Abstract
Allogeneic stem cell transplantation (SCT) is an established therapy for patients with relapsed lymphoma, but the role of positron emission tomography (PET) scanning preallogeneic and postallogeneic SCT is uncertain. We investigated whether pretransplantation PET status predicted outcome after allogeneic SCT and whether PET surveillance after transplantation provided additional information compared with computed tomography (CT) scanning. Eighty consecutive patients with lymphoma who received a reduced-intensity allogeneic SCT were entered onto a prospective trial. PET and CT scans were performed before transplantation and up to 36 months after transplantation. Forty-two patients were PET-positive before transplantation. Pretransplantation PET status had no significant impact on either relapse rate or overall survival. Thirty-four relapses were observed, of which 17 were PET-positive with a normal CT scan at relapse. Donor lymphocyte infusion (DLI) was administered in 26 episodes of relapse and was guided by PET alone in 14 patients. These findings suggest that, in contrast to autologous SCT, pretransplantation PET status is not predictive of relapse and survival after allogeneic SCT for lymphoma. Posttransplantation surveillance by PET detected relapse before CT in half of episodes, often allowing earlier administration of DLI in patients with recurrent lymphoma, and permitted withholding of potentially harmful DLI in those with PET-negative masses on CT scans.
Introduction
High-dose chemotherapy followed by allogeneic stem cell transplantation (SCT) has become an established approach in the management of selected patients with relapsed lymphoma often after the failure of autologous SCT.1 More recently, reduced-intensity allogeneic SCT has been shown to be effective in the treatment of patients with lymphoma but with less toxicity than standard myeloablative allogeneic SCT.2-4 Reduced-intensity regimens rely on immunosuppression of the recipient to allow allogeneic engraftment and then harness the alloreactivity of the donor T cells against any residual lymphoma cells (graft-versus-lymphoma [GVL] effect). Donor T cells may be administered either on the day of transplantation (T cell–replete graft) or many months after a T cell–depleted graft (or indeed a T cell–replete graft) as a donor lymphocyte infusion (DLI).
A number of published series in both autologous and reduced-intensity allogeneic SCT suggest that satisfactory outcomes are largely confined to those patients who show a response to salvage therapy before the transplantation.5-9 In recent years, assessment of response to therapy has been improved by the use of fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET). Several studies have shown that the results of autologous SCT are poor in patients with a positive pretransplantation PET scan, even if they have had a response using conventional computed tomography (CT) criteria.10-12 It is not known whether a positive PET scan immediately before a reduced-intensity allogeneic SCT is associated with a similarly poor outcome.
After reduced-intensity allogeneic SCT, the GVL effect may be augmented by the administration of DLI, typically at the first sign of disease persistence or relapse, when it may reinduce remission.13 Consequently, patients who have undergone reduced-intensity allogeneic transplantation for lymphoma may require close monitoring after transplantation to detect persistent or recurrent disease at an early stage, when DLI might be most effective. PET scanning may therefore be of value after allogeneic SCT by providing early evidence of recurrent lymphoma. A small retrospective analysis of patients at our center who underwent PET imaging for possible relapse after allogeneic transplantation found that administration of DLI was influenced by PET in 9 of 15 patients.14 There are, however, no prospective data on the utility of PET in this setting.
This prospective study aimed to investigate (1) whether the pretransplantation PET status was predictive of outcome in patients undergoing reduced-intensity allogeneic SCT for lymphoma, and (2) whether posttransplantation PET imaging would lead to earlier detection of relapse compared with standard CT scans, thereby potentially allowing earlier use of adoptive immunotherapy.
Methods
Study population
All patients being considered for allogeneic transplantation for lymphoma who are typically FDG-avid were eligible for entry to the study and were treated at University College London Hospital and the Royal Free Hospital, which together compose a single, integrated academic transplantation unit. Approval for the study was provided by the Multicenter Research Ethics Committee, and all patients gave written, informed consent in accordance with the Declaration of Helsinki.
Eighty patients were recruited (55 male; median age at transplantation, 44.1 years; range, 19-67 years), and these were a median of 45.6 months after transplantation (range, 10-84 months after transplantation) at the time of analysis in May 2009. The diagnoses were follicular lymphoma (n = 30), Hodgkin lymphoma (n = 22), diffuse large B-cell lymphoma (n = 7), transformed follicular lymphoma (n = 6), mantle cell lymphoma (n = 11), and peripheral T-cell lymphoma (n = 4). Forty-three patients underwent human leukocyte antigen–matched sibling donor transplantation, and 37 patients underwent matched unrelated donor transplantation.
Study design and timing of scans
An observational, prospective study design was followed. Imaging was performed before transplantation and then at 3, 6, 9, 15, 24, and 36 months after reduced-intensity SCT. At each time point, patients underwent both conventional CT scanning and fused PET-CT scanning. Before transplantation, conventional CT was used to determine chemosensitivity and suitability to proceed to transplantation. Concomitant pretransplantation PET was not used in the decision to proceed to transplantation. After transplantation, patients were eligible to receive DLI with or without chemotherapy if there was evidence of relapse, assessed either clinically, by CT or by PET.
Conditioning regimen and clinical follow-up
Seventy-eight patients were conditioned using alemtuzumab, fludarabine, and melphalan, and 2 received alemtuzumab, carmustine, cytarabine, etoposide, and melphalan.7,15 Unmanipulated peripheral blood stem cells were returned on day 0. Cyclosporine A was administered as immunosuppression and continued for 3 months after SCT. Patients were assessed clinically for the presence of graft-versus-host disease (GVHD), infective complications, and evidence of relapse. Lineage-specific chimerism was assessed routinely every 3 months.16
Fused PET-CT
PET was performed using the GE Advance PET scanner and the GE Light-speed multislice spiral CT (GE Medical Systems). The Light-speed CT acquires 4 5-mm slices at 140 kV with 80 mA and a large pitch of 6 (30 mm of table travel per gantry rotation). The CT and the PET studies were acquired during normal quiet breathing. Images were acquired 60 minutes after the injection of 370 MBq of FDG. The CT acquisition took 20 to 30 seconds, and the PET acquisition took 25 to 30 minutes (5 or 6 bed positions with an acquisition time of 5 minutes per position). PET data were reconstructed using iterative reconstruction (ordered subsets expectation maximization),17 and CT was used for attenuation correction of the PET images.
PET scans were assessed visually by a core team of experienced nuclear medicine physicians within our institution, in keeping with the most recent Consensus recommendations18 and reported as “positive” (suggestive of residual or recurrent lymphoma) or “negative” (suggestive of remission from lymphoma).
CT
CT was performed with intravenous contrast using a 64-multidetector row CT scanner and covered from base of neck to pelvis. CT appearances were reported as complete remission (CR), relapse/progression, or residual abnormality. The latter category combined the standard response criteria partial response and stable disease, on the grounds that either would prompt further investigation in the posttransplantation setting.
Definitions
Chemotherapy sensitivity was defined as the achievement of at least partial remission (a reduction in nodal bulk of at least 50% using standard CT volume criteria).
Primary progression of lymphoma was defined as the persistence of lymphoma at 3 months and at 6 months after transplantation, whether detected clinically, or by the presence of PET positivity, or the presence of progression on CT. Relapse was defined as the development of new findings on physical examination, new positivity on PET, and/or evidence of relapse/progression on CT, which were in keeping with the recurrence of lymphoma, in a patient previously in documented remission, and death occurring after relapse was always ascribed to disease. Progression-free survival (PFS) was defined as survival in continuous remission from transplantation until the time of analysis. Current progression-free survival (cPFS) was defined as survival in remission at the time of analysis and included patients in continuous remission since transplantation and those who relapsed after transplantation but reachieved remission after further therapy.
Posttransplantation DLI administration
Patients with relapse/progression had immunosuppression withdrawn and received DLI, unless they had ongoing GVHD. Escalating doses, ranging from 106 to 108 T cells/kg, were used according to our published regimen.13 DLIs were withheld in patients with stable CT abnormalities who had a normal PET scan and full donor chimerism.
Statistical analysis
Continuous variables are presented using mean (SD) and categorical variables using n (%). Baseline characteristics between PET-negative and PET-positive patients were compared using t tests or Fisher exact test as appropriate. There were 5 time-to-event outcomes of interest: overall survival (OS, primary endpoint), relapse, PFS, cPFS, and nonrelapse mortality, assessed at the start of May 2009. Survival distributions for OS, PFS, and cPFS were estimated using Kaplan-Meier curves, and differences between the PET-negative and PET-positive groups were tested using the log-rank test. Relapse and nonrelapse mortality events were analyzed using cumulative incidence estimates.19 A Cox proportional hazards model was used to estimate the hazard ratio for differences between the groups. McNemar test was used to assess agreement between PET and CT results. A P value of less than .05 was considered statistically significant. (Data analysis was performed by J.R.L., K.S.P., and M.R., and all authors had access to primary clinical data.)
Results
PET and CT concordance before reduced-intensity SCT
Seventy-eight of 80 patients were assessed to have chemosensitive lymphoma by CT criteria. Thirty-eight patients were PET-negative and 42 were PET-positive. Using CT criteria alone, 29 of 80 patients were in CR. There were no significant differences in patient characteristics between the PET-negative and PET-positive groups (Table 1). Concordance between pretransplantation PET and CT was 69%, with 8 patients who were PET-positive and CT-negative, and 17 who were PET-negative and CT-positive (P = .072; Table 2).
Baseline and transplantation-related characteristics according to pretransplantation PET status
| . | PET-negative (n = 38), mean (SD) . | PET-positive (n = 42), mean (SD) . | P . |
|---|---|---|---|
| Age at transplantation, y | 46.7 (10.6) | 42.1 (12.9) | .085 |
| Male | 27 (71.1) | 28 (66.7) | .810 |
| GVHD (before DLI) | 10 (26.3) | 12 (28.6) | .638 |
| Full donor chimerism (6 months after SCT) | 12 (31.6) | 13 (31.0) | 1.000 |
| Lymphoma subtype | |||
| Follicular | 14 (36.8) | 16 (38.1) | .387 |
| Hodgkin | 8 (21.1) | 14 (33.3) | — |
| Mantle cell | 5 (13.2) | 6 (14.3) | — |
| Diffuse large B-cell | 6 (15.8) | 1 (2.4) | — |
| Transformed follicular | 3 (7.9) | 3 (7.1) | — |
| Peripheral T-cell | 2 (5.3) | 2 (4.8) | — |
| Donor type | |||
| Matched unrelated | 13 (34.2) | 16 (38.1) | .761 |
| Mismatched unrelated | 3 (7.9) | 5 (11.9) | — |
| Sibling | 22 (57.9) | 21 (50.0) | — |
| . | PET-negative (n = 38), mean (SD) . | PET-positive (n = 42), mean (SD) . | P . |
|---|---|---|---|
| Age at transplantation, y | 46.7 (10.6) | 42.1 (12.9) | .085 |
| Male | 27 (71.1) | 28 (66.7) | .810 |
| GVHD (before DLI) | 10 (26.3) | 12 (28.6) | .638 |
| Full donor chimerism (6 months after SCT) | 12 (31.6) | 13 (31.0) | 1.000 |
| Lymphoma subtype | |||
| Follicular | 14 (36.8) | 16 (38.1) | .387 |
| Hodgkin | 8 (21.1) | 14 (33.3) | — |
| Mantle cell | 5 (13.2) | 6 (14.3) | — |
| Diffuse large B-cell | 6 (15.8) | 1 (2.4) | — |
| Transformed follicular | 3 (7.9) | 3 (7.1) | — |
| Peripheral T-cell | 2 (5.3) | 2 (4.8) | — |
| Donor type | |||
| Matched unrelated | 13 (34.2) | 16 (38.1) | .761 |
| Mismatched unrelated | 3 (7.9) | 5 (11.9) | — |
| Sibling | 22 (57.9) | 21 (50.0) | — |
PET indicates positron emission tomography; GVHD, graft-versus-host disease; DLI, donor lymphocyte infusion; SCT, stem cell transplantation; and —, not applicable.
Indication for transplantation according to PET and CT status before transplantation
| Subtype . | PET-negative (n = 38) . | PET-positive (n = 42) . | ||
|---|---|---|---|---|
| CT-positive (n = 17) . | CT-negative (n = 21) . | CT-positive (n = 34) . | CT-negative (n = 8) . | |
| Follicular lymphoma* | 8 | 6 | 12 | 4 |
| Hodgkin lymphoma | 4 | 4 | 11 | 3 |
| Mantle cell lymphoma | 1 | 4 | 6 | 0 |
| Diffuse large B-cell lymphoma | 2 | 4 | 1 | 0 |
| Transformed follicular | 1 | 2 | 2 | 1 |
| Peripheral T-cell lymphoma | 1 | 1 | 2 | 0 |
| Subtype . | PET-negative (n = 38) . | PET-positive (n = 42) . | ||
|---|---|---|---|---|
| CT-positive (n = 17) . | CT-negative (n = 21) . | CT-positive (n = 34) . | CT-negative (n = 8) . | |
| Follicular lymphoma* | 8 | 6 | 12 | 4 |
| Hodgkin lymphoma | 4 | 4 | 11 | 3 |
| Mantle cell lymphoma | 1 | 4 | 6 | 0 |
| Diffuse large B-cell lymphoma | 2 | 4 | 1 | 0 |
| Transformed follicular | 1 | 2 | 2 | 1 |
| Peripheral T-cell lymphoma | 1 | 1 | 2 | 0 |
PET indicates positron emission tomography; and CT, computed tomography.
Fourteen patients with follicular lymphoma were PET-negative before transplantation. Of these, 3 had evidence of FDG-avid disease at some stage before transplantation (but were rendered PET-negative immediately before transplantation by salvage chemotherapy). A further 3 patients relapsed with FDG-avid disease after transplantation. It is therefore possible that up to 8 patients with FL did not have FDG-avid disease.
Relationship between pretransplantation PET and survival
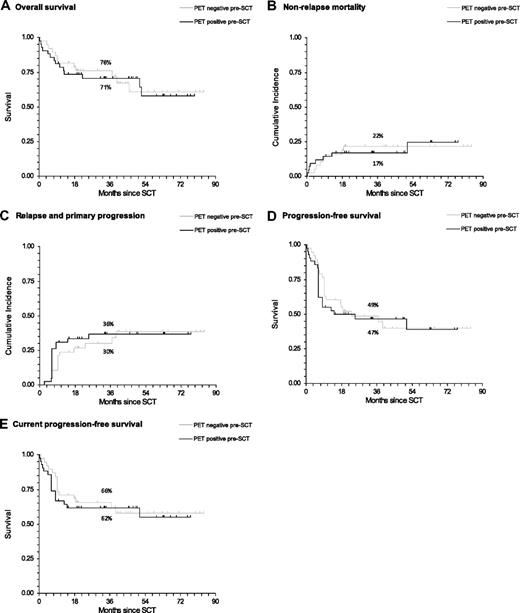
At the time of analysis, 54 patients were still alive and 26 had died (Table 3). There were 12 (31.6%) deaths in the PET-negative group and 14 (33.3%) in the PET-positive group, with no significant difference in survival between the 2 groups (hazard ratio [HR] = 1.08; 95% confidence interval [CI], 0.50-2.33; P = .851, Figure 1A). There was also no difference in nonrelapse mortality between the 2 groups (HR = 1.04; 95% CI, 0.40-2.69; P = .939, Figure 1B).
Cause of death according to PET status before transplantation
| Cause of death . | PET-negative . | PET-positive . |
|---|---|---|
| Infection | 5 | 7 |
| Disease progression | 4 | 5 |
| GVHD | 1 | 1 |
| Encephalomyelitis | 1 | 0 |
| Accidental | 0 | 1 |
| Pneumonitis | 1 | 0 |
| Cause of death . | PET-negative . | PET-positive . |
|---|---|---|
| Infection | 5 | 7 |
| Disease progression | 4 | 5 |
| GVHD | 1 | 1 |
| Encephalomyelitis | 1 | 0 |
| Accidental | 0 | 1 |
| Pneumonitis | 1 | 0 |
PET indicates positron emission tomography; and GVHD, graft-versus-host disease.
Survival estimates of outcome after reduced-intensity allogeneic SCT according to pretransplantation PET status. All lymphoma subtypes are shown (percentages on the graphs indicate 3-year rates for each outcome). (A) OS. (B) Nonrelapse mortality. (C) Relapse and primary progression. (D) PFS. (E) cPFS.
Survival estimates of outcome after reduced-intensity allogeneic SCT according to pretransplantation PET status. All lymphoma subtypes are shown (percentages on the graphs indicate 3-year rates for each outcome). (A) OS. (B) Nonrelapse mortality. (C) Relapse and primary progression. (D) PFS. (E) cPFS.
Relationship between pretransplantation PET and primary progression and relapse
Of the 80 patients, 7 fulfilled the criteria for primary progression, of whom 4 died of lymphoma, 2 entered remission after the administration of DLI, and 1 remains alive but with evidence of ongoing disease. A further 21 patients relapsed during follow-up.
In total, 15 of the 28 patients diagnosed with primary progression or relapse were PET-positive before transplantation. Overall, the rate of primary progression and relapse combined was not significantly different in those who were PET-positive compared with those who were PET-negative before transplantation (HR = 1.14; 95% CI, 0.54-2.41; P = .714, Figure 1C). Similarly, there was no difference in the PFS and cPFS between the 2 groups (HR = 1.12; 95% CI, 0.62-2.02; P = . 703; and HR = 1.15; 95% CI, 0.57-2.30; P = .699, respectively; Figure 1D-E).
PET and CT results and chimerism analysis at relapse
Thirty-four episodes of progression/relapse were observed in 28 different patients (4 patients relapsed twice and 1 relapsed 3 times). In 17 of these episodes, the diagnosis was made using PET alone, as they were judged to be negative on CT: in 16 of these, the relapse was at a site documented to be involved by lymphoma before transplantation, whereas in one the relapse was at a new site. In 2 of these 17 episodes, relapsed/progressive lymphoma was proven histologically. In the remaining 15 episodes, the presence of residual or relapsed lymphoma was diagnosed on the basis of persistently abnormal FDG uptake (at least 2 consecutive scans), in nodal areas documented to be involved by lymphoma before transplantation, and biopsy was not attempted for the following reasons: in 7 episodes, the PET positivity affected anatomic locations not readily amenable to biopsy (retroperitoneal or mediastinal); in 2 episodes, the persistently abnormal FDG uptake was so marked and so extensive (above and below the diaphragm) that it was considered unlikely to be the result of anything other than relapse; and in the other 6 episodes, the PET positivity was confined to a single nodal group but persisted for at least 6 months without any apparent infective or inflammatory cause.
There were 13 relapse episodes where both CT and PET were positive and 4 where both were initially negative (McNemar test, P < .001). Of the 4 patients whose relapse was first identified clinically, 1 was beyond 3 years, 1 relapsed intracranially, 1 had an isolated cutaneous relapse (not detected on the most recent imaging performed 4 months earlier), and the fourth developed new supraclavicular lymphadenopathy just a few days before his next imaging was due (his PET and CT scans 3 months earlier demonstrated CR).
Peripheral blood chimerism showed a full donor pattern in 13 of the 34 episodes of progression/relapse, mixed donor/recipient in 16 episodes, and was unknown in 5 episodes.
Nineteen patients had residual abnormalities on CT after transplantation, with a normal simultaneous PET. None of these patients received antilymphomatous therapy for the abnormal CT appearances. After a median follow-up of 36 months, 13 of them remained in continuous remission; of the 6 who relapsed, the site of relapse was within the region of CT abnormality in 4, and distant from it in 2.
Administration of DLI
Thirty-nine doses of DLI were administered for 26 episodes of relapse/primary progression, to 22 different patients. Of these 39 doses, 32 were collected by steady-state leukopheresis (for 19 patients), and 7 (for 3 patients) were collected after hematopoietic growth factor stimulation. In 16 of the 26 episodes, chemotherapy, rituximab, or radiotherapy (or a combination of these) was also administered, and in a further case (of cutaneous relapse) an excision biopsy was performed.
In 14 of the 26 episodes of relapse treated with DLI with or without chemotherapy, the relapse was diagnosed solely on the basis of an abnormal PET, and 13 of these 14 patients achieved CR. In the remaining 12 episodes (where there was both CT and PET evidence of relapse, n = 9; or the diagnosis was clinical, n = 3), CR was attained in 7 cases. In total, therefore, in 20 of the 26 episodes of relapse/primary progression, administration of DLI with or without chemotherapy resulted in CR within 6 months of administration.
PET positivity resulting from infective or inflammatory causes
There were 11 instances of PET positivity resulting from infective or inflammatory causes, of a total of 475 PET scans performed (2%). Three of these demonstrated increased pulmonary FDG uptake, and patients underwent tissue biopsy: in 2 cases, an infectious agent was identified (1 fungal infection, 1 tuberculosis), and in the third a nonspecific inflammatory appearance was observed histologically. A fourth patient had abnormal mediastinal lymph node uptake on PET (and lymphadenopathy on CT), which was found on biopsy to be the result of sarcoidosis. Patient 5 had femoral FDG avidity, which was histologically proven to be the result of bone remodeling at a site previously treated with radiotherapy. In the other 6 cases, the FDG avidity was observed in cervical lymph nodes in patients who were documented to have an upper respiratory tract infection at the time, and the abnormalities resolved spontaneously after their respiratory symptoms improved. In every case, spontaneous normalization of PET appearances was documented on subsequent scans in the absence of any antilymphomatous treatment.
Chimerism status and incidence of GVHD
There was no significant difference in the proportion of patients who showed full donor chimerism at 6 months between the PET-negative pretransplantation group and the PET-positive pretransplantation group (12 of 38 vs 13 of 42, P = 1.000).
The rate of clinically significant GVHD (ie, grade II-IV acute or any chronic) occurring before DLI administration was similar for patients who were PET-negative and those who were PET-positive before transplantation (10 of 38 vs 12 of 42, P = .638). However, the rate of clinically significant GVHD was significantly lower in the evaluable patients who relapsed compared with those who did not (4 of 28 vs 18 of 52, P = .018).
In total, DLIs were administered for 26 episodes of relapse: of these, 10 were complicated by GVHD (including 2 grade II-IV acute, 2 limited chronic, and 5 extensive chronic). Of the 14 episodes of DLIs administered for PET positivity alone, 4 were complicated by GVHD, of which none was grade II-IV acute, 2 were limited chronic, and 1 was extensive chronic. In the latter case, DLI administration had been prompted by 2 consecutive PET scans showing extensive FDG uptake, above and below the diaphragm, with progression between the 2 scans. Administration of DLI resulted in a rapid improvement in PET appearances but was followed by extensive chronic GVHD, manifest as arthritis.
Discussion
The present study is the first prospective examination of the role of PET imaging in allogeneic SCT. Our data indicate that, in patients with chemosensitive lymphoma, PET status before transplantation had no significant effect on OS, PFS, cPFS, and relapse/progression rate. However, after allogeneic SCT, surveillance with PET imaging did add clinically useful information to that provided by CT.
The observation that pretransplantation PET status did not predict outcome after reduced-intensity allogeneic SCT contrasts with the findings of most published studies that have investigated the role of PET before autologous transplantation.10-12 Each of these studies indicated that OS and PFS in patients with metabolic evidence of residual lymphoma preautologous SCT was significantly worse than in patients with negative PET scans before transplantation. By comparison, with a median follow-up of nearly 45 months, neither group had reached median OS in our study. It is possible that the relatively small number of patients in some histologic subgroups obscured a genuine correlation between pretransplantation PET status and outcome in certain types of lymphoma, but, overall, our data suggest that reduced-intensity SCT may surmount the unfavorable prognostic effects of a positive PET before transplantation. This is in keeping with the conclusion of a small retrospective study that investigated the prognostic role of PET imaging before myeloablative allogeneic transplantation,20 and is of considerable clinical significance: patients with chemosensitive relapsed lymphoma who are PET-positive after salvage chemotherapy should not be excluded from reduced-intensity SCT.
The difference between our findings and those from studies examining autologous transplantation implies that either the conditioning regimen or the GVL effect of the transplantation and subsequent DLI abrogates the negative effects of low-level persistent disease (as evidenced by PET positivity) before transplantation. Of the 10 episodes of relapse treated with DLI alone, CR was achieved within 6 months in 9 patients. This strongly suggests the presence of a GVL effect in this context and is consistent with previous observations from our center.14 The inverse association between the presence of clinically significant GVHD and relapse also points to the presence of a GVL effect.
Any GVL effect is most probable to be effective in the presence of relatively low disease burden; and consequently, relapse should ideally be detected early to maximize its benefit. We observed that posttransplantation PET surveillance detected 17 relapses (half of all episodes), which were not apparent on simultaneous CT scans. Further, of the 26 relapses treated with DLI with or without chemotherapy, PET alone directed therapy in 14 episodes. Early intervention, facilitated by PET scanning, may have contributed to the encouraging outcomes seen. By contrast, no relapses were detected on CT that were not evident on PET. Taken together, this might imply that, where available, routine posttransplantation surveillance with PET alone may be the preferred strategy.
The 11 instances of PET positivity resulting from inflammatory or infective causes demonstrate the importance of interpreting the findings from one imaging modality together with those from others and the clinical scenario. For example, the 6 cases with FDG-avid cervical nodes in the presence of an upper respiratory tract infection allowed watchful waiting instead of intervention.
The low rate of histologic confirmation of relapse in our study (only 3 were biopsy-proven, largely because of anatomic difficulties) raises the possibility that some of the cases of relapse diagnosed solely on the basis of a positive PET were actually false-positives. We think this is unlikely because of the following: in the majority of cases, the FDG-avid lymph nodes were at the site of previous disease; there was no concurrent clinical suggestion of an infective or inflammatory cause; and in all the patients, these changes either resolved after antilymphomatous therapy or progressed to overt relapse. Furthermore, administration of DLI in this situation appears relatively safe; of the 14 episodes of DLI administration guided by PET positivity alone, only 1 resulted in extensive chronic GVHD; and in this case, the PET appearances on repeated scans were highly suspicious of relapsed lymphoma. Therefore, although administration of DLI solely on the basis of PET abnormalities may theoretically risk overtreatment, with the potential to cause unnecessary GVHD, the observed rate of clinically significant GVHD was low, and we think was more than compensated by the probable benefit of treating relapses early with relatively low T-cell doses of DLI.
The characterization of residual posttherapy masses is a common problem in the management of patients with lymphoid malignancies. The findings of this study indicate that PET is a valuable tool in this situation: only a small minority of the patients with residual PET-negative abnormalities on CT relapsed at the site of these abnormalities. This suggests that some patients with a negative PET and stable abnormalities on CT can simply be observed without compromising their outcome.
It is possible that, by increasing the scanning interval after the first 9 months after transplantation, we might have diminished the potential benefit of using a sensitive imaging modality, such as PET. However, the schedule was based on our prior experience of the timing of relapse in similar patients, and it is notable that in this study 22 of the 34 relapses occurred within 9 months of transplantation. Further, of the 12 later relapses, it is probable that 7 would not have been diagnosed earlier by the use of more frequent routine scans between 9 and 36 months because 4 were detected less than 3 months after a scheduled scan (either clinically or on additional imaging requested in view of equivocal abnormalities on the scheduled scan) and 3 occurred after 36 months. Therefore, routine imaging of all 80 patients every 3 months for 3 years would have resulted in earlier detection of, at most, 5 relapses, and it is unlikely that such a surveillance schedule could be justified. It should also be noted that, in all 5 cases, therapy resulted in remission within 6 months.
In conclusion, a positive pretransplantation PET scan does not preclude a successful long-term outcome in patients with chemosensitive lymphoma. PET is a valuable imaging modality in the posttransplantation management of such patients and detected relapse before CT in half of episodes, often allowing earlier administration of DLI. It should therefore be considered in the routine surveillance of patients after reduced-intensity allogeneic SCT for lymphoma.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
J.R.L. is supported by the United Kingdom Leukaemia Research Fund. University College London Hospitals National Health Service Trust and Royal Free Hampstead National Health Service Trust received a proportion of funding from the United Kingdom Department of Health's National Institute for Health Research Biomedical Research Centre's funding scheme.
Authorship
Contribution: J.R.L. performed data collection and analysis and wrote the paper; J.B.B. and P.J.E. reviewed patient imaging, wrote the paper, and designed the study; K.S.P. performed data collection and analysis, wrote the paper, and participated in patient management; K.J.T., R.K.C., A.K.F., P.D.K., E.C.M., A.H.G., and D.C.L. performed data collection, participated in patient management, and revised the paper; M.R. performed data analysis and wrote the paper; and S.M. participated in patient management, wrote the paper, and designed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen Mackinnon, Department of Haematology, University College London Medical School–Royal Free Campus, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: s.mackinnon@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal