Abstract
The success of active immunotherapy is based on the vaccine's ability to overcome immune tolerance through recalibrating the immune system so that it is able to recognize tumor antigens as foreign rather than self. In this study, we used a lentiviral vector system to target human telomerase reverse transcriptase (lv-hTERT), a widely expressed tumor antigen. Immunization of HLA-A*0201 transgenic HHD mice with recombinant lv-hTERT vector induces potent and diversified cytotoxic T lymphocyte responses that recognize in vitro murine tumor cells, which overexpress telomerase. Compared with peptide-based vaccinations, the lv-hTERT vector triggers better and more sustained CD8+ T-cell response against self/TERT epitope in vivo. The study found that the additional use of a heterologous boosted vaccination drastically improves self/TERT-specific CD8 responses in lv-hTERT primed mice. Both primary and long-lasting self/TERT-specific CD8+ T-cell responses induced with Iv-hTERT vector required the presence of CD4 T cells in vivo. This lv-hTERT–based active immunotherapy efficiently inhibits the growth of telomerase expressing tumors (B16/HLA-A2.1 murine melanoma) in HHD mice. These data show that targeting hTERT with lentivector is highly effective in stimulating a broad range of CD8 T-cell immunity that can be exploited for cancer immunotherapy.
Introduction
The stimulation of tumor-specific T-cell responses with active immunotherapy has several theoretical advantages over other forms of cancer treatment.1,2 However, heterogeneous expression of most of the characterized tumor antigens limits the broad applicability of cancer vaccines that target such antigens. During the past few years, telomerase (TERT) has emerged as the first bona fide common tumor antigen and is actively investigated as the target for cancer immunotherapy.3,4 Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of telomerase enzyme that synthesizes telomeric DNA at the chromosome ends.5,6 hTERT is overexpressed in most human tumors (> 85%) and virtually all types of cancer.7 Telomerase activation has become one of the most important tumor escape mechanisms to circumvent the telomere-dependent pathways of cell death.8,9 It is well established that therapeutic strategies targeting antigens not involved in tumor growth can result in the selection of antigen-loss tumor mutants that are clinically progressive.10,11 Hence, down-regulation or loss of telomerase activity will severely inflict the growth potential of the tumor cells. All these findings justify the clinical applications of hTERT for anticancer immunotherapy. Broadly used in several anticancer vaccine trials, peptide vaccination is the most advanced strategy concerning hTERT antigen.3,12-16 Although the potential of a vaccination with minimal hTERT-derived peptides exceeds other vaccine strategies, several factors could influence the optimal success of this peptide-based strategy, such as (1) human leukocyte antigen (HLA) restriction, (2) natural processing of peptide on tumor cells, (3) antigen loss on tumors, (4) functionality of the antigen-specific T cells, and (5) persistence of the immune response in the host.1
One main obstacle of active anticancer immunotherapy is its inability to overcome immune tolerance. hTERT, like most tumor antigens, is a self-antigen; the induction of T-cell immunity against such shared self/tumor antigen could be controlled by mechanisms of central and peripheral tolerance that prevent detrimental immune responses against self. Consequently, it appears essential with regard to hTERT to evaluate novel approaches other than peptide vaccines, such as potent viral vectors capable of delivering hTERT-derived polypeptide vaccines to overcome immune tolerance.
Recently, recombinant lentiviral vectors (lentivectors) have gained substantial interest as an alternative method of antigen-specific immunization. These vectors have been able to effectively target the antigen to dendritic cells (DCs) in vivo and to elicit effective cell-mediated immune responses.17 Indeed, lentivector has been shown to trigger T-cell responses against some tumors antigens, such as melanoma-associated (NY-ESO-1 [New-York Esophageal 1], tyrosinase-related protein)18-20 and tumor-associated OVA foreign antigen.21,22 In our previous studies, we reported that the use of a lentiviral flap vector is very effective in stimulating CD8 T-cell responses against viral or tumor antigens.23,24 More recently, Liu et al showed that vaccination with a lentivector carrying mouse melanoma self/antigen can stimulate strong antiself/melanoma CD8 T-cell responses that induce successful tumor regression, thus supporting the ability of lentivector to break immune tolerance.25
In this study, taking advantage of the high homology between hTERT and mTERT,26 we constructed a lentivector expressing the full-length hTERT gene (lv-hTERT) and evaluated its immunogenicity and antitumor effect in an animal model of HLA transgenic mice. We compared the efficacy of the recombinant lv-hTERT vaccination with the hTERT peptide vaccination containing CD8-restricted epitopes. We show that vaccination with lv-hTERT permits to break tolerance against self and induce strong, broad, and highly functional hTERT-specific T CD8+ cells and confer a superior prophylactic and therapeutic antitumor response compared with hTERT-derived peptide plus adjuvant. Our results strongly support the use of the lentivector hTERT vaccine as a new potential option for telomerase-based active immunotherapy against cancers.
Methods
Mice
H-2 class I knockout, HLA-A*0201-transgenic mice (HHD) have been previously described.27 Eight- to 10-week-old female mice were bred and maintained under specific pathogen-free conditions in our animal facility (Institut Pasteur). All animal experiments were conducted in accordance with the guidelines of the Office Laboratory of Animal Care at the Pasteur Institute and were approved by the Pasteur Institute Institutional Review Board.
Cell lines and telomerase detection
HHD-transfected TAP-deficient RMAS cell lines (RMAS/HHD) have been described elsewhere.27 The HLA-A2.1-expressing B16F10 murine melanoma cells (B16/A2) were kindly provided by Dr N. Chaput (Institut Gustave Roussy). Wild-type B16F10 cell line (wt B16) was purchased from ATCC. Telomerase detection in tumor cells was achieved by flow cytometry as previously reported28 using anti-hTERT monoclonal antibody (Rockland), which cross reacts with mouse TERT.
Synthetic peptides
The HLA-A2.1-restricted, hTERT-derived peptides were previously described (Table 1). Briefly, p540 and p865 are high-affinity hTERT peptides.29,32 The peptides p152, p555, p572, and p988 were described as low-affinity cryptic hTERT epitopes. First amino acid substitution (with a tyrosine Y) of these cryptic peptides strongly increased their binding capacity to HLA-A2.1 and immunogenicity.30,31 The 2 peptides p572 and p988 are fully conserved in hTERT and mouse TERT (mTERT) and were used as self/TERT epitope in HHD mice.30,31 All peptides were synthetized by NeoMPS.
In vitro CTL responses in HHD mice immunized with recombinant lv-hTERT vector
| Peptides . | Sequence . | Relative avidity . | Protein of origin . | CTL response . | |
|---|---|---|---|---|---|
| R/T . | Percentage specific lysis . | ||||
| p152 | LLARCALPV | Low | hTERT | 0/6 | — |
| pY152 | YLARCALPV | High | hTERT | 0/6 | — |
| p540 | ILAKFLHWL | High | hTERT | 5/6 | 64, 48, 57, 65, 41 |
| p555 | ELLRSFFYV | Low | hTERT | 0/6 | — |
| pY555 | YLLRSFFYV | High | hTERT | 1/6 | 16 |
| p572 | RLFFYRKSV | Low | hTERT/mTERT | 5/6 | 41, 42, 48, 52, 26 |
| pY572 | YLFFYRKSV | High | hTERT/mTERT | 6/6 | 48, 51, 64, 71, 38, 21 |
| p865 | RLVDDFLLV | High | hTERT | 3/6 | 54, 49, 33 |
| p988 | DLQVNSLQTV | Low | hTERT/mTERT | 6/6 | 46, 39, 48, 15, 19, 34 |
| pY988 | YLQVNSLQTV | High | hTERT/mTERT | 6/6 | 61, 44, 79, 27, 33, 52 |
| Peptides . | Sequence . | Relative avidity . | Protein of origin . | CTL response . | |
|---|---|---|---|---|---|
| R/T . | Percentage specific lysis . | ||||
| p152 | LLARCALPV | Low | hTERT | 0/6 | — |
| pY152 | YLARCALPV | High | hTERT | 0/6 | — |
| p540 | ILAKFLHWL | High | hTERT | 5/6 | 64, 48, 57, 65, 41 |
| p555 | ELLRSFFYV | Low | hTERT | 0/6 | — |
| pY555 | YLLRSFFYV | High | hTERT | 1/6 | 16 |
| p572 | RLFFYRKSV | Low | hTERT/mTERT | 5/6 | 41, 42, 48, 52, 26 |
| pY572 | YLFFYRKSV | High | hTERT/mTERT | 6/6 | 48, 51, 64, 71, 38, 21 |
| p865 | RLVDDFLLV | High | hTERT | 3/6 | 54, 49, 33 |
| p988 | DLQVNSLQTV | Low | hTERT/mTERT | 6/6 | 46, 39, 48, 15, 19, 34 |
| pY988 | YLQVNSLQTV | High | hTERT/mTERT | 6/6 | 61, 44, 79, 27, 33, 52 |
The HLA-A*0201-restricted hTERT-derived peptides and their respective relative avidity have been already described.29-31 HHD mice were immunized subcutaneously with 2 × 107 TU of recombinant lv-hTERT vector, and their splenocytes were restimulated in vitro 10 days later with each peptide: p540 or p865, or with the pool of native and optimized form of indicated cryptic peptides (p152/pY152, p555/pY55, p572/pY572, and p988/pY988; 10 μg/mL). Peptide-specific CTLs were tested against RMAS/HHD cells pulsed or not with the corresponding peptide. Data represent the percentage of specific lysis of peptide-pulsed target cells at a 50:1 splenocyte/target ratio, after subtraction of nonspecific lysis of nonpulsed target cells (0%-8%).
CTL indicates cytotoxic T lymphocytes; HHD, HLA-A2.1 transgenic mice; R/T, number of responder/total number of immunized; and —, not applicable.
Recombinant lentivector preparation
Plasmid DNA expressing full-length hTERT was previously described.28 The hTERT DNA was subcloned into lentivector-transferred plasmid pTRIP.ΔU3.CMV.IRES.GFP23 by replacing the enhanced GFP gene to generate pTRIP.ΔU3.CMV.hTERT. Recombinant vector particles containing hTERT (hereafter referred to as lv-hTERT) were produced by a transient calcium phosphate cotransfection of 293T cells, and vector stock titration was done by real-time quantitative polymerase chain reaction as previously described.24
Immunizations
For recombinant lv-hTERT vector vaccination, HHD mice were immunized subcutaneously at the right abdominal flank with 2 × 107 transduction units (TU) of recombinant lv-hTERT vector particles (the same dose was used throughout the study). hTERT peptide immunization was performed using 100 μg of pY572 along with hepatitis B–derived helper peptide emulsified incomplete Freund adjuvant (IFA) (pY572/adjuv) at day 0 and day 14, as previously reported.31,33 For prime-boost vaccination, mice were first injected with lv-hTERT (2 × 107 TU) and then received 3 weeks later a boost injection with pY572 (100 μg) in IFA (pY572/IFA).
CD4 T-cell depletion
For CD4+ T-cell depletion, mice were injected with 100 μg of anti-CD4 antibody (GK1.5, kindly provided by Dr C. Leclerc, Institut Pasteur) 3 days before the first immunization as previously reported.24
Pentamer assay
To quantify p572-specific T cell, purified spleen CD8+ T lymphocytes (CD8+ T-cell isolation kit, Miltenyi Biotec; 5 × 105 cells) were incubated with phycoerythrin-conjugated pentamer recognizing the optimized pY572 peptide (pY572/pentamer; ProImmune) for 30 minutes at 4°C. The p572-specifc pentamer is not available because of the low affinity of this peptide.31 After 2 washes, cells were stained with anti–mouse CD8-APC (eBioscience) for 20 minutes at 4°C. Irrelevant HLA-A2.1 pentamer recognizing an HIV gag-peptide (HIVgag/A2) was used as a control. Samples were analyzed by flow cytometry on a FACSCalibur.
ELISpot
Ficoll-purified lymphocytes or purified spleen CD8+ T lymphocytes (CD8+ T-cell isolation kit, Miltenyi Biotec) were incubated (in triplicates) in ELISpot plates in the presence of medium or hTERT peptide.28 Plates were incubated for 20 to 30 hours at 37°C, and the interferon-γ (IFN-γ) spots were revealed following the manufacturer's instructions (Diaclone).
CTL generation and chrome assay
Splenocytes from immunized mice were stimulated with γ-irradiated lipopolysaccharide blasts loaded with hTERT peptides as described previously.30,31 The cytolytic activity of peptide-specific cytotoxic T lymphocytes (CTLs) against RMAS/HHD, wt B16, or B16/A2 cells was performed using standard 51chromium-release assay.28,31 The wt B16 and B16/A2 cell lines were treated with 20 ng/mL recombinant IFN-γ for 48 hours before use.
In vivo tumor protection
For therapeutic vaccination, HHD mice were challenged subcutaneously with 3 × 105 viable B16/A2 cells on the right abdominal flank. At day 5, tumor-bearing mice were subsequently vaccinated either with pY572/adjuv (days 0 and 14) or lv-hTERT, or lv-hTERT plus pY572/IFA boost as detailed previously. Control mice were immunized with lv-GFP (empty vector). Tumor size was monitored every 2 to 3 days using a caliper, and mice were killed when the tumor mass reached more than 200 mm2. The inhibition of lung pseudometastases experiment was performed as follows: HHD mice were vaccinated with pY572/adjuv (days 0 and 14) or with lv-hTERT plus pY572/IFA boost; and 7 days later, anesthetized mice were challenged intravenously with a high lethal dose of B16/A2 cells (106). Mice were killed at day 18 after tumor challenge and lung pseudometastases were counted. When these were too numerous (> 200 per mouse), they are shown as 200.
Statistics
The nonparametric Mann-Whitney test was used to compare CD8 T-cell responses among the groups of immunized mice. One-way analysis of variance and Neuman-Keuls tests were used to analyze the average tumor sizes among the respective groups of immunization. The Mann-Whitney test was used to study the effect of respective vaccinations on the number of lung pseudometastases. Mouse survival time was estimated using the Kaplan-Meier method, and the log-rank test was used. Statistically significant differences were considered at P less than .05.
Results
Induction of hTERT polyspecific CTL responses in HHD mice immunized with recombinant lv-hTERT vector
The recombinant lv-hTERT were produced by transient transfection of 293T cells with the lentivector plasmid pTRIP.ΔU3.CMV.HIV1 containing the full-length hTERT gene (Figure 1). HHD mice were immunized with a single dose of 2 × 107 TU of recombinant lv-hTERT. Results presented in Table 1 show that a single immunization with lv-hTERT induces strong CTL responses against most of the hTERT epitopes. Six of 10 epitopes induce strong CTL responses in the majority of HHD mice. Among them, 2 low-affinity peptides (p572 and p988) are fully conserved between hTERT and mTERT and can be considered as self-epitopes in the HHD mouse (Table 1). Interestingly, we found that the specific CTL responses against the low-affinity self/TERT epitopes (p572 and p988) were as strong as against the high-affinity non–self-epitopes, such as p540 and p865 in most mice. Thus, immunization of HHD mice with lv-hTERT vector induces a polyepitopic CTL response against HLA-A*0201–restricted peptides, including self/TERT epitopes.
Schematic diagram of lentiviral vector encoding hTERT. The human telomerase reverse transcriptase (hTERT) sequence was inserted into the TRIP.ΔU3.BR.IRES green fluorescent protein (GFP) lentiviral vector between the BamHI and EcoRI cloning sites. The ensuing bi-cistronic construct contains the full-length hTERT coding sequence under the control of the human cytomegalovirus immediate early promoter (CMVie), followed by the encephalomyocarditis virus internal ribosome entry site (IRES) and the enhanced green fluorescence protein (EGFP). LTR indicates long terminal repeat; cPPT, central polypurine tract; and CTS, central termination sequence.
Schematic diagram of lentiviral vector encoding hTERT. The human telomerase reverse transcriptase (hTERT) sequence was inserted into the TRIP.ΔU3.BR.IRES green fluorescent protein (GFP) lentiviral vector between the BamHI and EcoRI cloning sites. The ensuing bi-cistronic construct contains the full-length hTERT coding sequence under the control of the human cytomegalovirus immediate early promoter (CMVie), followed by the encephalomyocarditis virus internal ribosome entry site (IRES) and the enhanced green fluorescence protein (EGFP). LTR indicates long terminal repeat; cPPT, central polypurine tract; and CTS, central termination sequence.
The in vivo primed CTLs with lv-hTERT vector recognize B16/A2 tumor cells
Because lv-hTERT vector stimulates CTLs against peptides present in mTERT sequence, we then assessed the cytolytic activity of these CTLs against endogenously processed mTERT as previously reported. To this end, CTL lines specific for pY572, pY988, and p540 were generated from spleen cells of mice immunized with lv-hTERT vector, and their cytolytic activity was tested against B16/A2 tumor cells. Figure 2A shows that these cells express high amounts of mTERT protein while also possessing sufficient cell- surface HLA-A2.1 complex after IFN-γ treatment. Peptide recognition by CTLs was checked using the RMAS/HHD cells loaded with the corresponding peptide (Figure 2B-D). We found that both CTLpY572 (Figure 2B) and CTLpY988 (Figure 2C) were able to lyse B16/A2 cells, but no cytotoxicity was observed against the HLA-A2.1− parental tumor cells (wt B16). In contrast, we showed that CTLp540 failed to kill B16/A2 cells (Figure 2D), confirming that p540 epitope is not processed from mTERT protein. Thus, CTLs primed in vivo with lv-hTERT immunization are able to recognize and kill tumor cells in a HLA-A2.1–restricted manner. Our data also support previous studies showing that these 2 shared cryptic epitopes (p572 and p988) are naturally presented on HLA-A*0201+ tumors from both humans and mice.31,33 Consequently, we decided in this study to focus specifically on the immune response against p572 epitope.
CTLs stimulated with lv-hTERT vector kill TERT-expressing tumor cells in vitro. (A) Flow cytometric analysis of HLA-A2.1 and TERT expressions on the B16/A2 cell line. (B-D) Splenocytes from lentiviral vector (lv)–hTERT–immunized HHD mice were restimulated twice in vitro with the indicated hTERT peptide. The peptide-specific cytotoxic T lymphocytes (CTLs), CTLpY572 (B), CTLpY988 (C), and CTLp540 (D), were then tested against IFN-γ–treated tumor cells. Peptide recognition of CTLs was confirmed using RMAS/HHD cells pulsed with respective peptides (B-D). Results represent the percentage of lysis at various CTL/target ratios. The summarized data from 2 independent experiments are shown.
CTLs stimulated with lv-hTERT vector kill TERT-expressing tumor cells in vitro. (A) Flow cytometric analysis of HLA-A2.1 and TERT expressions on the B16/A2 cell line. (B-D) Splenocytes from lentiviral vector (lv)–hTERT–immunized HHD mice were restimulated twice in vitro with the indicated hTERT peptide. The peptide-specific cytotoxic T lymphocytes (CTLs), CTLpY572 (B), CTLpY988 (C), and CTLp540 (D), were then tested against IFN-γ–treated tumor cells. Peptide recognition of CTLs was confirmed using RMAS/HHD cells pulsed with respective peptides (B-D). Results represent the percentage of lysis at various CTL/target ratios. The summarized data from 2 independent experiments are shown.
The lv-hTERT vector induces a stronger and long-lasting self/TERT-specific CD8 T-cell response than hTERT peptide-base vaccination
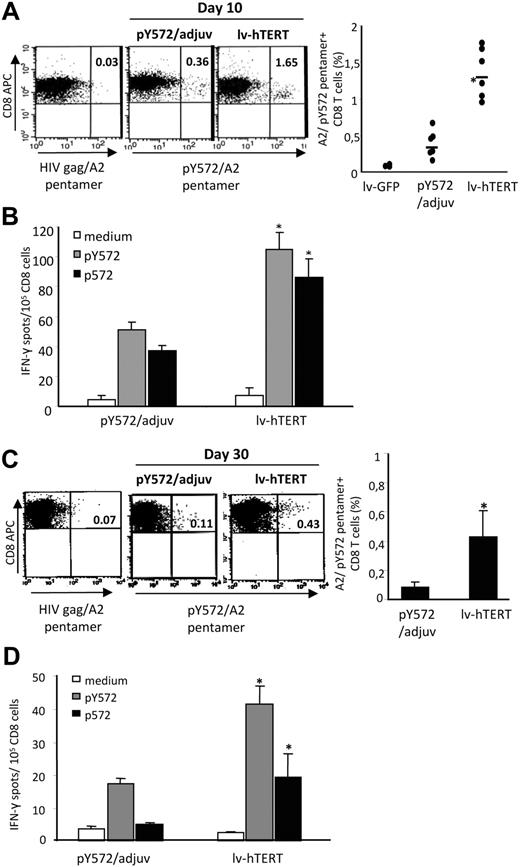
We immunized HHD mice with a single dose of lv-hTERT particles and focused on the immune response against the shared human and mouse self/TERT epitope p572. The lentivector strategy was compared with hTERT-based peptide vaccination using the cryptic optimized hTERT peptide pY572 plus adjuvant (pY572/adjuv), already reported to stimulate CTL responses against its native counterpart p572 (self/epitope) in both mice and humans.31,33,34 The induction of p572-specific CD8+ T cells was evaluated ex vivo 10 days after vaccination using the A2/pY572 pentamer. We showed that a single injection with lv-hTERT vector induced greater p572-specific CD8+ T-cell response compared with pY572/adjuv vaccination (Figure 3A). The frequencies of A2/pY572 pentamer-specific CD8+ T cells detected in HHD mice were approximately 5-fold higher after lv-hTERT injection, 1.34% plus or minus 0.33% versus 0.32% plus or minus 0.23% A2/pY572 pentamer+ CD8+ T cells in the pY572/adjuv group. No A2/pY572 pentamer+ cells were detected in mice immunized with lv-GFP (empty vector; Figure 3A right). Because pentamer analysis does not discriminate between anergic and functional T cells, we also monitored antigen-specific CD8 T-cell response by ex vivo IFN-γ ELISpot. In agreement with pentamer analysis, we showed a greater number of IFN-γ–producing CD8 T cells in response to pY572 and p572 in the lv-hTERT–vaccinated mice in contrast with the pY572/adjuv group (Figure 3B). These results demonstrate the efficient priming of functional anti-self/TERT CD8 T cells using the recombinant lv-hTERT vector.
Comparison of primary and long-term self/TERT-specific CD8 T-cell responses in mice immunized with lv-hTERT vector or with pY572/adjuv. Spleen lymphocytes from HHD mice immunized with recombinant lv-hTERT or with pY572/adjuv 10 days later were stained ex vivo either with pY572/pentamer or with irrelevant HIVgag/pentamer (N = 3-6 mice per group). Control mice were vaccinated with lv-GFP (empty vector). Representative fluorescence-activated cell sorter (FACS) plots of pentamer staining are presented (A left), and the mean percentages of pY572/pentamer+ CD8 T cells after subtraction of irrelevant pentamer are shown (A right). (B) Freshly isolated spleen CD8 T cells were cultured with p572 or pY572 peptide (5 μg/mL), and the IFN-γ–producing CD8 T cells were detected by IFN-γ ELISpot. The mean of specific IFN-γ spots plus or minus SD in each group are shown (n = 6 mice/group). The long-lasting responses are measured 30 days after the respective immunization with pentamer and IFN-γ ELISpot done ex vivo. (C) Representative FACS plots of pentamer staining and the mean of pY572/pentamer+ CD8 T cells are presented (n = 6 mice/group). (D) The mean of IFN-γ–producing CD8 T cells in response to p572 or pY572 peptide are shown (n = 4 mice/group). *Statistically significant value (P < .05, Mann-Whitney test).
Comparison of primary and long-term self/TERT-specific CD8 T-cell responses in mice immunized with lv-hTERT vector or with pY572/adjuv. Spleen lymphocytes from HHD mice immunized with recombinant lv-hTERT or with pY572/adjuv 10 days later were stained ex vivo either with pY572/pentamer or with irrelevant HIVgag/pentamer (N = 3-6 mice per group). Control mice were vaccinated with lv-GFP (empty vector). Representative fluorescence-activated cell sorter (FACS) plots of pentamer staining are presented (A left), and the mean percentages of pY572/pentamer+ CD8 T cells after subtraction of irrelevant pentamer are shown (A right). (B) Freshly isolated spleen CD8 T cells were cultured with p572 or pY572 peptide (5 μg/mL), and the IFN-γ–producing CD8 T cells were detected by IFN-γ ELISpot. The mean of specific IFN-γ spots plus or minus SD in each group are shown (n = 6 mice/group). The long-lasting responses are measured 30 days after the respective immunization with pentamer and IFN-γ ELISpot done ex vivo. (C) Representative FACS plots of pentamer staining and the mean of pY572/pentamer+ CD8 T cells are presented (n = 6 mice/group). (D) The mean of IFN-γ–producing CD8 T cells in response to p572 or pY572 peptide are shown (n = 4 mice/group). *Statistically significant value (P < .05, Mann-Whitney test).
It has been proposed that the efficacy of active immunotherapy may be related to the capacity of the vaccine to promote sustained T-cell responses in vivo.35 We therefore investigated the capacity of recombinant lv-hTERT immunization to induce long-lasting CD8 T-cell responses. HHD mice were immunized with a single injection of recombinant lv-hTERT or with pY572/adjuv, and the CD8 T-cell responses were measured 30 days after vaccination by pentamer and ELISpot assay. As shown in Figure 3C, A2/pY572 pentamer staining detected greater amounts of p572-specific CD8 T cells in lv-hTERT–immunized mice than in the pY572/adjuv-vaccinated group (0.43% ± 0.17% vs 0.08% ± 0.03% A2/pY572 pentamer+ CD8 T cells, respectively). When the long-lasting CD8 T-cell responses were evaluated by ex vivo ELISpot assay, we found that significant IFN-γ–producing CD8 T cells in response to self/epitope p572 can only be detected in mice injected with lv-hTERT (Figure 3D). We did not detect p572-specific cells in mice vaccinated with pY572/adjuv 30 days earlier. However, a minor response against pY572 was found in these mice (Figure 3D). Thus, recombinant lv-hTERT stimulates better and sustained CD8 T-cell responses against self/TERT epitope than pY572/adjuv vaccination.
Heterologous prime boost greatly improves the self/TERT-specific CD8 T-cell responses
Previous data showed that heterologous prime-boost vaccination improves the breadth of CD8 T-cell responses in lentivector-primed mice and allows circumvention of antivector immunity.19,36 Therefore, we investigated the effect of heterologous boost on self/TERT-specific CD8 T-cell response. To this end, HHD mice received lv-hTERT immunization followed 3 weeks later by pY572/IFA boost vaccination. The antigen-specific CD8 T-cell responses were monitored 7 days after the boost. We showed that the pY572/IFA boost drastically increased the CD8 T-cell responses in lentivector-primed mice. The A2/pY572 pentamer+ CD8 T cells reach more than 3% within spleen CD8 T cells compared with mice primed with lv-hTERT alone (Figure 4A). Accordingly, we found high numbers of IFN-γ–producing cells in response to p572 peptide stimulation in mice that received the boost injection (Figure 4B). To measure more precisely the function of the CD8 T cells, we performed ex vivo cytotoxicity test using 51Cr-labeled target cells. Results in Figure 4C show that freshly isolated CD8 T cells from prime-boost immunized mice were able to kill pY572-pulsed RMAS/HHD target cells and also exert direct cytotoxicity against B16/A2 tumor cells. Although the CD8 T cells from lv-hTERT–primed mice could kill peptide-loaded target cells, they failed to recognize B16/A2 tumor ex vivo (Figure 4C). Altogether, the peptide-based heterologous boost vaccination strongly enhances the magnitude and the avidity of self/TERT-specific CD8 T cells primed with lv-hTERT vector.
Heterologous boost vaccination strongly increases p572-specific CD8 T-cell responses in lentivector-primed mice. HHD mice (n = 4-6/group) were immunized with lv-hTERT vector alone or with pY572/IFA boost done 3 weeks later. (A) Enumeration of pY572/pentamer+ CD8 T cells by pentamer staining. Representative FACS dot plots for each group is shown (left) and the mean percentage of pY572/pentamer+ CD8 T cells after subtraction of irrelevant pentamer (right). *Statistically significant value (P < .05). (B) IFN-γ–producing spleen lymphocytes were detected by an ex vivo IFN-γ ELISpot in response to p572 peptide. Responses from individual mice are shown. (C) The ex vivo cytotoxicity of CD8 T cells was tested against RMAS/HHD cells pulsed with pY572 (left) or against B16/A2 tumor cells (right). Results represent the specific lysis (percentage) plus or minus SD in each immunized group of mice (n = 3 mice/group), after subtraction of nonspecific lysis measured in the presence of nonpulsed RMAS/HHD or wt B16 cells. The CD8 T cells from mice immunized with lv-GFP were used as negative control.
Heterologous boost vaccination strongly increases p572-specific CD8 T-cell responses in lentivector-primed mice. HHD mice (n = 4-6/group) were immunized with lv-hTERT vector alone or with pY572/IFA boost done 3 weeks later. (A) Enumeration of pY572/pentamer+ CD8 T cells by pentamer staining. Representative FACS dot plots for each group is shown (left) and the mean percentage of pY572/pentamer+ CD8 T cells after subtraction of irrelevant pentamer (right). *Statistically significant value (P < .05). (B) IFN-γ–producing spleen lymphocytes were detected by an ex vivo IFN-γ ELISpot in response to p572 peptide. Responses from individual mice are shown. (C) The ex vivo cytotoxicity of CD8 T cells was tested against RMAS/HHD cells pulsed with pY572 (left) or against B16/A2 tumor cells (right). Results represent the specific lysis (percentage) plus or minus SD in each immunized group of mice (n = 3 mice/group), after subtraction of nonspecific lysis measured in the presence of nonpulsed RMAS/HHD or wt B16 cells. The CD8 T cells from mice immunized with lv-GFP were used as negative control.
The self/TERT-specific CD8 T-cell responses required CD4 T-cell help in lv-hTERT–immunized mice
We next addressed the role of the CD4 T cell on the induction of the p572-specific CD8 T cells with lv-hTERT immunization. After immunization of CD4-depleted or nondepleted HHD mice, p572-specific CD8 T cells were evaluated by ex vivo IFN-γ ELISpot. Ten days after lv-hTERT injection, we showed lower numbers (2-3 times) of p572-specific CD8 T cells in CD4 T cell–depleted mice than in nondepleted mice (Figure 5A). In mice immunized 30 days earlier, the p572-specific CD8 T-cell responses were strongly reduced in CD4 T cell–depleted mice, whereas significant numbers of IFN-γ–producing cells were detected ex vivo in control mice as shown in Figure 5B. Thus, CD4+ T cells are required for optimal priming and persistence of p572-specific CD8 T cells in lv-hTERT–immunized mice. We could not discriminate in our study whether help was the result of CD4 response against hTERT, mTERT-specific epitopes, or both. Nevertheless, these results support previous findings showing that delivery of antigen with lentivector allows access to major histocompatibility complex (MHC) class II antigen-presenting pathways and triggers CD4 T-cell responses.37,38
CD4 T-cell depletion reduces the self/TERT-specific CD8 T cells in lv-hTERT–immunized mice. HHD mice (4/group) were treated either with anti-CD4 monoclonal antibody (GK1.5; CD4 depleted) or with saline (control), 3 days before immunization. Ten (A) or 30 days (B) later, spleen-isolated CD8 T cells were cultured with p572 peptide or medium. The p572-specific CD8 T cells were detected ex vivo by IFN-γ ELISpot.
CD4 T-cell depletion reduces the self/TERT-specific CD8 T cells in lv-hTERT–immunized mice. HHD mice (4/group) were treated either with anti-CD4 monoclonal antibody (GK1.5; CD4 depleted) or with saline (control), 3 days before immunization. Ten (A) or 30 days (B) later, spleen-isolated CD8 T cells were cultured with p572 peptide or medium. The p572-specific CD8 T cells were detected ex vivo by IFN-γ ELISpot.
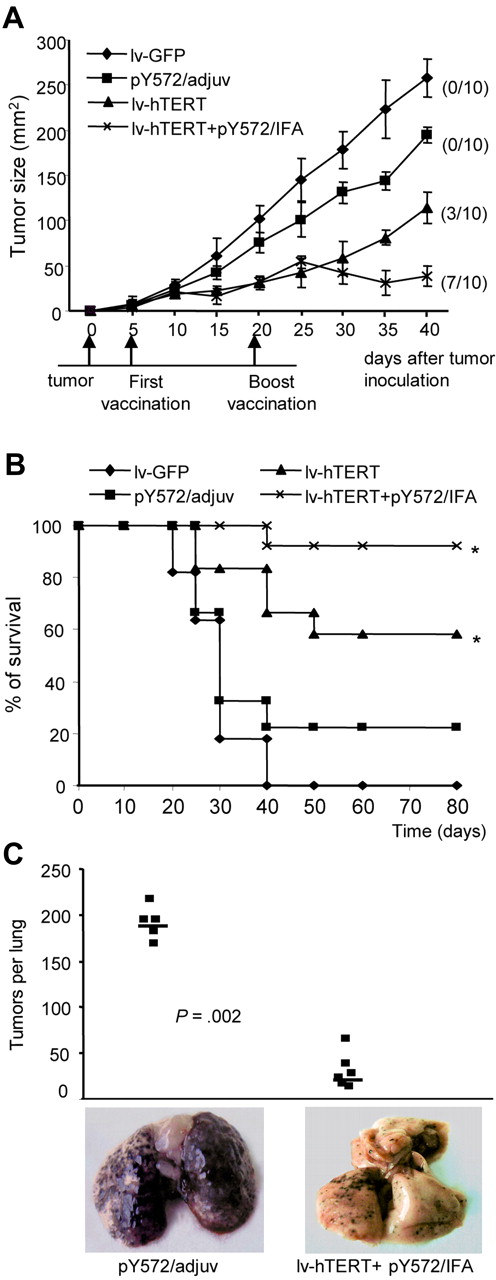
Recombinant lv-hTERT vaccination protects HHD mice against the growth of B16/A2 tumor
The therapeutic effect of lv-hTERT vaccination was then investigated using the B16/A2 melanoma in HHD mice.39 Five days after tumor inoculation, tumor-bearing mice were immunized as indicated. We found that a single injection of lv-hTERT could induce tumor regression in 3 of 10 mice and reduce the size of tumor in the remainder (Figure 6A). When tumor-bearing mice were treated with lv-hTERT plus pY572/IFA boost, B16/A2 tumors were cured in 7 of 10 mice and significant reduction of tumor size was seen in the remainder (Figure 6A). In contrast, no tumor regression was found in the pY572/adjuv group, but we observed a minor delay of B16/A2 tumor growth compared with the control mice (Figure 6A). Furthermore, the lv-hTERT–based vaccinations increased the survival of tumor-bearing mice. Whereas unvaccinated mice died at day 40, the survival time in the group immunized with lv-hTERT–based vaccinations was significantly prolonged compared with pY572/adjuv-immunized mice (Figure 6B).
Lentivector-based immunizations inhibit B16/A2 tumor growth in vivo. HHD mice were inoculated with 3 × 105 B16/A2 cells and immunized 5 days later as indicated (“In vivo tumor protection”). (A) Tumor sizes in each vaccinated group of mice are shown. The numbers in parentheses indicate the number of mice that have tumor recurrence of a total of 10. (B) The mouse survival time was estimated using the Kaplan-Meier method and recorded until 80 days. *Statistically significant survival value between lentivector groups and peptide group (P < .05; log-rank test). (C) HHD mice (n = 6/group) were prior immunized either with pY572/adjuv or with lv-hTERT plus pY572/IFA and subsequently challenged intravenously with 106 B16/A2 cells. Results represent the number of lung metastases counted after 18 days in each mouse, and representative photographs of lung from 1 mouse per group are shown.
Lentivector-based immunizations inhibit B16/A2 tumor growth in vivo. HHD mice were inoculated with 3 × 105 B16/A2 cells and immunized 5 days later as indicated (“In vivo tumor protection”). (A) Tumor sizes in each vaccinated group of mice are shown. The numbers in parentheses indicate the number of mice that have tumor recurrence of a total of 10. (B) The mouse survival time was estimated using the Kaplan-Meier method and recorded until 80 days. *Statistically significant survival value between lentivector groups and peptide group (P < .05; log-rank test). (C) HHD mice (n = 6/group) were prior immunized either with pY572/adjuv or with lv-hTERT plus pY572/IFA and subsequently challenged intravenously with 106 B16/A2 cells. Results represent the number of lung metastases counted after 18 days in each mouse, and representative photographs of lung from 1 mouse per group are shown.
We also determined whether lv-hTERT–based vaccination could mediate prophylactic protection against a high dose of B16/A2 tumors. To this end, HHD mice were previously immunized with recombinant lv-hTERT plus pY572/IFA or with pY572/adjuv vaccine as control, and then challenged intravenously with a high intravenous dose of B16/A2 cells that induce pseudometastases in lung. Eighteen days later, lungs were recovered and pseudometastases were counted. As shown in Figure 6C, animals injected with pY572/adjuv developed very aggressive tumors in their lungs. In contrast, we found a stronger inhibition of pseudometastases growth in all mice immunized with lv-hTERT plus pY572/IFA boost (Figure 6C). Overall, these results showed that interference with tumor growth of mTERT-expressing B16/A2 cells was obtained by active immunization with recombinant lv-hTERT vector.
Discussion
In our study, taking advantage of the high homology between hTERT and mTERT,26 we have explored an hTERT-based active immunotherapy using a lentivector delivery system. We showed that HLA-A*0201 transgenic HHD mice immunized with lv-hTERT simultaneously developed CTL against several HLA-A*0201-restricted hTERT epitopes, notably against low-affinity self/TERT epitopes. The lv-hTERT vector was capable of triggering robust and long-lasting self/TERT-specific CD8 T-cell response compared with the hTERT peptide-based vaccination, and provided protective and therapeutic immunity against TERT+ murine tumor B16, engineered to express HLA-A*0201.
Active cancer immunotherapy relies on the fundamental concept that tumor antigens exist and are presented in the context of MHC molecules for recognition by specific effector T cells. However, heterogeneous expression of most of the characterized tumor antigens limits the broad applicability of cancer vaccines that target such antigens. Telomerase, on the other hand, represents a prototype of a universal tumor antigen because of both its expression by the majority of tumors and its inherent functional involvement in oncogenic transformation.9 Given these attractive features, several active immunotherapy studies targeting hTERT have been conducted and hTERT peptide-based vaccinations have been the most used approach during the past few years.4 The relative ease of construction and production, chemical stability, and lack of oncogenic potential have made the peptide-based vaccine very popular. However, several obstacles limit the widespread use of peptide vaccines. These include HLA restriction, low immunogenicity, the need for adjuvants and carriers, failure or absence of epitope expression on tumor cells, low level of memory responses, and their inability to break immune tolerance.40
Recently, the use of potent viral vectors, such as lentiviral vectors, can allow us to circumvent many of these obstacles. Numerous studies have shown that lentivectors efficiently targeted tumor antigens to DC and trigger more potent and longer specific CTL responses than other viral vectors as well as genetic immunization approaches.21,22,37,38,41 In this study, we showed that vaccination of mice with a lentivector carrying the full-length hTERT gene allowed the generation of broad, multifunctional, and sustained hTERT-specific CTL responses and provided better antitumor immunity than peptide-based vaccination. The hTERT-specific CTL responses induced with this lv-hTERT vector were obtained without addition of any adjuvant, suggesting efficient in vivo cross-presentation of the hTERT protein. Because we did not engineer the lv-hTERT vector to carry a “danger” signal, we speculated that the lentivector itself was responsible for the DC activation in vivo. Indeed, in vitro experiments have shown that lentivectors are capable of providing the antigen and the appropriate signals to activate immature DCs.42 In addition, lentivector constructions contain tubulovesicular structures that can trigger innate responses through TLR9 and induce maturation in vivo.43 Similar results were reported by Chapatte et al showing that vaccination of HLA-A2/Kb mice with lentivector carrying a Melan-A antigen, was more effective for antigen-specific memory CD8+ T-cell induction than peptide-based vaccines.36 The authors also showed that the high expression of the survival/memory marker interleukin-7 receptor α chain on antigen-specific CD8 T cells induced with lentivector may contribute to the efficacy of this strategy compared with peptide-based vaccine.36,44
Another major point concerning the success of active cancer immunotherapy is the capacity of vaccine to overcome mechanisms of immune tolerance. To break this immune tolerance to self/tumor antigens and induce high-avidity antitumor T cells, several approaches, such as altered peptide ligands, xenoantigens, blockade of immune suppression pathways, or the use of potent viral vector system, have been used.2,45 About the lentiviral vectors, recent data from Liu et al showed the capacity of a lentivector carrying self/melanoma antigen to promote rupture of the immune tolerance against self/melanoma derived epitopes in mice.25 In this study, we found that immunization of HHD mice with recombinant lentivirus carrying hTERT induced CTLs against hTERT epitopes and also resulted in high expansion of CD8 T cells specific for self/mouse TERT epitopes (p572 and p988) shared between the 2 species.31,33 Furthermore, the self/p572-specific CD8 T response generated with lv-hTERT vector was stronger than one elicited with the heteroclitic peptide pY572 plus adjuvant vaccination (pY572/adjuv). It should be noted that HHD mice are knockout for their endogenous MHC class I (H-2); consequently, only the HLA-A*0201–restricted self/TERT-specific CD8 responses are probably responsible for the in vivo therapeutic effects of lv-hTERT vector compared with heteroclitic pY572/adjuv vaccination. As outlined, we especially focused on the CD8 T-cell responses against the self/TERT peptide p572 or its analog pY572 because they have already been shown to induce CTL responses in HHD mice and are used to vaccinate humans.30,31,33,34 However, our lentivector carries hTERT that is a heterologous antigen in mouse. Therefore, this xenogenic vaccination was able to break self-tolerance and to inhibit the progression of established tumor as previously reported.46,47 On the other hand, we found also a decrease of both primary and long-lasting self/p572-specific CD8 responses when CD4 T cells were depleted before lv-hTERT vector vaccination. Because the endogenous MHC class II molecules IA/E are still constitutively expressed in HHD mice,27 these results suggest that hTERT-specific CD4 T cells were induced after lv-hTERT immunization and could participate in the optimal priming of self/p572-specific CD8 T-cell response in vivo. Nonetheless, we cannot discriminate whether the help is provided from nonself or self/TERT-specific CD4 T-cell responses. This question would be addressed in future studies using transgenic mouse for human class II molecules and class II, IA/E knockout.
The challenge with regard to telomerase-based active immunotherapy is to develop a strategy capable of generating immune responses against this self/tumor antigen as robust as safe. As already reported, there are differences in telomerase expression in normal versus tumor tissues, making telomerase a potential safe cancer target.9 In the present study, we did not observe any deleterious effects in lv-hTERT–treated animals despite evidence of strong self/TERT-specific CTLs in vivo. In line with our observations, Mennuni et al recently showed that repeated immunizations with mTERT-encoding DNA resulted in anti-self/mTERT T-cell immunity and important delay of tumor growth in mouse models of prostate and colon cancers without adverse effects.48 These recent data support other cumulative observations in animal models and in humans concerning the safety of telomerase-based immunotherapy.9,33,49,50 However, future investigations should be conducted to address whether the duration of telomerase inhibition by active immunotherapy can affect normal tissues.
In conclusion, vaccination with recombinant lv-hTERT is highly effective to break tolerance against hTERT and elicits antitumor CD8+ T-cell immunity in HHD mice. Delivery of hTERT using lentivector may represent a potential approach to improve telomerase-based active immunotherapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr F. Lemonnier and Dr N. Chaput for providing HHD mice and B16/HLA-A*0201 cell lines, respectively, and Drs Sylvie Rusakiewicz and Federico Sandoval for critical reading of the manuscript and editorial assistance.
This work was supported by Institut National du Cancer, Paris, France.
Authorship
Contribution: O.A. designed and performed research, analyzed data, and wrote the paper; C.N. and P.C. designed research and performed lentivector expressing hTERT construction; K.M. and M.D. performed immunologic studies and analyzed data; P.R. performed statistical analysis; W.-H.F., E.T., and S.W.-H. designed research and analyzed data; and P.L.-D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Langlade-Demoyen, Institut Pasteur, Rétrovirologie Moléculaire, Unité de Rétrovirologie Moléculaire, Inserm U579; 25-28 rue du Dr Roux, 75724 Paris Cedex 15, France; e-mail: planglad@pasteur.fr; or Olivier Adotévi, Hôpital Européen Georges Pompidou AP-HP, Immunologie Biologique, 20 rue Leblanc 75015 Paris, France; e-mail: olivier.adotevi@egp.aphp.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal