Abstract
The discovery of JAK2 and MPL mutations in patients with myeloproliferative neoplasms (MPNs) provided important insight into the genetic basis of these disorders and led to the development of JAK2 kinase inhibitors for MPN therapy. Although recent studies have shown that JAK2 kinase inhibitors demonstrate efficacy in a JAK2V617F murine bone marrow transplantation model, the effects of JAK2 inhibitors on MPLW515L-mediated myeloproliferation have not been investigated. In this report, we describe the in vitro and in vivo effects of INCB16562, a small-molecule JAK2 inhibitor. INCB16562 inhibited proliferation and signaling in cell lines transformed by JAK2 and MPL mutations. Compared with vehicle treatment, INCB16562 treatment improved survival, normalized white blood cell counts and platelet counts, and markedly reduced extramedullary hematopoeisis and bone marrow fibrosis. We observed inhibition of STAT3 and STAT5 phosphorylation in vivo consistent with potent inhibition of JAK-STAT signaling. These data suggest JAK2 inhibitor therapy may be of value in the treatment of JAK2V617F-negative MPNs. However, we did not observe a decrease in the size of the malignant clone in the bone marrow of treated mice at the end of therapy, which suggests that JAK2 inhibitor therapy, by itself, was not curative in this MPN model.
Introduction
Polycythemia vera (PV), essential thrombocytosis (ET), and primary myelofibrosis (PMF) are classified as BCR-ABL− myeloproliferative neoplasms (MPNs), typified by clonal proliferation of 1 or more myeloid lineages.1,2 There are approximately 130 000 to 150 000 patients with MPN in the United States, which makes these disorders among the most common hematopoietic malignancies.3 Patients with MPN are at high risk for several disease-related complications, including bleeding, thrombosis, splenomegaly, progressive bone marrow failure, and transformation to acute myeloid leukemia (AML). Current therapies for PV and ET are largely empiric, and include antiplatelet therapy, phlebotomy, hydroxyurea, anagrelide, and IFN-γ.4 For patients with PMF or with post-PV/ET myelofibrosis, treatment options are limited, with the notable exception of allogeneic stem cell transplantation for the subset of patients in which age and/or comorbidities do not exclude transplantation as a therapeutic option.5,6 There is therefore a need for novel therapies for patients with these disorders.
Although previous studies had demonstrated the clonal stem cell origin of these disorders,7,8 the genetic basis of these disorders was not known until several groups reported the identification of a recurrent somatic mutation in JAK2 (JAK2V617F) in approximately 90% to 95% of patients with PV and in approximately 50% to 60% of patients with ET and PMF.9-14 Expression of JAK2V617F in vitro transforms hematopoietic cells to cytokine-independent growth and leads to constitutive activation of downstream signaling pathways.9,15 In addition, expression of JAK2V617F in vivo using the murine bone marrow transplantation (BMT) assay results in a short latency, fully penetrant MPN notable for marked polycythemia, hepatosplenomegaly, and variable progression to myelofibrosis.16-19 These data demonstrate the importance of JAK2V617F to the pathogenesis of JAK2V617F-positive MPN.
Although the discovery of JAK2V617F mutations in almost all patients with PV and approximately half of those with ET and PMF provided important insight into the molecular basis of these MPNs, the etiology of JAK2V617F− MPN remained unknown. Investigators subsequently identified somatic activating mutations in exon 12 of JAK2 in patients with JAK2V617F− PV;20 however, alternate JAK2 mutations were not identified in JAK2V617F− ET and PMF. Based on the observation that the JAK2V617F kinase requires expression of a type I homodimeric cytokine receptor (EPOR, MPL, GCSFR) to efficiently transform hematopoietic cells,15 investigators sequenced these cytokine receptors in patients with MPN and identified somatic mutations at codon 515 of the thrombopoietin receptor (MPLW515L) in ET and PMF.21 Subsequent to the initial identification of the MPLW515L allele, additional somatic mutations at codon 515 (MPLW515K, MPLW515A)22,23 and at codon 505 (MPLS505N)24 have been identified in patients with ET/PMF. Analysis of large patient cohorts suggests that somatic MPL mutations are present in approximately 3% of patients with ET and 8% of patients with PMF.24,25 Expression of MPLW515L transforms murine and human hematopoietic cell lines to cytokine-independent growth, and results in constitutive activation of several downstream molecules, including STAT3, STAT5, ERK, and PI3K/Akt pathways.21 More importantly, overexpression of MPLW515L in the murine BMT assay results in development of an acute myeloproliferative neoplasm characterized by features of human ET and PMF, including marked thrombocytosis, leukocytosis, and the rapid development of extramedullary hematopoeisis and reticulin fibrosis in all mice expressing this mutant allele.21
Based on the identification of activating JAK2 and MPL mutations in these MPNs, many groups have initiated efforts aimed at developing small-molecule inhibitors of JAK2 signaling for the treatment of MPN.26 These compounds inhibit growth and signaling in cell lines transformed by JAK2V617F and MPLW515L27 and in primary MPN patient samples,28 and have demonstrated efficacy in a murine BMT model of JAK2V617F-induced PV.29 Based on these data, different JAK2 inhibitors have entered early-stage clinical trials for patients with PMF and post-PV/ET PMF,30 and at this early stage it is difficult to ascertain whether JAK2 inhibition will lead to significant hematologic and molecular responses in the different MPNs, and if responses will differ based on mutational context. Given that previous in vivo studies have focused on the effects of JAK2 inhibition in a JAK2V617F-dependent model of PV, we sought to ascertain whether JAK2 inhibition would improve thrombocytosis, myelofibrosis, and survival in a MPLW515L-dependent model of ET/PMF.
Methods
Reagents
INCB16562 was synthesized by Incyte Corporation. A total of 1mM stock solutions were prepared and stored in DMSO and diluted in RPMI-1640 with 10% fetal bovine serum (FBS) just before use. Antibodies used for Western blotting included phosphorylated and total JAK2, STAT3, STAT5, and MAPK (Cell Signaling), and actin (Santa Cruz Biotechnology). Luminex assay kits (mouse cytokine 32-plex) were used to quantify plasma cytokine levels (Millipore). The hMPL wild-type plasmid was generously provided by K. Kaushansky (University of California San Diego) and cloned into the MSCV-IRES-EGFP retroviral vector. The MPLW515L mutation was generated using site-directed mutagenesis (Quickchange-XL; Stratagene) and confirmed by full-length DNA sequencing. The MSCV-mJAK2-GFP, MSCV-mJAK2V617F-GFP and MSCV-mJAK2K539L-GFP plasmids have been previously described.16,20
Cell lines
293T cells were grown in Dulbecco-modified Eagle medium with 10% FBS. Transient cotransfection of 293T cells and generation of retroviral supernatant were performed using Fugene (Roche) according to the manufacturer's guidelines. Ba/F3 cells were maintained in RPMI-1640 with 10% FBS and 1 ng/mL murine IL-3 (R&D Systems). Ba/F3 cells expressing the murine EPOR (BaF3-EPOR)31 were grown in RPMI medium with 10% FBS and 1 U/mL EPO. Ba/F3 cells were transduced with viral supernatant containing MSCV-hMPLW515L-GFP, whereas Ba/F3-EPOR cells were transduced with retroviral supernatant containing MSCV-mJAK2-GFP or MSCV-mJAK2V617F-GFP and MSCV-mJAK2 K539L-GFP vectors, respectively, sorted for green fluorescent protein (GFP) positivity by flow cytometry or by growth in the absence of cytokines. The human leukemic cell lines THP-1, NOMO-1, and HEL were cultured in RPMI-1640 with 10% FBS, whereas SET-2 cells were maintained in RPMI-1640 with 20% FBS. UKE-1 cells were cultured in RPMI-1640 supplemented with 10% horse serum and 1μM hydrocortisone.
In vitro inhibitor assays and Western blot analysis
A total of 10 000 viable cells were plated in 96-well microtiter plates in 200 μL of RPMI media with different concentrations of INCB16562 in triplicate. The 48-hour proliferation was assessed using the Cell Viability Luminescent Assay Kit (CellTiter-Glo; Promega). Results were normalized to growth of cells in media containing an equivalent volume of DMSO. The concentration at which 50% inhibition in proliferation occurred was determined using GraphPad Prism 5.0 software. For Western blot analysis of signaling pathways, cell lines were exposed to different concentrations of INCB16562 or to DMSO for 4 hours. Cells were then collected and lysed in lysis buffer and separated by electrophoresis as described previously.32 Nitrocellulose membrane was blocked in TBST/5% milk and incubated with antibodies as described in “Reagents.” Immunoprecipitation experiments were carried out as described previously.13
Murine model and analysis of mice
The MPL wild-type and W515L murine BMT assay was performed as described previously.21 Briefly, bone marrow cells from 5FU-treated male donors were harvested and transduced with viral supernatants containing either MSCV-hMPLW515L-GFP or MSCV-hMPLWT-GFP, and 750 000 bone marrow cells of each type were injected into the tail veins of lethally irradiated female BALB/c mice. Nonlethal bleeds were performed 11 days after transplantation to assess disease severity in all mice. MPLW515L mice were then randomized to receive once-daily treatment with INCB16562 (20 mg/kg, 60 mg/kg, 120 mg/kg) or with vehicle by oral gavage beginning 12 days after transplantation. We also observed 5 MPLW515L mice not treated with either vehicle or drug to study the course of the disease. MPL wild-type (MPLWT) mice were randomized to receive either vehicle (n = 5 mice) or INCB16562 (60 mg/kg) per day. With the exception of mice killed at specific time points for flow cytometric analysis, all mice were treated for 28 days or until any one of several criteria for killing were met, including severe lethargy, more than 10% body weight loss, and palpable splenomegaly that extended across the midline in accordance with our Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee–approved animal protocol. Animal care was in strict compliance with institutional guidelines established by the Memorial Sloan-Kettering Cancer Center, the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences 1996),33 and the Association for Assessment and Accreditation of Laboratory Animal Care International. Differential blood counts were assessed by mandibular bleeds before the trial, after 14 days of therapy/vehicle, and at study endpoints. Cytokine levels in mouse serum were determined using the Luminex multiplex bead-based assay system and the Millipore Mouse Cytokine 32-plex kit on a Luminex 200 following the manufacturer's protocols. Data analysis included with the Luminex 200 was used to conduct a 5-parameter logistic fitting method to determine cytokine concentrations. Bone marrow and spleen samples were subjected to Gordon and Sweeds stain for reticulin fibers (ammoniacal silver procedure). For histopathology, tissues were fixed in formalin and then embedded in paraffin for analysis as previously described.15 Immunohistochemistry for pSTAT3 was performed as previously described.34 We performed blinded immunohistochemical analysis of slides from 5 different mice in each group and counted the number of cells with positive-nuclear staining for pSTAT3 at ×20 magnification in cohorts of 5 mice treated with vehicle, 60 mg/kg or 120 mg/kg, respectively. Tissue sections were examined with a 2×/0.6, 10×/0.6, 20×/0.6, or 40×/0.6 objective. Photomicrographs were examined using a Zeiss Axio2 imaging microscope. Final images were assembled using a Zeiss Miramax scanner viewer.
Flow cytometry
For surface flow cytometry of mouse bone marrow and spleen, cells were washed in phosphate-buffered saline (PBS) plus 1% BSA and stained with monoclonal antibodies in PBS plus 1% BSA for 20 minutes on ice. Antibodies used were CD41a (PE), CD11b (PECy7), Gr1 (APCCy7), CD71 (PE), and Ter119 (PECy7; all from BD Pharmingen). Cells were gated for viability after incubation with 7-amino-actinomycin (7AAD) and GFP positivity, and more than 100 000 events were analyzed from this subset for marker expression using a FACSCalibur. For phospho-flow analysis, freshly isolated bone marrow from animals that had received transplants of hMPLW515L was harvested and exposed ex vivo to either DMSO or INCB16562 (1μM) at 37°C for 2 hours. After this, cells were stimulated with rhGCSF (375 ng/mL; R&D Systems), rhTPO (1250 ng/mL; R&D Systems), or vehicle for 10 minutes. Cells were then fixed immediately with 2% paraformaldehyde (BD Pharmingen) and permeabilized with 90% ice-cold methanol. Briefly, cells were incubated with CD11b (APCCy7) and CD61 (PE) in combination with anti–phospho-STAT3Y705 (Ax647), anti–phospho-STAT3S727 (Ax647), or anti–phospho-STAT5Y694 (Ax647; BD Pharmingen). For progenitor sorts, bone marrow cells and splenocytes were stained with the following antibodies for 20 minutes on ice: CD34 (PE), FcγR (Pacific Blue), CD117 (APCH7), Sca1 (PECy7), CD127 (biotin), CD4 (biotin), CD5 (biotin), CD8a (biotin), and CD19 (biotin; all from BD Pharmingen). Cells were then washed and restained with streptavidin (PerCP; BD Pharmingen) and propidium iodide. After a final wash, cells were analyzed by flow cytometry on a FACSAria to enumerate the common myeloid progenitor (CMPs), granulocyte-macrophage progenitor (GMPs), and megakaryocyte-erythroid progenitor populations (MEPs) as previously described.35 The gates for defining various subsets were set in the following manner using (1) unstained controls, (2) “fluorescence-minus one” (FMO) controls for experiments when more than 2 surface markers were used simultaneously, and (3) by the use of gating on discrete cell populations, when present, and then application of this exact gate to other groups stained with the same fluorophore. Also, all fluorescence-activated cell sorter (FACS) data presented is gated on living cells followed by gating for GFP+ cells. All data were analyzed using FlowJo software (TreeStar).
Statistical analyses
Pooled data were displayed as means plus or minus SD. Statistical significance between 2 groups was assessed using the nonparametric exact 1-tailed (Wilcox–Mann-Whitney) test.
Results
Effects of INCB16562 on proliferation of hematopoietic cell lines
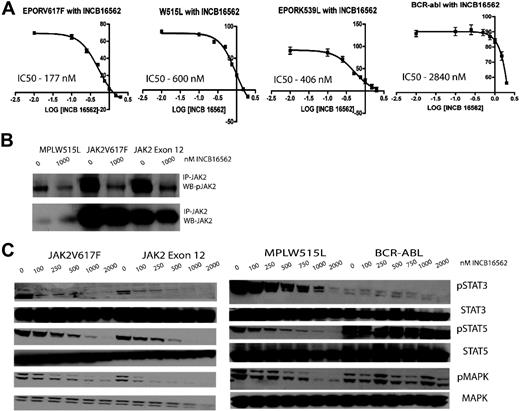
INCB16562 is an orally bioavailable, selective small-molecule inhibitor of JAK2 with a cell-free half maximal inhibitory concentration (IC50) of approximately 0.3nM. In enzyme assays, INCB16562 shows maximal inhibitory activity against JAK2, followed by JAK1 (2.2nM) and then JAK3 (10nM). Activity of the compound was evaluated in a variety of Ba/F3 isogenic cell lines and human leukemic cell lines (Figure 1; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We first evaluated the ability of INCB16562 to inhibit the proliferation of Ba/F3 isogenic cell lines expressing the Tel-JAK1/2/3 fusion proteins. Ba/F3 cells expressing Tel-JAK2 were most sensitive to INCB16562 with an IC50 of 168nM, whereas Ba/F3 cell lines expressing Tel-JAK1 (IC50 = 2310nM) and Tel-JAK3 (IC50 = 2494nM) were much less sensitive (supplemental Figure 1A). INCB16562 inhibited the proliferation of Ba/F3-EPOR cells expressing JAK2V617F (IC50 = 177nM) or the exon 12 mutant JAK2K539L (IC50 = 406nM) and of Ba/F3 cells expressing hMPLW515L (IC50 = 600nM), but Ba/F3 cells expressing BCR-ABL were much less sensitive, with an IC50 of 2840nM (Figure 1A). Similar results were observed in leukemic cell lines; the JAK2V617F+ cell lines SET-2 (IC50 = 46nM) and UKE-1 (573nM) were sensitive to growth inhibition by INCB16562, whereas the JAK2-wild-type cell lines THP-1 (IC50 = 3460nM) and NOMO-1 (IC50 > 3000nM) were much less sensitive (supplemental Figure 1B).
Viability curves, immunoprecipitation analysis, and Western blots revealing viability and down-modulation of signaling intermediates downstream of JAK2 with various concentrations of INCB16562 in Ba/F3 isogenic cell lines bearing mutant MPN alleles. (A) Cells bearing mutations which result in constitutive activation of the JAK-STAT signaling pathway (JAK2V617F, JAK2K539L, and MPLW515L) have a lower IC50 compared with Ba/F3 cells bearing BCR-Abl. \E Error bars denote SEM. (B) Immunoprecipitation analysis demonstrated decrease in JAK2 phosphorylation in Ba/F3 isogenic cell lines with INCB16562 treatment. A total of 20 million cells were incubated with either DMSO or INCB16562 for 4 hours, followed by immunoprecipitation as described previously.13 (C) Western blots revealing a dose-dependent down-modulation of signaling intermediates in the JAK-STAT pathway after treatment with INCB16562.
Viability curves, immunoprecipitation analysis, and Western blots revealing viability and down-modulation of signaling intermediates downstream of JAK2 with various concentrations of INCB16562 in Ba/F3 isogenic cell lines bearing mutant MPN alleles. (A) Cells bearing mutations which result in constitutive activation of the JAK-STAT signaling pathway (JAK2V617F, JAK2K539L, and MPLW515L) have a lower IC50 compared with Ba/F3 cells bearing BCR-Abl. \E Error bars denote SEM. (B) Immunoprecipitation analysis demonstrated decrease in JAK2 phosphorylation in Ba/F3 isogenic cell lines with INCB16562 treatment. A total of 20 million cells were incubated with either DMSO or INCB16562 for 4 hours, followed by immunoprecipitation as described previously.13 (C) Western blots revealing a dose-dependent down-modulation of signaling intermediates in the JAK-STAT pathway after treatment with INCB16562.
Effects of INCB16562 on signal transduction
We investigated the effects of INCB16562 on signal transduction pathways in sensitive and resistant hematopoietic cell lines. Treatment with INCB16562 markedly reduced phosphorylation of JAK2 in Ba/F3-EPOR-JAK2V617F, Ba/F3-EPOR-JAK2K539L, and Ba/F3-MPLW515L cells (Figure 1B). We also observed dose-dependent inhibition of downstream signaling pathways, including phosphorylation of STAT3, STAT5, and MAPK at doses comparable with those required for growth inhibition (250nM-1000nM). In contrast, signaling in Ba/F3-BCR-ABL cells was not affected, with persistent STAT5 and MAPK phosphorylation seen in the presence of 2000nM INCB16562 (Figure 1C). We observed similar results in JAK2V617F+ and JAK2V617F− human leukemia cell lines, with potent inhibition of downstream signaling pathways in JAK2V617F+ SET-2 and UKE-1 cells but not in JAK2V617F− NOMO-1 cells (supplemental Figure 1C).
INCB16562 improves survival in the MPLW515L model of ET/PMF
We have previously shown that expression of MPLW515L using the murine BMT assay induces a rapid and lethal myeloproliferative disease in mice that recapitulates many features of human ET and PMF, including marked thrombocytosis, myelofibrosis, and extramedullary hematopoiesis.21 Given the in vitro efficacy of INCB16562 in JAK2-dependent cell lines, we assessed the efficacy of INCB16562 in the MPLW515L retroviral model of ET/PMF (supplemental Figure 2). We assessed engraftment and disease severity by measuring blood counts in all mice 11 days after tail vein injection; at this time point, all mice had evidence of myeloproliferation with leukocytosis (110.7 ± 64.8 K/μL) and thrombocytosis (1595 ± 556.4 K/μL). Animals were then randomized to begin treatment with vehicle or with 20 mg/kg per day, 60 mg/kg per day, or 120 mg/kg per day of INCB16562 administered by oral gavage beginning on day 12. All mice receiving vehicle died within 21 days of treatment initiation, consistent with previous transplantation experiments with MPLW515L. The survival, body weight, liver/spleen weight, and blood counts of mice treated with or without vehicle were not significantly different (supplemental Figure 3), arguing against an adverse effect of vehicle on disease severity.
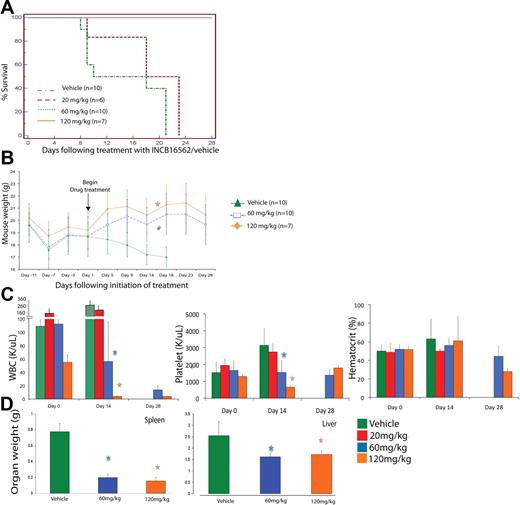
Although all mice receiving 20 mg/kg per day died soon thereafter, all mice randomized to receive 60 mg/day or 120 mg/kg per day were alive on day 28 of drug therapy (P < .001; Figure 2A). We noted a rapid decline in the weight of mice treated with vehicle (or untreated W515L mice), whereas mice receiving 60 mg/kg or 120 mg/kg INCB16562 regained weight lost after transplantation and maintained their weight throughout the rest of the trial. This difference in weights was statistically significant starting at 17 days of treatment with 120 mg/kg per day (P = .007) and after 18 days of treatment with 60 mg/kg per day (P = .004; Figure 2B). This observation is consistent with the improvement in body weight and constitutional symptoms of patients with PMF who have received JAK2 inhibitors in early clinical trials.36
Phenotypic characteristics of hMPLW515L mice treated with vehicle versus INCB16562 at varying doses over time. (A)Treatment with INCB16562 resulted in significant increase in survival at 60 mg/kg and 120 mg/kg doses compared with vehicle as shown by Kaplan-Meier survival curve (P < .001, log-rank test). (B) Mice treated with INCB16562 regained weight lost after tail vein injections compared with vehicle-treated mice, which continued to lose weight. This difference in weights were statistically significant starting at 17 days of treatment with 120 mg/kg per day (P = .007, Mann-Whitney U test) and after 18 days of treatment with 60 mg/kg per day (P = .004, Mann-Whitney U test). (C) Measurement of hematologic parameters at various time points in INCB16562 treatment reveal significant improvement in WBC and platelet counts with 60 mg/kg and 120 mg/kg of INCB16562 at day 14 after treatment initiation. (D) Treatment with INCB16562 resulted in decreased hepatosplenomegaly as measured in organ weight at 12 days of treatment. *P < .05 compared with vehicle-treated mice. (B-D) Error bars denote SD.
Phenotypic characteristics of hMPLW515L mice treated with vehicle versus INCB16562 at varying doses over time. (A)Treatment with INCB16562 resulted in significant increase in survival at 60 mg/kg and 120 mg/kg doses compared with vehicle as shown by Kaplan-Meier survival curve (P < .001, log-rank test). (B) Mice treated with INCB16562 regained weight lost after tail vein injections compared with vehicle-treated mice, which continued to lose weight. This difference in weights were statistically significant starting at 17 days of treatment with 120 mg/kg per day (P = .007, Mann-Whitney U test) and after 18 days of treatment with 60 mg/kg per day (P = .004, Mann-Whitney U test). (C) Measurement of hematologic parameters at various time points in INCB16562 treatment reveal significant improvement in WBC and platelet counts with 60 mg/kg and 120 mg/kg of INCB16562 at day 14 after treatment initiation. (D) Treatment with INCB16562 resulted in decreased hepatosplenomegaly as measured in organ weight at 12 days of treatment. *P < .05 compared with vehicle-treated mice. (B-D) Error bars denote SD.
INCB16562 improves thrombocytosis, leukocytosis, and extramedullary hematopoeisis in MPLW515L model
To assess the effects of INCB16562 on MPLW515L-induced thrombocytosis and leukocytosis, we performed peripheral blood analyses of all mice on the day that they were randomized to receive treatment (day 0), 14 days after treatment initiation (day 14), and at the end of treatment (day 28). Mice receiving INCB16562 had a dose-dependent reduction in leukocytosis and thrombocytosis over time (Figure 2C). Specifically, mice receiving INCB16562 60 mg/kg per day had a 50% reduction in white blood cell (WBC) counts compared with their counts before therapy, and their platelet counts were unchanged, whereas platelet counts in vehicle-treated mice increased by more than 100% by day 14 of the trial. Mice receiving INCB16562 120 mg/kg per day had a 98% reduction in WBC counts compared with before therapy, and their platelet counts were normalized by day 14 of drug therapy. By day 28, platelet counts in mice treated with 60 mg/kg or 120 mg/kg INCB16562 increased compared with levels at 14 days after treatment, but still remained within normal limits (Figure 2C). In contrast, drug treatment of mice who received transplants of MPLWT cells did not result in any difference in weights (supplemental Figure 4A), blood counts (supplemental Figure 4B), or in liver/spleen weights (supplemental Figure 4C) at time of death. These data demonstrate that expression of MPLW515L, but not MPLWT, results in marked leukocytosis and thrombocytosis that is reversed by JAK2 inhibitor therapy.
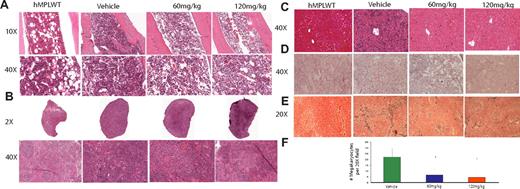
Planned killing of a subset of mice 12 days after treatment initiation allowed us to assess the effects of INCB16562 on extramedullary hematopoeisis and on histology in target organs. We observed a dose-dependent decrease in spleen and liver weights of W515L mice receiving 60 mg/kg or 120 mg/kg INCB16562 compared with vehicle-treated mice (P < .001; Figure 2D). Histologic evaluation of bone marrow revealed a decrease in overall cellularity compared with vehicle-treated mice (Figure 3A). In addition to the reduction in spleen size (Figure 2D), INCB16562 therapy was associated with partial normalization of splenic histology and periarteriolar lymphatic sheaths, whereas vehicle-treated mice showed neutrophilic infiltration of the spleen with complete effacement of normal splenic architecture (Figure 3B). There was a dose-dependent decrease in the number of megakaryocytes in treated mice versus vehicle (P < .01; Figure 3F). We also noted marked infiltration of neutrophils in the livers of vehicle-treated mice which greatly distorted the normal hepatic architecture; in contrast, hepatic histology was preserved in mice receiving 60 mg/kg or 120 mg/kg INCB16562 (Figure 3C). Histopathologic analysis of lung tissue revealed neutrophilic and megarkyocytic extramedullary infiltrates in the lungs of vehicle control but not INCB16562-treated mice (data not shown). Mice that received transplants of MPLWT-transduced cells, regardless of their assigned treatment (vehicle or INCB16562) had bone marrow, spleen, and liver histopathology similar to that seen in INCB16562-treated MPLW515L mice (Figure 3A-D; first panel).
H+E staining of bone marrow, spleen, and liver, and reticulin of hMPLWT mice and hMPLW515L mice treated with vehicle or INCB16562 for 12 days. Hematoxylin and eosin (H+E) stains demonstrate reduced cellularity in the (A) bone marrow and (B) reduced myeloproliferation in the spleen of INCB16562-treated W515L mice. Enumeration of megakaryocytes per ×20 field in 5 fields from the spleens of 2 to 3 mice killed on day 12 after beginning treatment reveals significant dose-dependent decrease in number of megakaryocytes with 60 mg/kg and 120 mg/kg INCB16562 (F). (C) Liver histology reveals greatly reduced extramedullary hematopoeisis in the liver with INCB16562 treatment at either 60 mg/kg or 120 mg/kg W515L mice. Reticulin staining of marrow (D) and spleen (E) reveals a decrease in myelofibrosis with 12 days of INCB16562 treatment. *P < .05 compared with vehicle-treated mice. hMPLWT mice on the other hand, show normal bone marrow (A left panel) and splenic (B left panel) architecture with minimal fibrosis (D-E left panels), and a normal hepatic structure (D left panel). (F) Error bars denote SD.
H+E staining of bone marrow, spleen, and liver, and reticulin of hMPLWT mice and hMPLW515L mice treated with vehicle or INCB16562 for 12 days. Hematoxylin and eosin (H+E) stains demonstrate reduced cellularity in the (A) bone marrow and (B) reduced myeloproliferation in the spleen of INCB16562-treated W515L mice. Enumeration of megakaryocytes per ×20 field in 5 fields from the spleens of 2 to 3 mice killed on day 12 after beginning treatment reveals significant dose-dependent decrease in number of megakaryocytes with 60 mg/kg and 120 mg/kg INCB16562 (F). (C) Liver histology reveals greatly reduced extramedullary hematopoeisis in the liver with INCB16562 treatment at either 60 mg/kg or 120 mg/kg W515L mice. Reticulin staining of marrow (D) and spleen (E) reveals a decrease in myelofibrosis with 12 days of INCB16562 treatment. *P < .05 compared with vehicle-treated mice. hMPLWT mice on the other hand, show normal bone marrow (A left panel) and splenic (B left panel) architecture with minimal fibrosis (D-E left panels), and a normal hepatic structure (D left panel). (F) Error bars denote SD.
Most importantly, reticulin-staining of bone marrow and spleen from mice treated with 60 mg/kg or 120 mg/kg INCB16562 showed a marked reduction in fibrosis compared with vehicle-treated mice (Figure 3D-E). We also assessed bone marrow fibrosis in MPLW515L mice killed just before treatment initiation (day 11) and found that all MPLW515L mice had at least moderate bone marrow and spleen fibrosis at this early time point. These data suggest that fibrosis in hMPLW515L mice is reduced, and not merely prevented, by INCB16562 treatment (Figure 3D-E; supplemental Figure 5). A minimum of 6 reticulin slides (each from bone marrow and spleen) taken from at least 3 different animals per treatment group was reviewed.
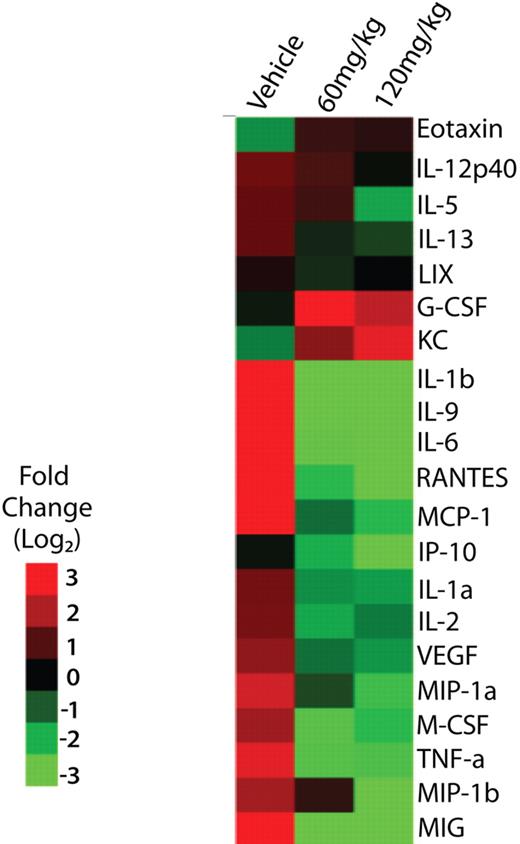
In addition to improving body weight and reducing WBC and platelet counts, treatment with INCB16562 produced profound decrements (≥ 2 log2) in inflammatory cytokines and angiogenic growth factors, many of which have been associated with the hypercatabolic state and constitutional symptoms observed in patients with MPN, including IL-6, TNFα, interleukin 1β (IL-1β), and VEGF (Figure 4).37
Effect of INCB16562 treatment on serum cytokine levels in hMPLW515L mice. Displayed are log2 fold changes in serum cytokine levels in vehicle-treated hMPLW515L mice after 9 days of treatment compared with levels in normal BALB/c mice as well as serum cytokine levels in hMPLW515L in mice treated with 9 days of 60 mg/kg or 120 mg/kg INCB16562 relative to vehicle-treated mice. Treatment with INCB16562 led to a greater than 2 log2–fold decrease in inflammatory cytokines such as IL-1β, IL-9, IL-6, and TNF-α.
Effect of INCB16562 treatment on serum cytokine levels in hMPLW515L mice. Displayed are log2 fold changes in serum cytokine levels in vehicle-treated hMPLW515L mice after 9 days of treatment compared with levels in normal BALB/c mice as well as serum cytokine levels in hMPLW515L in mice treated with 9 days of 60 mg/kg or 120 mg/kg INCB16562 relative to vehicle-treated mice. Treatment with INCB16562 led to a greater than 2 log2–fold decrease in inflammatory cytokines such as IL-1β, IL-9, IL-6, and TNF-α.
Flow cytometric analyses of INCB16562-treated mice versus vehicle control MPLWT and MPLW515L mice
Consistent with histopathologic analyses, we noted a decrease in myeloid cell burden in the spleens and bone marrows of INCB16562-treated MPLW515L mice by surface flow cytometry (supplemental Figure 6). We noted a reduction in the proportion of Gr1/Mac-1 double-positive population in the spleen, but not bone marrow, of drug-treated mice (supplemental Figure 6A), and noted a decrease in the proportion of CD41+ megakaryocytes in the bone marrow and spleen of drug-treated MPLW515L mice (supplemental Figure 6C). We also observed a decrease in the proportion of early erythroid precursors (CD71 single-positive and CD71/Ter119 double-positive cells) in mice treated with 60 mg/kg and 120 mg/kg INCB16562 compared with vehicle-treated mice (supplemental Figure 6B). No differences were seen in the proportion of B-cell populations in mice treated with 60 mg/kg and 120 mg/kg INCB16562 compared with vehicle-treated mice (data not shown). MPLWT mice treated with 60 mg/kg per day INCB16562 did not show any significant differences in the granulocyte, neutrophil, or erythroid populations between vehicle-treated or drug-treated samples either in the bone marrow or spleen (supplemental Figure 7A-C).
To further determine whether the decrease in myelopoiesis in bone marrow and spleens of treated MPLW515L mice could be attributed to a decrease at the level of the myeloid precursors, we performed multiparameter flow cytometric analysis of stem and progenitor populations on bone marrow and spleen from MPLW515L mice treated with 60 mg/kg INCB16562 compared with vehicle-treated MPLW515L mice. First, compared with control Balb/C mice and mice transduced with MPLWT bone marrow cells, we noted a marked increase in the proportion of megakaryocyte-erythroid precursor cells (MEPs) in vehicle-treated MPLW515L mice such that they accounted for the majority of progenitor cells in the spleens of diseased mice (supplemental Figure 8A). In contrast, mice treated with 60 mg/kg INCB16562 demonstrated a distribution of cells within the GMP, CMP, and MEP gates in the spleen consistent with normalization of the size of the MEP compartment (supplemental Figure 8A). We consistently observed a significant decrease in the size of the GFP+ spleen MEP compartment with INCB16562 treatment of MPLW515L mice: the average proportion of GFP+ spleen MEPs in 3 independent experiments was 0.0117 plus or minus 0.009 in drug-treated mice compared with 0.155 plus or minus 0.035 in vehicle-treated mice (P = .04, Mann-Whitney U test; supplemental Figure 8B). In contrast to the marked effects seen in the spleen MEP population, the absolute number of GFP+ MEP cells in bone marrow was not statistically significant after 12 days of INCB16562 treatment at 60 mg/kg per day (supplemental Figure 8B). We also assessed the percentage of GFP+ cells within the MEP population in bone marrow and spleen of vehicle and treated mice. The average percentage of GFP+ MEP cells without treatment was 68.0% with vehicle treatment compared with 31.3% with INCB16562 (P = .043, Mann-Whitney U test). This suggests that although the effects of INCB16562 on the bone marrow MEP population are modest compared with the spleen MEP population, there is a relative decrease in the relative proportion of mutant MEPs in bone marrow and spleen relative to wild-type MEPs. We did not observe significant differences in the bone marrow or splenic MEP population of MPLWT vehicle-treated or MPLWT INCB16562-treated mice (supplemental Figure 9), and stem/progenitor populations of mice that received transplants of MPLWT 12 days after transplantation, regardless of vehicle/drug treatment, were similar to control BALB/c mice.
Inhibition of JAK-STAT signaling with INCB16562 treatment in vivo
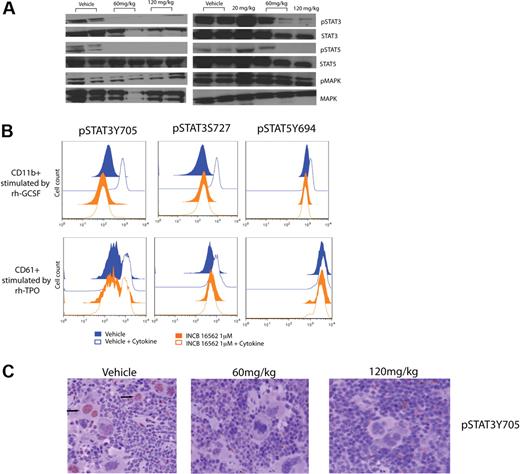
To investigate the effects of INCB16562 on signal transduction in vivo, we performed Western blotting and phosphoprotein-specific flow cytometry on spleen and bone marrow cells from mice treated with INCB16562 or with vehicle. Western blot analysis demonstrated marked reduction of STAT3 and STAT5 phosphorylation in splenocytes from mice treated with 60 mg/kg or 120 mg/kg INCB16562 compared with mice treated with vehicle or with 20 mg/kg INCB16562 (Figure 5A). We observed a near-complete or complete inhibition of STAT3 and STAT5 phosphorylation in vivo in 3 of 4 60 mg/kg-treated mice and in all 3 120 mg/kg-treated mice; in contrast, we observed persistent, marked STAT3 and STAT5 phosphorylation in all vehicle-treated mice and in mice treated with 20 mg/kg INCB16562. Of note, pMAPK levels did not differ between vehicle-treated and drug-treated mice consistent with persistent activation of the MAPK pathway in vivo in the setting of JAK2 inhibition. We then asked whether INCB16562 treatment of mouse hematopoietic cells inhibited JAK2-mediated phosphorylation of STAT3 and STAT5 in specific myeloid cell subsets by phospho-flow cytometry. A total of 2 hours of ex vivo INCB16562 treatment resulted in inhibition of phosphorylation of STAT3 and STAT5 in response to stimulation by rhGCSF and rhTPO in CD11b+ and CD61+ cells (Figure 5B). These results were representative of 3 separate experiments, and we consistently observed a greater effect of INCB16562 on inhibition of STAT3 phosphorylation compared with STAT5 phosphorylation in purified megakaryocyte and neutrophil populations. In addition, immunohistochemical staining for pSTAT3Y705 in mice spleen revealed a significant decrease in pSTAT3Y705 with INCB16562 therapy compared with vehicle-treated mice (Figure 5C). Specifically, the average number of pSTAT3-stained positive nuclei was 14.8 plus or minus 5.2 in the vehicle-treated group compared with 4.2 plus or minus 1.5 and 3.8 plus or minus 1.9 in the 60 mg/kg and 120 mg/kg INCB16562-treated groups, respectively (P < .05, Mann-Whitney U test).
Inhibition of JAK-STAT signaling with INCB16562 treatment of primary cells from hMPLW515L mice. (A) Western blotting of JAK-STAT signaling intermediates in hMPLW515L splenocytes after 12 days of INCB16562 treatment reveals abrogation of phosphorylation of STAT3, STAT5, and MAPK with INCB16562 treatment compared with vehicle. (B) Phosphoprotein-specific flow cytometry of CD11b+ and CD61+ myeloid cells in hMPLW1515L bone marrow cells treated ex vivo with INCB16562 at 1μM or with vehicle for 2 hours. Treatment with INCB16562 greatly decreased phosphorylation of STAT3 and STAT5 in response to 10-minute ex vivo stimulation with rhGCSF (375 ng/mL) and rhTPO (1250 ng/mL). (C) Immunohistochemical staining (×40) of pSTAT3Y705 in mouse spleen after 12 days of treatment with vehicle or INCB16552 at 60 mg/kg or 120 mg/kg reveals a decrease in pSTAT3 in treated versus control mice tissue.
Inhibition of JAK-STAT signaling with INCB16562 treatment of primary cells from hMPLW515L mice. (A) Western blotting of JAK-STAT signaling intermediates in hMPLW515L splenocytes after 12 days of INCB16562 treatment reveals abrogation of phosphorylation of STAT3, STAT5, and MAPK with INCB16562 treatment compared with vehicle. (B) Phosphoprotein-specific flow cytometry of CD11b+ and CD61+ myeloid cells in hMPLW1515L bone marrow cells treated ex vivo with INCB16562 at 1μM or with vehicle for 2 hours. Treatment with INCB16562 greatly decreased phosphorylation of STAT3 and STAT5 in response to 10-minute ex vivo stimulation with rhGCSF (375 ng/mL) and rhTPO (1250 ng/mL). (C) Immunohistochemical staining (×40) of pSTAT3Y705 in mouse spleen after 12 days of treatment with vehicle or INCB16552 at 60 mg/kg or 120 mg/kg reveals a decrease in pSTAT3 in treated versus control mice tissue.
Twenty-eight days of therapy with INCB16562 does not eradicate MPLW515L-expressing cells
Once-daily therapy with INCB16562 improved survival, thrombocytosis, leukocytosis, extramedullary hematopoeisis, and myelofibrosis in MPLW515L mutant mice. We then asked if INCB16562 therapy resulted in a reduction in the relative size of the mutant clone by assessing the GFP percentage of different hematopoietic subsets in drug-treated mice. We did not note a decrease in the proportion of GFP+ peripheral neutrophils in drug-treated mice over time (supplemental Figure 10B). Treatment with INCB16562 did not result in a significant decrease in the percentage of GFP+ bone marrow lineage-negative, Sca-1+, c-Kit+ (LSK) cells (P = .9). There was a nonsignificant reduction in the proportion of GFP+ LSK cells in the spleen with INCB16562 treatment (P = .06, Mann-Whitney U test; supplemental Figure 10A). We have also observed 3 mice after discontinuation of INCB16562 (60 mg/kg) treatment after 28 days of treatment and found that these mice survived an average of 20 days after discontinuation of INCB16562 treatment. These data suggest that once-daily therapy with INCB16562 for 28 days at efficacious doses does not eradicate the mutant clone in this model of ET/PMF.
Discussion
Mutations in the JAK-STAT signaling pathway are the most common somatic genetic events in PV, ET, and PMF, and MPL mutations are the most frequent class of disease alleles encountered in patients with MPN after JAK2V617F.17,18 This has led to the development of small-molecule inhibitors of JAK2 for the treatment of PV, ET, and PMF, and several of these agents are in late preclinical studies or in early-phase clinical trials. The agent with the most extensive clinical experience to date is INCB18424, a selective JAK1/2 inhibitor that is currently in phase 1/2 clinical trials for patients with PMF or with post-PV or ET MF.36 Most patients treated to date have experienced rapid reductions and marked improvements in constitutional symptoms; however, it remains to be seen whether INCB18424 therapy will allow more than the occasional patient to become transfusion independent and whether there will be reductions in JAK2V617F allele burden over time. Based on preclinical data in JAK2V617F+ patient samples and preclinical models, several other JAK kinase inhibitors are currently being evaluated in phase 1/2 studies for patients with MPN.28,29 However, there remain several important questions regarding the use of JAK kinase inhibitors in patients with MPN: (1) Do the existing JAK2 inhibitors completely abrogate JAK2 kinase activity and downstream signaling at clinically achievable doses? (2) Do different JAK inhibitors have similar efficacy/side effects, or will differences in selectivity or pharmacokinetics/pharmacodynamics alter activity of different compounds? (3) Will JAK inhibitors demonstrate efficacy regardless of JAK2/MPL mutational status? and (4) Will these agents offer more significant benefit to patients with PV and ET compared with patients with PMF or with post-PV or ET MF?
In this report, we evaluate the effects of INCB16562, a selective, small-molecule JAK2 kinase inhibitor, on a mouse model of MPLW515L-induced thrombocytosis and myelofibrosis. Both INCB16562 and INCB18424, the clinical compound used in PMF patient trials, inhibit JAK2 most potently, followed by JAK1 (INCB16562 is 5-fold less potent against JAK1 compared with JAK2 in enzyme-based assays versus 2-fold for INCB18424), TYK2 (14-fold less potent versus 7-fold for INCB18424), and then JAK3 (30-fold less potent versus 150-fold for INCB18424). We believe therefore that the observations made regarding the effects of JAK2 inhibition with INCB16562 in our in vitro and in vivo studies are relevant to clinical experiences with INCB18424 (and Li and colleagues38 ) and with other JAK2 inhibitors (TG101348, CEP701).28,29 We demonstrate that treatment once daily with doses of INCB16562 that inhibit JAK-STAT signaling in vivo, markedly improve survival in the MPLW515L murine model. Moreover, we found that INCB16562 therapy resulted in dose-dependent improvement in leukocytosis and thrombocytosis with normalization of WBC counts and platelet counts in all mice treated with 60 mg/kg and 120 mg/kg once daily. We also observed marked improvements in extramedullary hematopoeisis, including reductions in liver and spleen size and marked improvement in liver and spleen architecture. Treatment with INCB16562 also resulted in normalization of multiple inflammatory cytokines and angiogenic factors elevated in MPLW515L-mutant control mice and in patients with MPN. Mice receiving INCB16562 gained weight consistent with what has been observed in the initial clinical trials with INCB18424 in patients with PMF. We also noted a significant effect of INCB16562 therapy on the proliferation of specific myeloid progenitor populations, including normalization of the size of the MEP compartment in spleens of INBC16562-treated mice. Most importantly, INCB16562 therapy led to significant, dose-dependent reduction of reticulin fibrosis in bone marrow and spleen of all mice treated with doses of INCB16562 that inhibited signaling in vivo.
We were able to demonstrate that INCB16562 specifically abrogated JAK-STAT signaling induced by constitutively activated JAK2 and MPL alleles. In vitro studies demonstrated that INCB16562 inhibited proliferation, JAK2 phosphorylation, and downstream signaling pathways in cell lines transformed by JAK2/MPL mutations, but not in hematopoietic cell lines without activating JAK2/MPL alleles. Moreover, we were able to demonstrate potent, dose-dependent inhibition of JAK-STAT signaling in vivo, including potent inhibition of STAT3 and STAT5 phosphorylation in primary tissues from MPLW515L mice treated with INCB16562. The observation that INCB16562 potently inhibits JAK2 activity and downstream signaling in vitro and in vivo provides important insights into the effects of potent JAK2 inhibition in a model of MPN based on a human disease allele. Moreover, we did not see significant benefit on blood counts, histopathology, and survival until we used INCB16562 doses sufficient to inhibit JAK-STAT signaling in vivo, suggesting more attention should be paid to the degree of target inhibition achieved with JAK2 inhibitors in the clinic, potentially using flow cytometric analysis of phosphorylated signaling intermediates to assess pathway inhibition in specific myeloid cell subsets.
Although INCB16562 improved survival and reduces myeloproliferation in the MPLW515L murine BMT model, we did not observe a reduction in the proportion of GFP+ cells in peripheral blood or in the LSK population with 28 days of treatment. Furthermore, all previously treated mice developed fatal thrombocytosis/leukocytosis within 2 to 3 weeks after cessation of therapy. These data demonstrate INCB16562 does not fully eradicate the MPN clone in our murine model despite in vivo evidence of pathway inhibition and improvements in clinical signs of disease. This may in part reflect the acute nature of the myeloproliferation induced by MPLW515L in the retroviral BMT assay, compared with the chronic myeloproliferation seen in human ET/PMF. However, our experience with INCB16562 in vivo parallels the observations seen thus far in the initial clinical trials of JAK2 inhibitors, where there has been improvement in hematologic parameters and organomegaly with JAK2 inhibition but minimal decrease in allele burden with JAK2 inhibitor therapy. This may reflect the relatively short duration of therapy, insufficient pathway inhibition, insensitivity of the stem cell compartment to targeted therapies, or the possible emergence of resistant/persistent clones over time due to JAK2-dependent or -independent mechanisms. The preclinical and clinical experience with these compounds suggest that treatment with JAK2 inhibitors alone may not be truly curative for these MPNs, and preclinical studies combining JAK2 inhibitors with other existing and novel therapeutic approaches are warranted. Of note, we did not observe in vivo inhibition of MAPK signaling with INCB16562 therapy, and future studies into the basis for persistent in vivo MAPK activation in the setting of JAK2 inhibition and into the potential efficacy of combined JAK2/MAPK inhibition are warranted in MPN models and in MPN patient samples. Taken together, our data show that JAK2 inhibitor therapy offers significant efficacy and limited toxicity in a MPL-mutant model of ET/PMF, and suggest that JAK2 inhibitors may offer significant clinical benefit to patients with JAK2V617F− ET/PMF, particularly those with MPL mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Denise Hertel and Rebecca Stewart from Incyte Corporation for their help with immunohistochemistry and Richard Sparks, Argyrios Arvanitis, and James Rodgers for the synthesis of INCB16562. We also thank Eian Caulder from Incyte Corporation for help with the cytokine data.
This work was supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute to R.L.L. O.A.-W. is supported by the Clinical Scholars Program at Memorial Sloan-Kettering Cancer Center.
R.L.L. is an Early Career Award recipient of the Howard Hughes Medical Institute, a Clinical Scientist Development Award recipient of the Doris Duke Charitable Foundation, and is the Geoffrey Beene Junior Chair at Memorial Sloan-Kettering Cancer Center.
National Institutes of Health
Authorship
Contribution: P.K., O.A.-W., J.S.F., and R.L.L. designed the study; P.K., O.A.-W., S.M., J.P., N.K., J.R.G., T.C.B., E.d.S., P.J.H., A.G., A.P.C., and M.R. performed the experiments; P.K., O.A.-W., J.P., C.H., J.S.F., M.L.H., and R.L.L. analyzed data; K.V. and J.F.B. provided critical reagents; and P.K., O.A.-W., and R.L.L. wrote the manuscript.
Conflict-of-interest disclosure: K.V., P.J.H., T.C.B., M.R., A.P.C., and J.S.F. are employed by the Incyte Corporation. The remaining authors declare no competing financial interests.
Correspondence: Ross L. Levine, Human Oncology and Pathogenesis Program, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 20, New York, NY 10065; e-mail: leviner@mskcc.org.
References
Author notes
P.K. and O.A.-W. contributed equally to this article.