts remains unexplained. OS did not differ between TG and MP in this study, probably reflecting successful salvage therapy after iCNS relapse on MP.
At the inception of this study, the principal cytotoxic effect of thiopurines was presumed to be the formation of thioguanine nucleotides (TGNs) and their incorporation into DNA (Figure 1). Emerging preclinical and pharmacologic data suggested that TG may have a therapeutic advantage over MP. However, recent research now suggests that methylated compounds derived from MP, and in particular methyl-thiopurine inosinic monophosphate (meTIMP), exert substantial cytotoxicity through inhibition of de novo purine synthesis.6,7 TG is not metabolized to TIMP or meTIMP and, thus, is not cytotoxic through this mechanism.24 Furthermore, recent evidence indicates that RBCs lack the enzyme, IMP dehydrogenase, which is required for conversion of MP, but not TG, to TGNs.7 Thus, previous comparisons reporting higher levels of RBC TGNs in patients on TG than on MP may have been misleading.
In addition to the CCG-1952 study reported here, 2 European trials, the German Cooperative Study Group COALL-05-92 (1992-1997) and the United Kingdom Medical Research Council (UK MRC) ALL97, ALL97/99 (1997-2002) studied substitution of TG for MP in the treatment of childhood ALL.25,26 Neither trial found a statistical EFS advantage for TG over MP. However, their study designs, patient populations, and total accrual differed from our study, as did the daily target dose of thiopurines. Both VOD and DT occurred in patients receiving TG on the UK MRC protocols, despite the lower starting dose of 40 mg/m2.26,27 No VOD was reported on the German Cooperative Study Group COALL study, with TG doses of 40 to 50 mg/m2. However, patients on TG had a 7.5-fold higher incidence of thrombocytopenia without neutropenia than those on MP,25 similar to the DT described in our study. The MRC study also contained a randomization between oral steroids, wherein dexamethasone provided significantly higher EFS than prednisolone. Notably, the TG group on the UK MRC study experienced 5 times more infectious deaths in remission than the MP group (P = .01), primarily among patients on TG and dexamethasone. In contrast, patients on CCG-1952 received prednisone in maintenance, and the rare number of infectious deaths were equivalent with either thiopurine. TG may be more immunosuppressive than MP, as suggested by the greater degree of lymphopenia seen with TG than MP in the UK MRC study.28 Dexamethasone may be more immunosuppressive than prednisone at equivalent anti-inflammatory doses.29,30 Thus, TG likely compounded the immunosuppressive effects of dexamethasone on the MRC study.
Similar to results observed in the present study, the incidence of iCNS relapse on the UK MRC study was reduced by nearly half in patients randomized to TG compared with MP (P = .02).26 In the CCG-1942 study reported by Jacobs et al, substitution of intravenous and oral TG for oral MP resulted in no iCNS relapses among 58 patients after 8 years of follow-up, despite BM and/or testicular recurrences in 18% of subjects.31 In the same study, the mean steady-state CSF TG concentration achieved by intravenous infusion was 0.05 μM,32 a level shown to be cytotoxic in vitro.8 Because 10-fold higher concentrations of MP than TG are required for cytotoxicity in vitro, it is unlikely that cytotoxic MP levels are achieved in CSF with an oral dose of 75 mg/m2, despite a similar degree of CSF penetration.8 As reported previously, CNS-2 status was a significant predictor of iCNS relapse for the entire CCG-1952 randomized cohort.4 TG overcame the negative prognostic impact of CNS-2, as the iCNS relapse rate was similar for subjects on TG with CNS-1 or CNS-2 status. In contrast, the iCNS relapse rate was significantly higher for patients with CNS-2 status on MP compared with CNS-1.
Hepatic VOD, or SOS as coined by DeLeve et al,13 is characterized by painful congestive hepatomegaly, jaundice, ascites, and weight gain from fluid retention. Damage to sinusoidal endothelium is the primary pathologic event in VOD, followed by a series of processes that cause hepatic venous outflow obstruction, circulatory compromise to centrilobular hepatocytes, and hepatocellular necrosis.13,33 VOD is a well-recognized complication of bone marrow transplantation (BMT).34 Various chemotherapy agents, alone or in combination, have also been implicated in the development of VOD.33 These drugs typically undergo first-pass hepatic metabolism, and the parent compound or a hepatic metabolite appear directly responsible for the toxicity. In addition, evidence in the rat model suggests that agents causing VOD deplete sinus endothelial cells of glutathione, an endogenous antioxidant, and that repletion of glutathione prevents VOD.35
VOD was anticipated to be a rare side effect of TG at the inception of the CCG-1952 protocol. Few reports of TG-associated VOD were reported in the medical literature, and most episodes occurred with exposure to additional drugs.36-39 One case of reversible VOD developed in a patient receiving TG alone for treatment of psoriasis, but at very high oral doses.40 Although several patients on the CCG-1942 intravenous TG pilot developed reversible VOD while receiving daily oral TG, prior intravenous TG was presumed to be a predisposing factor.31 Furthermore, no TG-induced VOD had been observed on the COALL-92 protocol, which was ongoing since 1992.25 Episodes of VOD on CCG-1952 were typically milder than those seen in BMT. Although hyperbilirubinemia is a hallmark of VOD in BMT, it occurred in fewer than half the cases of VOD diagnosed in our study. The etiology of VOD in the BMT setting is multifactorial, compounded by injury from prior high-dose chemotherapy, total body irradiation, and concomitant infections. Unlike cases after transplantation, the offending agent can be readily discontinued in TG-induced VOD. Similar to BMT, vigilant supportive care and fluid management are the mainstay of treatment in TG-induced VOD.
The disproportionate thrombocytopenia (DT) experienced by 5% of patients on TG in this study likely reflects insidious TG-mediated sinusoidal injury and endotheliitis, but without the congestive hepatopathy of full-blown VOD. A previous study reported greater thrombocytopenia in children on TG than MP and assumed this disparity to reflect preferential suppression of thrombopoiesis by TG.28 In retrospect, consumption of platelets at sites of sinusoid endothelial damage is the more likely explanation. Factors contributing to the incidence and severity of TG-induced VOD and DT remain unclear. TG monotherapy induces sinusoidal damage, as reported in 2003 among patients receiving TG for inflammatory bowel disease.41 In our study and in that of the UK MRC, VOD or DT correlated with longer duration of exposure and higher cumulative dose of TG.27,42 Data from the latter study, and from the CCG-1942 intravenous TG pilot study, showed no temporal correlation between high plasma levels of TGNs or TG and episodes of VOD.27,31,32,43 Considering the extensive first-pass intestinal and hepatic metabolism that follows oral dosing of TG, VOD likely reflects damage of sinusoidal endothelium caused directly by parent compound or a hepatic metabolite(s) not formed from MP.
Some investigators have questioned whether low thiopurine methyltransferase (TPMT) activity, indicative of heterozygosity or homozygosity for TPMT mutations, predisposes to VOD.27,43 None of the 6 patients who experienced VOD on CCG-1942 had low TPMT activity.31 In one study of 99 subjects on the UK MRC protocol, the 12 patients who developed VOD had TPMT activity levels comparable with those without the toxicity.27 In contrast, an analysis of different subjects on the same protocol found that 18% of patients who developed VOD were heterozygous for a TPMT mutation compared with 10% in those without VOD.43 Interestingly, 1 patient on CCG-1952 with homozygous TPMT deficiency developed severe skin eruptions and prolonged pancytopenia within 2 weeks of starting TG, but no VOD.23 A complex interplay among various metabolic enzymes and their genetic polymorphisms, such as TPMT, glutathione synthetase, methyl tetrahydrofolate reductase, and the X-linked hypoxanthine guanine phosphoribosyltransferase (HGPRT), may set the stage for TG-induced toxicities.
The results of this study underscore the sex disparity in EFS among children with ALL.44-46 Despite an additional year of treatment, 7-year estimated EFS for the entire male cohort was significantly worse than for females. Girls had a significantly better outcome on MP than boys and no statistical benefit from TG. The higher rate of iCNS relapse in boys than girls on MP, along with a 4% incidence of testicular relapse, accounts for the poorer male outcome. Because iCNS relapse is largely a local manifestation of systemic failure and occurs earlier than BM relapse, the increased incidence of iCNS relapse in boys does not imply less residual marrow leukemia in boys than girls. The host and disease factors contributing to the poorer outcome in boys is unclear. Nonetheless, data from CCG-1952 suggest that an intervention other than extended maintenance is needed for boys to achieve an EFS similar to that of girls with SR-ALL.
In the design and conduct of a clinical trial, a study chair or principle investigator, along with the data monitoring committee, assumes fundamental responsibilities to the subjects, investigators, and the specific aims of the study itself. CCG-1952 proved especially challenging in this regard, as the burden of reversible VOD was continually weighed against early indications of better EFS with TG. Recognition of portal hypertension as a possible life-threatening complication of TG created an unambiguous imbalance between its risks and potential benefits.47 Although use of TG for a short time frame early in the treatment of boys with ALL may seem an attractive therapeutic strategy, the side effects of TG limit this approach. Furthermore, the therapeutic benefit of TG may be redundant in the face of dexamethasone treatment in SR-ALL. The acute and insidious toxicities of TG preclude its prolonged use in the treatment of ALL.
Four-year EFS data presented in abstract form at the 44th Annual Meeting of the American Society of Hematology, Philadelphia, PA, December 9, 2002.48
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors express gratitude to all the patients and families who participated in this trial. We also thank Dr Greg Reaman, COG chair; Dr Stephen Hunger, COG ALL committee chair; Meenakshi Devidas, PhD, COG ALL statistician; Martha Sensel, PhD, scientific writer for COG; Marilyn Blake, study clinical research associate; CCG/COG staff; and all CCG institutional investigators and their clinical research staff.
This study was supported in part by the Children's Oncology Group Chairman's grants CA-98543 and CA-98413 from the National Cancer Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: L.C.S. was the study chair of CCG-1952 and the primary author, and designed and conducted the study; Y.M. was a study committee member, and reviewed and edited the paper; E.B., R.Y., A.D.S., and M.M. were study committee members and reviewed the paper; M.L. was study statistician, performed statistical analyses, designed Kaplan-Meier figures, and reviewed the paper; H.S. was a study statistician, designed and monitored the study, and performed statistical analyses; R.H. was study vice chair, designed the study, and reviewed the paper; N.A.H. was a study cytogeneticist and reviewed the paper; A.B. was a CCG group chair and mentor to L.C.S., monitored the study, and reviewed and edited the paper; P.S.G. was CCG ALL committee chair and mentor to L.C.S., designed the study, and reviewed and edited the paper; M.L. and H.S. performed the statistical analyses; and all authors had access to the primary clinical trial data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Linda C. Stork, OHSU, Department of Pediatrics, 3181 SW Sam Jackson Park Rd, CDRCP, Portland, OR 97239; e-mail: storkl@ohsu.edu.