Abstract
Selectins mediate leukocyte rolling, trigger β2-integrin activation, and promote leukocyte recruitment into inflamed tissue. E-selectin binding to P-selectin glycoprotein ligand 1 (PSGL-1) leads to activation of an immunoreceptor tyrosine-based activation motif (ITAM)–dependent pathway, which in turn activates the spleen tyrosine kinase (Syk). However, the signaling pathway linking Syk to integrin activation after E-selectin engagement is unknown. To identify the pathway, we used different gene-deficient mice in autoperfused flow chamber, intravital microscopy, peritonitis, and biochemical studies. We report here that the signaling pathway downstream of Syk divides into a phospholipase C (PLC) γ2– and phosphoinositide 3-kinase (PI3K) γ–dependent pathway. The Tec family kinase Bruton tyrosine kinase (Btk) is required for activating both pathways, generating inositol-3,4,5-trisphosphate (IP3), and inducing E-selectin–mediated slow rolling. Inhibition of this signal-transduction pathway diminished Gαi-independent leukocyte adhesion to and transmigration through endothelial cells in inflamed postcapillary venules of the cremaster. Gαi-independent neutrophil recruitment into the inflamed peritoneal cavity was reduced in Btk−/− and Plcg2−/− mice. Our data demonstrate the functional importance of this newly identified signaling pathway mediated by E-selectin engagement.
Introduction
Leukocyte recruitment into inflamed tissue is required for host defense and proceeds in a coordinated sequence of different steps. The first contact of neutrophils with the endothelium is mediated by selectins and their counter-receptors, followed by rolling and integrin-mediated arrest. While rolling, neutrophils collect different inflammatory signals that can activate several pathways and mediate integrin activation, arrest, crawling, and extravasation of leukocytes into inflamed tissue.1
E-selectin is expressed on inflamed endothelial cells and can bind to different glycosylated ligands on leukocytes, including CD44,2 P-selectin glycoprotein ligand-1 (PSGL-1),3 CD43,4 E-selectin ligand-1 (ESL-1),5 macrophage antigen-1 (Mac-1; αMβ2),6 and other unknown ligands. E-selectin engagement induces the activation of a receptor-proximal Src family immunoreceptor tyrosine-based activation motif (ITAM)–containing adaptor protein-Syk signaling pathway, which induces lymphocyte function–associated antigen-1 (LFA-1)–dependent slow rolling in vitro and in vivo.7,8 Selplg−/− and Syk−/− neutrophils cannot reduce their rolling velocity when rolling on E-selectin and intercellular adhesion molecule-1 (ICAM-1) in flow chamber experiments.8 In neutrophils from Tyrobp−/−Fcrg−/− (DAP12- and FcRγ-deficient) mice, E-selectin engagement fails to phosphorylate Syk and does not induce slow rolling.7 Binding of neutrophils to E-selectin under shear induces the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK),7 and blocking of p38 MAPK elevates the rolling velocity on E-selectin and ICAM-1 compared with the control.8 Blocking of p38 MAPK in isolated human neutrophils inhibits their adhesion to cells transfected with E-selectin and ICAM-1.9
Neutrophils mainly express 2 β2-integrins, LFA-1 (αLβ2) and Mac-1 (αMβ2), which regulate the rolling velocity of leukocytes in postcapillary venules of the cremaster muscle after TNF-α injection.10 In autoperfused flow chamber experiments, neutrophils show a reduced rolling velocity on E-selectin and ICAM-1 compared with E-selectin alone.8 E-selectin–mediated slow rolling is LFA-1 dependent.8 LFA-1 can undergo partial activation to an intermediate-affinity state or full activation to a high-affinity state.11 Stabilization of LFA-1 in the extended conformation associated with intermediate affinity11 still allows LFA-1–dependent slow rolling on E-selectin and ICAM-1, but inhibits chemokine-induced firm adhesion.8 However, the distal signaling pathway linking Syk to LFA-1, the integrin responsible for controlling the rolling velocity on E-selectin and ICAM-1,8 is still unknown.
Bruton tyrosine kinase (Btk), a member of the Tec family kinases, has a unique NH2-terminal region containing a pleckstrin homology (PH) domain and a proline-rich stretch, followed by Src-homology 3 (SH3), SH2, and kinase domains (for a review, see Mohamed et al12 ). It is known that Btk is involved in regulating signaling through the B-cell receptor (BCR),13 and defects in this molecule lead to X-linked agammaglobulinemia in humans.14 After BCR engagement, Btk translocates to the plasma membrane, where it is phosphorylated by Src family kinases.12 Because Btk is a multidomain protein, it can interact with and activate different molecules, including phospholipase C (PLC) γ2. Btk is also expressed in the myeloid lineage,15,16 and experiments with gene-deficient mice or inhibitors indicate that Btk in neutrophils is involved in G-protein–coupled receptor signaling and other functions.17,18 A recently published paper demonstrated that Btk-deficient mice have a reduced inflammatory response in complex disease models due to a leukocyte recruitment defect.19
PLCγ is a member of the family of phosphoinositide-specific PLCs (PI-PLC) that catalyzes the breakdown of phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol-3,4,5-trisphosphate (IP3), inducing a subsequent activation of protein kinase C (PKC) and increase of intracellular calcium levels.20 There are 2 isoforms of PLCγ, PLCγ1, and PLCγ2. PLCγ1 is ubiquitously expressed, but the expression of PLCγ2 is primarily limited to cells of the hematopoietic lineage.20 PLCγ has a domain structure common to other mammalian PLCs consisting of the catalytic region, an amino terminal PH domain, a number of EF hands, and a PKC homology (C2) domain. The catalytic domain is split into 2 regions being connected by the X/Y linker and is the site of DAG and IP3 production. Special features of PLCγ, like additional domains situated between the X and Y catalytic regions, separate them from other PLC isoforms and are responsible for their regulation by tyrosine kinases. PLCγ can be activated by receptor as well as nonreceptor tyrosine kinases. It has been shown that PLCγ2 is involved in Fcϵ receptor–mediated degranulation of mast cells21 and integrin signaling in platelets22,23 as well as neutrophils.24 A recently published study demonstrated that PLCγ2 is critically involved in integrin and Fc receptor–mediated neutrophil functions.25
Phosphoinositide 3-kinases (PI3Ks) are important cellular lipid kinases that convert PIP2 to PIP3, a second messenger important for different cellular functions and responses. Members of the PI3K family consist of a regulatory subunit (p85α, p85β, or p55γ) and a catalytic subunit (p110α, p110β, p110γ, or p110δ).26 PI3Kγ is mainly expressed in leukocytes and is activated by G-protein–coupled receptor signaling via the βγ subunit.27,28 Previous research about PI3Kγ has focused on its function in leukocyte activation, migration, and superoxide production. Leukocytes lacking PI3Kγ show an impaired respiratory burst,29 defective sustained adhesion after arrest,30 reduced chemotactic response toward a number of chemoattractants,29,31 and attenuated leukocyte recruitment into inflamed tissue.29,31,32
The present study was designed to identify the E-selectin–induced signaling pathway linking Syk to β2-integrins. Using ex vivo flow chamber assays, in vitro phosphorylation assays in neutrophils, and in vivo inflammation experiments with several gene-targeted mouse strains, we found that the signaling pathway downstream of Syk divides into PLCγ2- and PI3Kγ-dependent pathways. The activation of these pathways is controlled by the Tec family kinase Btk, which is required for E-selectin–mediated slow rolling.
Methods
Animals and bone marrow chimeras
C57BL/6 mice aged 8 to 12 weeks (Janvier), Plcg2−/− mice,21 Pik3cg−/− mice,29 Itgb2−/−mice,33 Syk−/− mice,34 and Btk−/− mice (The Jackson Laboratory) were housed in a specific pathogen–free facility. The Animal Care and Use Committees of the University of Münster (Germany) approved all animal experiments.
For further information on the generation of bone marrow chimeras, the autoperfused flow chamber assay, the peritonitis model, the selectin engagement assay, and the measurement of IP3, see supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Autoperfused flow chamber
Intravital microscopy
At 2 hours before cremaster muscle exteriorization, mice received an intrascrotal injection of 500 ng TNF-α (R&D Systems) in 0.3 mL of saline. Some animals also received tail vein injections of 4 μg pertussis toxin (PTx; Sigma-Aldrich) suspended in 0.3 mL of saline, 5 minutes before TNF-α injection. Mice were anesthetized using an intraperitoneal injection of ketamine hydrochloride (125 mg/kg; Sanofi Winthrop Pharmaceuticals), xylazine (12.5 mg/kg; Tranqui Ved; Phonix Scientific), and atropine sulfate (0.025 mg/kg; Fujisawa), and the cremaster muscle was prepared for intravital imaging as previously described.7,8 Intravital microscopy was performed on an upright microscope (Axioskop; Carl Zeiss) with a 40 × 0.75 NA saline immersion objective. Leukocyte rolling velocity, leukocyte rolling flux fraction, and leukocyte arrest were determined by transillumination intravital microscopy, whereas leukocyte extravasation was investigated by reflected light oblique transillumination microscopy as previously described.37 Recorded images were analyzed offline using ImageJ and AxioVision (Carl Zeiss) software. Leukocyte rolling flux fraction was calculated as a percentage of total leukocyte flux. Emigrated cells were determined in an area reaching out 75 μm to each side of a vessel over a distance of 100 μm vessel length (representing 1.5 × 104 μm2 tissue area). The microcirculation was recorded using a digital camera (Sensicam QE). Postcapillary venules with a diameter between 20 and 40 μm were investigated. Blood flow centerline velocity was measured using a dual photodiode sensor system (Circusoft Instrumentation). Centerline velocities were converted to mean blood flow velocities by multiplying with an empirical factor of 0.625.8
Statistics
Statistical analysis was performed with SPSS (Version 14.0). Differences between the groups were evaluated by 1-way analysis of variance, Student-Newman-Keuls test, and t test where appropriate. Data are presented as means plus or minus SEM, and P values less than .05 were considered statistically significant.
Results
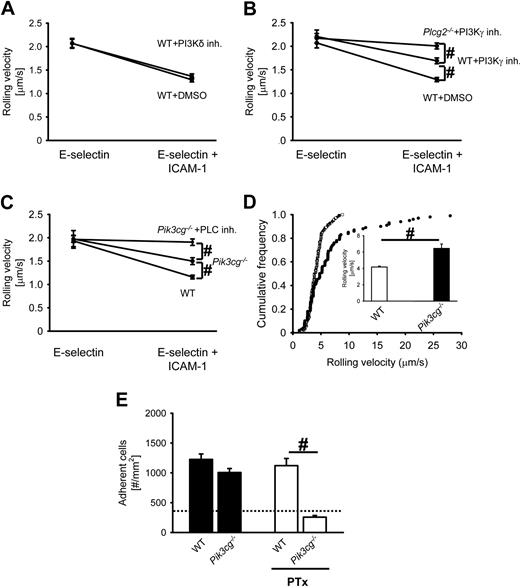
The Tec family kinase Btk is required for E-selectin–mediated slow rolling and Gαi-independent adhesion, but not for chemokine-induced arrest in vivo
It has been shown that Btk−/− mice have a leukocyte recruitment defect in complex disease models.19 To test whether Btk is involved in E-selectin–mediated slow rolling, we investigated the rolling velocity of neutrophils from Btk−/− mice and wild-type (WT) mice in an autoperfused flow chamber. The advantage of this system is that neutrophils can be investigated in whole blood without isolating the cells. We have demonstrated7,8 that the rolling velocity of WT neutrophils rolling on E-selectin– and ICAM-1–coated autoperfused flow chambers is significantly reduced compared with E-selectin alone.7,8 Btk−/− neutrophils showed a similar rolling velocity on E-selectin as WT neutrophils but failed to reduce their rolling velocity on E-selectin plus ICAM-1 (Figure 1A). To confirm these data, isolated bone marrow neutrophils7 were perfused through a flow chamber coated with either E-selectin or E-selectin plus ICAM-1, and rolling velocity was determined. Neutrophils from WT mice showed a reduction of the rolling velocity on E-selectin and ICAM-1 compared with E-selectin alone, but neutrophils from Btk−/− mice failed to reduce the rolling velocity on E-selectin and ICAM-1 (Figure 1B).
The Tec family kinase Btk is required for E-selectin–mediated slow rolling and Gαi-independent adhesion, but not for chemokine-induced arrest in vivo. (A) The carotid artery of chimeric mice reconstituted with bone marrow from WT mice (n = 3) or Btk−/− mice (n = 3) was cannulated with a catheter, which was connected to autoperfused flow chambers. Average rolling velocity of neutrophils on E-selectin (left) and E-selectin and ICAM-1 (right) is presented as means ± SEM. The wall shear stress in all flow chamber experiments was 5 to 6 dyn/cm2. (B) Isolated bone marrow neutrophils were resuspended in plasma, and the rolling velocity on either E-selectin alone or E-selectin plus ICAM-1 was measured. In these experiments, a shear stress of 3 dyn/cm2 was used (n = 3). (C) Mixed chimeric mice were generated by injecting bone marrow cells from LysM-GFP+ WT mice and Btk−/− mice into lethally irradiated WT mice. Cumulative histogram of rolling velocities of 100 GFP+ (WT; ●) and 100 GFP− (Btk−/−; ○) leukocytes in inflamed cremaster muscle venules of mixed chimeric mice (n = 4) treated with PTx and a monoclonal blocking P-selectin antibody (RB40.34). Inset data are means ± SEM. (D) Numbers of adherent cells per square millimeter in murine cremaster muscle venules. The cremaster muscle was exteriorized 2 hours after intrascrotal injection of 500 ng TNF-α in chimeric mice reconstituted with bone marrow from WT mice or Btk−/− mice. The dotted line indicates the number of adherent cells in WT mice treated with PTx and monoclonal blocking E-selectin antibody (9A9). #P < .05; *P < .05 vs other groups.
The Tec family kinase Btk is required for E-selectin–mediated slow rolling and Gαi-independent adhesion, but not for chemokine-induced arrest in vivo. (A) The carotid artery of chimeric mice reconstituted with bone marrow from WT mice (n = 3) or Btk−/− mice (n = 3) was cannulated with a catheter, which was connected to autoperfused flow chambers. Average rolling velocity of neutrophils on E-selectin (left) and E-selectin and ICAM-1 (right) is presented as means ± SEM. The wall shear stress in all flow chamber experiments was 5 to 6 dyn/cm2. (B) Isolated bone marrow neutrophils were resuspended in plasma, and the rolling velocity on either E-selectin alone or E-selectin plus ICAM-1 was measured. In these experiments, a shear stress of 3 dyn/cm2 was used (n = 3). (C) Mixed chimeric mice were generated by injecting bone marrow cells from LysM-GFP+ WT mice and Btk−/− mice into lethally irradiated WT mice. Cumulative histogram of rolling velocities of 100 GFP+ (WT; ●) and 100 GFP− (Btk−/−; ○) leukocytes in inflamed cremaster muscle venules of mixed chimeric mice (n = 4) treated with PTx and a monoclonal blocking P-selectin antibody (RB40.34). Inset data are means ± SEM. (D) Numbers of adherent cells per square millimeter in murine cremaster muscle venules. The cremaster muscle was exteriorized 2 hours after intrascrotal injection of 500 ng TNF-α in chimeric mice reconstituted with bone marrow from WT mice or Btk−/− mice. The dotted line indicates the number of adherent cells in WT mice treated with PTx and monoclonal blocking E-selectin antibody (9A9). #P < .05; *P < .05 vs other groups.
To support our flow chamber data, we conducted intravital microscopy in mixed chimeric mice generated by injecting bone marrow cells from Btk−/− mice (green fluorescent protein–negative [GFP−]) and WT LysM-GFP+ mice38 into lethally irradiated WT mice. After TNF-α injection, leukocyte rolling velocity was analyzed in inflamed venules of the cremaster muscle after blocking Gαi signaling and P-selectin to focus on E-selectin–dependent slow rolling.8 The mean blood flow velocity and the wall shear rates in these venules were 2.6 mm/second plus or minus 0.2 mm/second and 2100 second−1 plus or minus 180 second−1, respectively. Under these conditions, mean rolling velocity (Vavg) of Btk−/− leukocytes in vivo was 7.9 μm/second plus or minus 1.5 μm/second, whereas the mean rolling velocity of LysM-GFP+ control leukocytes was significantly lower (4.0 ± 1.0 μm/s, P < .05; Figure 1C). The rolling velocity seen in leukocytes from Btk−/− mice is similar to the rolling velocity of leukocytes seen in WT mice after blocking LFA-1, the integrin responsible for the reduction of the rolling velocity on E-selectin and ICAM-1.8 These findings suggest that Btk is involved in β2-integrin activation and subsequent slow rolling after E-selectin engagement.
Transition from leukocyte rolling to firm adhesion after TNF-α pretreatment is mediated in an overlapping fashion by G-protein–coupled receptor signaling and E-selectin.39 In agreement with a previously published study,39 pretreatment of WT mice with a blocking E-selectin antibody (9A9) or PTx did not affect the number of adherent cells (1173 ± 360 cells/mm2 and 990 ± 142 cells/mm2, respectively; data not shown). Blocking of both E-selectin and G-protein–coupled receptor signaling in WT mice significantly reduced leukocyte adhesion (347 ± 172 cells/mm2, P < .05; Figure 1D dotted line). Btk−/− mice showed the same number of adherent leukocytes as WT mice 2 hours after TNF-α injection (Figure 1D). Blocking of E-selectin by a monoclonal antibody in Btk−/− mice did not change the number of adherent cells compared with WT mice treated with a blocking E-selectin antibody (1003 ± 149 cells/mm2 and 1173 ± 360 cells/mm2, respectively). In contrast to WT mice, blocking of G-protein–coupled receptor signaling in Btk−/− mice significantly reduced leukocyte adhesion after TNF-α application (Figure 1D). Microvascular parameters (vessel diameters, centerline velocities, wall shear rates) and white blood cell (WBC) counts were similar among the groups (data not shown). These data suggest that the E-selectin–mediated pathway in Btk-deficient leukocytes is defective.
To test whether Btk is involved in chemokine-induced arrest, we conducted intravital microscopy of the untreated cremaster muscle.8 In this model, neutrophils roll in cremaster venules due to P-selectin expression on the endothelium, but rarely adhere. Injection of 600 ng of recombinant murine CXCL1, which binds CXCR2, induced the same number of adherent leukocytes in venules of chimeric mice reconstituted with bone marrow from WT mice or Btk−/− mice (272 ± 44 adherent cells/mm2 and 271 ± 38 adherent cells/mm2, respectively). These data suggest that Btk is required for E-selectin–mediated slow rolling, but is not involved in chemokine-induced leukocyte arrest.
Elimination of PLCγ2 partially abrogates E-selectin–mediated slow rolling and consequently reduces leukocyte adhesion in vivo
In several immunoreceptor signaling pathways, PLCγ can be activated by Btk.12 To address whether PLCγ2 is involved in E-selectin–mediated slow rolling, we investigated the rolling velocity of Plcg2−/− and WT neutrophils using the autoperfused flow chamber. Plcg2−/− neutrophils showed less reduction of their rolling velocity on E-selectin plus ICAM-1 compared with neutrophils from WT mice, but E-selectin–mediated slow rolling was not completely blocked (Figure 2A). These data suggest that PLCγ2 only partially regulates E-selectin–mediated slow rolling. To investigate the role of PLCγ2 in E-selectin–dependent slow rolling in vivo, we performed intravital microscopy on mixed chimeric mice generated by injecting bone marrow from Plcg2−/− mice and LysM-GFP+ mice into lethally irradiated WT mice. To investigate E-selectin–mediated slow rolling in vivo, we blocked Gαi-coupled signaling and P-selectin in TNF-α–treated mice. In TNF-α–induced inflamed postcapillary venules, the rolling velocity of Plcg2−/− leukocytes was 6.4 μm/second plus or minus 1.1 μm/second, which is significantly higher than the rolling velocity of LysM-GFP+ control leukocytes (4.2 ± 0.7 μm/second, P < .05; Figure 2B). To show that Plcg2−/− leukocytes have a partial defect, we investigated the rolling velocity of Syk−/− leukocytes in vivo. In a previous study, we demonstrated that Syk is required for integrin activation after E-selectin engagement.8 Compared with Plcg2−/− leukocytes, the rolling velocity of Syk−/− leukocytes is further increased (Figure 2B). These findings reveal that β2-integrin activation after E-selectin engagement is partially regulated by PLCγ2.
Elimination of PLCγ2 partially abrogates E-selectin–mediated slow rolling and consequently reduces leukocyte adhesion in vivo. (A) Carotid cannulas were placed in chimeric mice reconstituted with bone marrow from Plcg2−/− mice (n = 3) or WT mice (n = 3) and connected to autoperfused flow chambers. Average rolling velocity of neutrophils on E-selectin (left) and E-selectin and ICAM-1 (right) is presented as means ± SEM. The wall shear stress in all flow chamber experiments was 5 to 6 dyn/cm2. (B) Mixed chimeric mice were generated by injecting bone marrow cells from LysM-GFP+ WT mice and Plcg2−/− mice or Syk−/− mice into lethally irradiated WT mice. Cumulative histogram of rolling velocities of 150 WT leukocytes (●), 150 Plcg2−/− leukocytes (▾), and 150 Syk−/− leukocytes (○) in inflamed cremaster muscle venules of mixed chimeric mice (n = 4) treated with PTx and a monoclonal blocking P-selectin antibody (RB40.34). Inset data are means ± SEM. (C) Numbers of adherent cells per square millimeter in murine cremaster muscle venules. Cremaster muscle exteriorized 2 hours after intrascrotal injection of 500 ng TNF-α in chimeric mice reconstituted with bone marrow from WT mice or Btk−/− mice. Dotted line indicates the number of adherent cells in WT mice treated with PTx and monoclonal blocking E-selectin antibody (9A9). #P < .05.
Elimination of PLCγ2 partially abrogates E-selectin–mediated slow rolling and consequently reduces leukocyte adhesion in vivo. (A) Carotid cannulas were placed in chimeric mice reconstituted with bone marrow from Plcg2−/− mice (n = 3) or WT mice (n = 3) and connected to autoperfused flow chambers. Average rolling velocity of neutrophils on E-selectin (left) and E-selectin and ICAM-1 (right) is presented as means ± SEM. The wall shear stress in all flow chamber experiments was 5 to 6 dyn/cm2. (B) Mixed chimeric mice were generated by injecting bone marrow cells from LysM-GFP+ WT mice and Plcg2−/− mice or Syk−/− mice into lethally irradiated WT mice. Cumulative histogram of rolling velocities of 150 WT leukocytes (●), 150 Plcg2−/− leukocytes (▾), and 150 Syk−/− leukocytes (○) in inflamed cremaster muscle venules of mixed chimeric mice (n = 4) treated with PTx and a monoclonal blocking P-selectin antibody (RB40.34). Inset data are means ± SEM. (C) Numbers of adherent cells per square millimeter in murine cremaster muscle venules. Cremaster muscle exteriorized 2 hours after intrascrotal injection of 500 ng TNF-α in chimeric mice reconstituted with bone marrow from WT mice or Btk−/− mice. Dotted line indicates the number of adherent cells in WT mice treated with PTx and monoclonal blocking E-selectin antibody (9A9). #P < .05.
Leukocyte adhesion in chimeric mice reconstituted with either bone marrow from WT mice or Plcg2−/− mice was investigated 2 hours after TNF-α injection. WT mice and Plcg2−/− mice showed the same number of adherent leukocytes (Figure 2C). Blocking of E-selectin in Plcg2−/− mice did not change the number of adherent cells compared with WT mice treated with a blocking E-selectin antibody (1016 ± 161 cells/mm2 and 1173 ± 360 cells/mm2, respectively). WT mice treated with 4 μg of PTx via tail vein injection before intrascrotal injection of 500 ng of TNF-α showed no reduction in leukocyte adhesion compared with WT mice only treated with 500 ng of TNF-α (1121 ± 339 cells/mm2 and 1226 ± 221 cells/mm2, respectively; Figure 2C). After blocking Gαi signaling in Plcg2−/−, the reduction of leukocyte adhesion is similar to the reduction seen in WT mice pretreated with PTx and the blocking E-selectin antibody (9A9; Figure 2C, dotted line). Shear rates and diameters were similar between different groups, excluding a hemodynamic contribution to reduced leukocyte adhesion (data not shown).
Blocking PI3Kγ in Plcg2−/− neutrophils completely abolishes E-selectin–mediated slow rolling
Binding of P-selectin to PSGL-1 induces the phosphorylation of Nef-associated factor 1, which recruits the phosphoinositide-3-OH kinase p38-p110δ heterodimer and results in leukocyte integrin activation.40 Pretreatment of mice with a specific PI3Kδ inhibitor36 did not influence the rolling velocity of neutrophils on E-selectin or E-selectin plus ICAM-1 compared with neutrophils from WT control mice (Figure 3A). Blocking of PI3Kδ in Plcg2−/− neutrophils did not influence the rolling velocity on E-selectin alone and E-selectin plus ICAM-1 compared with Plcg2−/− neutrophils treated with DMSO (data not shown). The rolling velocity of neutrophils from WT mice pretreated with a specific PI3Kγ inhibitor35 and WT control mice on E-selectin alone was similar (Figure 3B). Neutrophils from WT control mice showed a reduction of the rolling velocity on E-selectin plus ICAM-1 (Figure 3B). Neutrophils from WT mice pretreated with the specific PI3Kγ inhibitor showed a reduced rolling velocity on E-selectin plus ICAM-1, but the reduction was significantly less compared with WT control neutrophils (Figure 3B). Pretreatment of Plcg2−/− mice with a specific PI3Kγ inhibitor had no effect on rolling velocity on E-selectin, but completely abolished the decrease of the rolling velocity on E-selectin and ICAM-1 compared with rolling velocity of neutrophils from WT mice pretreated with the PI3Kγ inhibitor (Figure 3B). To confirm these data, we used Pik3cg−/− mice in autoperfused flow chamber and intravital microscopy experiments. Pik3cg−/− neutrophils showed less reduction of their rolling velocity on E-selectin/ICAM-1 compared with WT neutrophils, but E-selectin–mediated slow rolling was not completely blocked (Figure 3C). Blocking of PLC (Figure 3C) or Btk (data not shown) in Pik3cg−/− mice completely abolished E-selectin–mediated slow rolling on E-selectin/ICAM-1. To investigate the role of PI3Kγ in E-selectin–dependent slow rolling in vivo, we performed intravital microscopy on mixed chimeric mice generated by injecting bone marrow from Pik3cg−/− mice and LysM-GFP+ mice into lethally irradiated WT mice. After blocking Gαi-coupled signaling and P-selectin in TNF-α–treated mice, the rolling velocity of Pik3cg−/− leukocytes was significantly higher compared with control leukocytes (Figure 3D).
Blocking PI3Kγ in Plcg2−/− neutrophils completely abolishes E-selectin–mediated slow rolling. (A) Rolling velocity of WT neutrophils on E-selectin alone or E-selectin/ICAM-1 of WT mice pretreated with either a PI3Kδ-inhibitor (PI3Kδ-inh.) or DMSO. (B) Rolling velocity of WT and Plcg2−/− neutrophils on E-selectin alone or E-selectin/ICAM-1 of either PI3Kγ-inhibitor (PI3Kγ inh.)– or DMSO-pretreated mice. (C) Rolling velocity of WT and Pik3cg−/− neutrophils on E-selectin or E-selectin/ICAM-1 of either untreated or PLC-inhibitor–pretreated mice. Data are presented as means ± SEM from 3 mice. (D) Mixed chimeric mice were generated by injecting bone marrow cells from Pik3cg−/− mice and LysM-GFP+ WT mice into lethally irradiated WT mice. Cumulative histogram of rolling velocities of 100 GFP+ (WT; ●) and 100 GFP− (Pik3cg−/−; ○) leukocytes in inflamed cremaster muscle venules of mixed chimeric mice (n = 3) treated with PTx and a monoclonal blocking P-selectin antibody (RB40.34). Inset data are means ± SEM. (E) Numbers of adherent cells per square millimeter in murine cremaster muscle venules. The cremaster muscle was exteriorized 2 hours after intrascrotal injection of 500 ng TNF-α in chimeric mice reconstituted with bone marrow from Pik3cg−/− mice or WT mice. The dotted line indicates the number of adherent cells in WT mice treated with PTx and monoclonal blocking E-selectin antibody (9A9). #P < .05.
Blocking PI3Kγ in Plcg2−/− neutrophils completely abolishes E-selectin–mediated slow rolling. (A) Rolling velocity of WT neutrophils on E-selectin alone or E-selectin/ICAM-1 of WT mice pretreated with either a PI3Kδ-inhibitor (PI3Kδ-inh.) or DMSO. (B) Rolling velocity of WT and Plcg2−/− neutrophils on E-selectin alone or E-selectin/ICAM-1 of either PI3Kγ-inhibitor (PI3Kγ inh.)– or DMSO-pretreated mice. (C) Rolling velocity of WT and Pik3cg−/− neutrophils on E-selectin or E-selectin/ICAM-1 of either untreated or PLC-inhibitor–pretreated mice. Data are presented as means ± SEM from 3 mice. (D) Mixed chimeric mice were generated by injecting bone marrow cells from Pik3cg−/− mice and LysM-GFP+ WT mice into lethally irradiated WT mice. Cumulative histogram of rolling velocities of 100 GFP+ (WT; ●) and 100 GFP− (Pik3cg−/−; ○) leukocytes in inflamed cremaster muscle venules of mixed chimeric mice (n = 3) treated with PTx and a monoclonal blocking P-selectin antibody (RB40.34). Inset data are means ± SEM. (E) Numbers of adherent cells per square millimeter in murine cremaster muscle venules. The cremaster muscle was exteriorized 2 hours after intrascrotal injection of 500 ng TNF-α in chimeric mice reconstituted with bone marrow from Pik3cg−/− mice or WT mice. The dotted line indicates the number of adherent cells in WT mice treated with PTx and monoclonal blocking E-selectin antibody (9A9). #P < .05.
Leukocyte adhesion in chimeric mice reconstituted with either bone marrow from Pi3kcg−/− mice or WT mice was investigated 2 hours after TNF-α injection. WT mice and Pik3cg−/− mice had the same number of adherent leukocytes (Figure 3E). Blocking of Gαi-coupled signaling in TNF-α–treated WT mice did not change the number of adherent leukocytes (Figure 3E). After blocking Gαi signaling in Pi3kcg−/− mice, the reduction of leukocyte adhesion is similar to the reduction seen in WT mice pretreated with the blocking E-selectin antibody and PTx (9A9; Figure 3E, dotted line). Shear rates and diameters were similar between different groups, excluding a hemodynamic contribution to reduced leukocyte adhesion (data not shown).
Gαi-independent leukocyte extravasation and neutrophil recruitment is defective in Btk−/− and Plcg2−/− mice
Leukocyte adhesion to the inflamed endothelium of the cremaster muscle and neutrophil recruitment into the peritoneal cavity after thioglycollate injection is promoted by E-selectin– and chemokine-dependent pathways.39
To investigate the contribution of Btk and PLCγ2 on the number of transmigrated leukocytes, we visualized extravasated leukocytes in the cremaster muscle using reflected-light oblique transillumination (RLOT) microscopy.37 WT mice treated with 4 μg PTx via tail vein injection before intrascrotal injection of 500 ng TNF-α showed no reduction in leukocyte extravasation (11 ± 1/1.5 × 104 μm2) versus WT mice that did not receive PTx treatment (13 ± 2/1.5 × 104 μm2; Figure 4A). However, the treatment of WT mice with PTx and a blocking E-selectin antibody caused a significant reduction in leukocyte extravasation (3 ± 1/1.5 × 104 μm2) relative to WT mice treated with the blocking E-selectin antibody alone (11 ± 1/1.5 × 104 μm2; Figure 4A). In chimeric mice reconstituted with bone marrow from Plcg2−/− mice or Btk−/− mice, the number of extravasated leukocytes was similar to that observed in WT mice (Figure 4A). Blocking of E-selectin in Plcg2−/− mice or Btk−/− mice did not change the number of transmigrated leukocytes compared with WT mice (data not shown). In contrast to this, the number of extravasated leukocytes in chimeric mice reconstituted with bone marrow from Pi3kcg−/− mice was significantly reduced compared with WT mice (Figure 4A). However, pretreating chimeric mice reconstituted with bone marrow from Plcg2−/− mice, Pi3kcg−/− mice, or Btk−/− mice with PTx almost abolished leukocyte extravasation (Figure 4A). Representative RLOT microscopic images of PTx pretreated chimeric mice reconstituted with bone marrow from WT mice, Btk−/− mice, or Plcg2−/− mice 2 hours after TNF-α application are shown in supplemental Figure 2A, B, and C, respectively.
Gαi-independent neutrophil recruitment is defective in Btk−/−and Plcg2−/− mice. (A) Number of extravasated leukocytes in cremasteric venules of TNF-α–treated chimeric mice reconstituted with bone marrow from WT mice (n = 4), Btk−/− mice (n = 4), Pik3cg−/− mice (n = 3), or Plcg2−/− mice (n = 4) per 1.5 × 104 μm2 tissue area. The measurements were performed 2 hours after intrascrotal TNF-α injection. The same groups were also analyzed after pretreatment with 4 μg PTx intravenously (+PTx; WT mice + PTx [n = 4], Btk−/− mice + PTx [n = 4], Pik3cg−/− mice + PTx [n = 3], Plcg2−/− mice + PTx [n = 4]). In addition to this, we analyzed WT mice after pretreatment with a blocking E-selectin antibody alone (+ anti–E-sel. ab [n = 3]) and in combination with PTx (WT mice + anti–E-sel. ab + PTx [n = 4]). (B) Neutrophil influx into the peritoneal cavity 8 hours after 1 mL injection of 3% thioglycollate into chimeric mice reconstituted with bone marrow from WT mice (n = 5), Btk−/− mice (n = 5), or Plcg2−/− mice (n = 5). The same groups were also analyzed after pretreatment with 4 μg PTx intravenously (+PTx; WT mice + PTx [n = 5], Btk−/− mice + PTx [n = 4], and Plcg2−/− mice + PTx [n = 5]). Total numbers of neutrophils in the peritoneal lavage fluid were determined by flow cytometry and hemocytometer count. #P < .05.
Gαi-independent neutrophil recruitment is defective in Btk−/−and Plcg2−/− mice. (A) Number of extravasated leukocytes in cremasteric venules of TNF-α–treated chimeric mice reconstituted with bone marrow from WT mice (n = 4), Btk−/− mice (n = 4), Pik3cg−/− mice (n = 3), or Plcg2−/− mice (n = 4) per 1.5 × 104 μm2 tissue area. The measurements were performed 2 hours after intrascrotal TNF-α injection. The same groups were also analyzed after pretreatment with 4 μg PTx intravenously (+PTx; WT mice + PTx [n = 4], Btk−/− mice + PTx [n = 4], Pik3cg−/− mice + PTx [n = 3], Plcg2−/− mice + PTx [n = 4]). In addition to this, we analyzed WT mice after pretreatment with a blocking E-selectin antibody alone (+ anti–E-sel. ab [n = 3]) and in combination with PTx (WT mice + anti–E-sel. ab + PTx [n = 4]). (B) Neutrophil influx into the peritoneal cavity 8 hours after 1 mL injection of 3% thioglycollate into chimeric mice reconstituted with bone marrow from WT mice (n = 5), Btk−/− mice (n = 5), or Plcg2−/− mice (n = 5). The same groups were also analyzed after pretreatment with 4 μg PTx intravenously (+PTx; WT mice + PTx [n = 5], Btk−/− mice + PTx [n = 4], and Plcg2−/− mice + PTx [n = 5]). Total numbers of neutrophils in the peritoneal lavage fluid were determined by flow cytometry and hemocytometer count. #P < .05.
Neutrophil recruitment in thioglycollate-induced peritonitis was also investigated in chimeric mice reconstituted with bone marrow from WT mice, Plcg2−/− mice, or Btk−/− mice with or without PTx treatment to block Gαi signaling. In the presence of intact GPCR signaling, Plcg2−/− and Btk−/− mice showed a normal number of neutrophils in the peritoneal cavity 8 hours after thioglycollate injection (Figure 4B). Pretreating WT mice with PTx reduced neutrophil recruitment into the peritoneal cavity by approximately 50%. However, blocking of Gαi signaling by PTx in Plcg2−/− mice and Btk−/− mice completely abolished neutrophil recruitment into the peritoneal cavity after thioglycollate injection (Figure 4B).
E-selectin engagement induces phosphorylation of Btk, PLCγ2, and PI3K
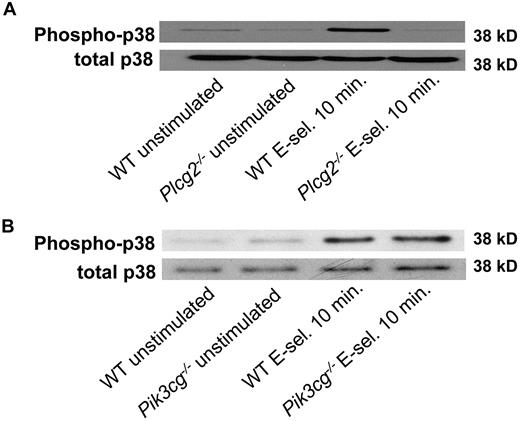
To investigate whether our previously published in vitro selectin engagement assay7 is shear stress dependent, we incubated WT neutrophils on immobilized E-selectin with and without shear stress. Incubation of WT neutrophils on E-selectin without shear stress did not induce phosphorylation of p38 MAPK, whereas the application of shear stress led to phosphorylation of p38 MAPK (supplemental Figure 1A). To exclude that integrin-mediated outside-in signaling is studied with the selectin engagement assay, we stimulated neutrophils from WT mice and Itgb2−/−mice and investigated the phosphorylation of p38 MAPK. After stimulation with E-selectin, WT and Itgb2−/− neutrophils showed the same phosphorylation of p38 MAPK, suggesting that phosphorylation of p38 MAPK is integrin-independent (supplemental Figure 1B-C).
Engagement of immunoreceptors (eg, T-cell receptor, B-cell receptor, GPVI) induces the activation of Src-family kinases, ITAM-containing adaptor proteins, Syk, and Tec family kinases, which in turn lead to phosphorylation of PLCγ2.20 The role of the different PI3K isoforms in these signaling pathways are not fully understood.41 Therefore, we tested whether E-selectin engagement leads to activation of Btk, PLCγ2, and PI3K. Stimulation of WT neutrophils with E-selectin under shear stress conditions induced phosphorylation of Btk (Figure 5A), PLCγ2 (Tyr1217), Akt as a target of PI3K, and p38 MAPK (Figure 5B). To demonstrate that these molecules are downstream of Syk, Syk−/− neutrophils were stimulated with E-selectin under shear stress conditions. In Syk−/− neutrophils phosphorylation of Btk (Figure 5A), PLCγ2 (Tyr1217), Akt as a target of PI3K, and p38 MAPK (Figure 5B) was absent, suggesting that Syk is upstream of Btk, PLCγ2, and PI3Kγ.
E-selectin engagement induces phosphorylation of Btk, PLCγ2, and PI3K. Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared. (A) Lysates were immunoprecipitated with anti-Btk, followed by immunoblotting (IB) with a general phosphotyrosine (PY; 4G10) antibody. (B) Lysates were immunoblotted with antibody to phosphorylated PLCγ2 (phospho-PLCγ2 [Tyr1217]), total PLCγ2 (n = 3), phosphorylated Akt (n = 3), total Akt (n = 3), phosphorylated p38 MAPK (phospho-p38), or total p38 (n = 3).
E-selectin engagement induces phosphorylation of Btk, PLCγ2, and PI3K. Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared. (A) Lysates were immunoprecipitated with anti-Btk, followed by immunoblotting (IB) with a general phosphotyrosine (PY; 4G10) antibody. (B) Lysates were immunoblotted with antibody to phosphorylated PLCγ2 (phospho-PLCγ2 [Tyr1217]), total PLCγ2 (n = 3), phosphorylated Akt (n = 3), total Akt (n = 3), phosphorylated p38 MAPK (phospho-p38), or total p38 (n = 3).
Btk is required for the activation of the PLCγ2- and PI3Kγ-dependent pathways
Our in vivo data indicate that Btk regulates PLCγ2- and PI3Kγ-dependent branches of a pathway leading to β2-integrin activation. Consequently, we tested whether Btk is required for downstream signaling of Syk after E-selectin engagement. As shown before, stimulation of WT neutrophils with E-selectin induced phosphorylation of PLCγ2 (Tyr1217), Akt, and p38 MAPK(Figure 6A). Stimulation of Btk−/− neutrophils with E-selectin did not induce the phosphorylation of PLCγ2 (Tyr1217), Akt, or p38 MAPK (Figure 6A), suggesting that Btk is required for activating PLCγ2- and PI3Kγ-dependent pathways. Phosphorylation of Syk in Btk−/− neutrophils was unaffected after E-selectin engagement (Figure 6B).
Btk is required for the activation of the PLCγ2- and PI3Kγ-dependent pathways. Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared. (A) Lysates were immunoblotted with antibodies to phosphorylated PLCγ2 (phospho-PLCγ2 [Tyr1217]), total PLCγ2 (n = 3), phosphorylated Akt (n = 3), total Akt (n = 3), phosphorylated MAPK (phospho-p38), or total p38 (n = 3). (B) Lysates were immunoprecipitated with anti-Syk, followed by immunoblotting (IB) with a general phosphotyrosine (PY; 4G10) antibody. (C) Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then intracellular IP3 levels were determined using a competitive binding assay. *P < .05 vs other groups.
Btk is required for the activation of the PLCγ2- and PI3Kγ-dependent pathways. Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared. (A) Lysates were immunoblotted with antibodies to phosphorylated PLCγ2 (phospho-PLCγ2 [Tyr1217]), total PLCγ2 (n = 3), phosphorylated Akt (n = 3), total Akt (n = 3), phosphorylated MAPK (phospho-p38), or total p38 (n = 3). (B) Lysates were immunoprecipitated with anti-Syk, followed by immunoblotting (IB) with a general phosphotyrosine (PY; 4G10) antibody. (C) Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then intracellular IP3 levels were determined using a competitive binding assay. *P < .05 vs other groups.
Phosphorylation of PLCγ2 is not strictly related to its enzymatic activity.20 To show that the activity of PLCγ2 is up-regulated after E-selectin engagement, we measured the concentration of the second messenger IP3, which is produced by activated PLC, in unstimulated and stimulated WT, Btk−/−, and Plcg2−/− neutrophils. After activation of WT neutrophils with E-selectin, the intracellular IP3 concentration increased compared with unstimulated WT neutrophils (Figure 6C). However, no increase in IP3 levels could be detected in Btk−/− and Plcg2−/− neutrophils stimulated with E-selectin (Figure 6C).
PLCγ2, but not PI3Kγ, is required for p38 MAPK phosphorylation
p38 MAPK is phosphorylated after E-selectin engagement and is involved in neutrophil slow rolling and adhesion.7-9 To investigate whether p38 MAPK is located in the PLCγ2- or PI3Kγ-dependent pathway, we looked for p38 MAPK phosphorylation in neutrophils from Plcg2−/− mice (Figure 7A) and Pi3kgc−/− (Figure 7B) after stimulation with E-selectin for 10 minutes. Phosphorylation of p38 was detectable in Pi3kgc−/− neutrophils after E-selectin engagement (Figure 7B), whereas stimulation of Plcg2−/− neutrophils with E-selectin did not induce p38 MAPK phosphorylation (Figure 7A). However, stimulation of Plcg2−/− and Pik3cg−/− neutrophils with E-selectin–induced phosphorylation of Syk (data not shown). These data suggest that p38 MAPK is downstream of PLCγ2.
PLCγ2, but not PI3Kγ, is required for p38 MAPK phosphorylation. Bone marrow–derived neutrophils from Plcg2−/− mice or Pik3cg−/− mice were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared (A-B). Lysates were immunoblotted with antibody to phosphorylated p38 MAPK (phospho-p38) or total p38 (n = 3).
PLCγ2, but not PI3Kγ, is required for p38 MAPK phosphorylation. Bone marrow–derived neutrophils from Plcg2−/− mice or Pik3cg−/− mice were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared (A-B). Lysates were immunoblotted with antibody to phosphorylated p38 MAPK (phospho-p38) or total p38 (n = 3).
Discussion
Binding of selectins to their glycoconjugate ligands on neutrophils induces the phosphorylation of Src-family kinases,7,40,42 ITAM-containing adaptor proteins,7 and Syk,7,42 resulting in integrin activation,8 slow rolling,8,43 and adhesion.9 However, the signaling pathway linking Syk to β2-integrins was still unknown. Here, we show that the Tec family kinase Btk is indispensable for E-selectin–mediated slow rolling. After E-selectin engagement Btk is phosphorylated in a Syk-dependent manner, and the signaling pathway downstream of Btk divides into PLCγ2- and PI3Kγ-dependent branches, which both regulate β2-integrin–mediated slow rolling (supplemental Figure 3). Eliminating Btk blocked Gαi-independent neutrophil recruitment into the inflamed cremaster muscle and peritoneal cavity, demonstrating the physiologic relevance of our findings.
Here, we identified that Btk has a key function for regulating the rolling velocity and Gαi-independent neutrophil recruitment into inflamed tissue. Neutrophils express the Tec family kinases Btk, Tec, and Bmx,12 which can be directly activated by Src family kinases.12 In the E-selectin–mediated signaling pathway, Btk is located downstream of Syk, as phosphorylation of Btk was absent in Syk−/− neutrophils after E-selectin engagement. In some signaling pathways,12 the generation of PIP3 by PI3K is necessary for the recruitment of Btk to the plasma membrane and subsequent activation. Because we used specific PI3K inhibitors (PI3Kγ and PI3Kδ), we cannot exclude the possibility that PIP3 generation is necessary for Btk activation, as other PI3K isoforms may produce PIP3. Alternatively, Btk may be recruited and activated by Src homology-2 domain–containing leukocyte protein of 76 kDa (SLP76), which is able to bind and activate Tec family kinases.44 It has previously been demonstrated that Btk−/− mice show reduced neutrophil recruitment into inflamed tissue.19 Btk is also involved in fMLP-induced production of superoxide anions, stimulation of adhesion, and chemotaxis,18 but not in chemokine-induced leukocyte arrest in vivo (see “The Tec family kinase Btk is required for E-selectin–mediated slow rolling and Gαi-independent adhesion, but not for chemokine-induced arrest in vivo”).
Mangla et al19 demonstrated that Btk−/− mice have reduced neutrophil recruitment into the peritoneal cavity after thioglycollate injection compared with WT mice. In the presence of intact Gαi-coupled signaling, we were unable to detect defective neutrophil recruitment in Btk−/− mice 8 hours after thioglycollate injection. However, Gαi-independent neutrophil recruitment is completely abolished in Btk−/− mice. The different results between the 2 studies can probably be explained by the use of different stimuli. It can be assumed that Mangla et al19 used a stronger stimulus, as they observed approximately 5-fold more neutrophils in the peritoneal cavity than we did (∼7.5 × 106 per mouse vs 1.5 × 106 per mouse, respectively), but the concentration of thioglycollate was not stated.
Downstream of Btk, the E-selectin signaling pathway divides into PLCγ2- and PI3Kγ-dependent pathways, whereas blocking the PLCγ2 pathway partially abrogates E-selectin–mediated slow rolling, but completely abolished leukocyte extravasation and neutrophil recruitment into inflamed tissue. These data, together with a previously published study25 showing that neutrophils from Plcg2−/− mice migrate and respond normally to chemoattractants, demonstrate that PLCγ2 may possess distinct and unique functions in different signaling pathways. Elimination of PLCγ2 completely inhibits integrin-mediated outside-in signaling,25 whereas the E-selectin–mediated slow rolling is only partially affected. The phosphorylation of PLCγ2 is absent in neutrophils from Syk−/− and Btk−/− mice, suggesting that these molecules are upstream of PLCγ2. In BCR signaling, Syk facilitates activation of PLCγ2 by Btk.44 Consistent with a putative role for these tyrosine kinases in activation of PLCγ2, E-selectin–mediated slow rolling was abolished in Btk−/− mice (Figure 1B) and Syk−/− mice (Figure 2B).8 The observed critical role of PLCγ2 in IP3 production and E-selectin–mediated slow rolling points toward an important downstream role for calcium and calcium-dependent signaling molecules. Indeed, increased intracellular calcium levels were observed after stimulation of neutrophils with E-selectin.45 Calcium-dependent signaling molecules likely are the classical and novel isoforms of protein kinase C and the RasGRP family of exchange factors, which act on different RAS family GTPases, including Ras and Rap1.46 Kindlins and talin directly interact with the tails of integrin-β subunits and regulate their affinity.47,48 The role of these molecules in selectin-mediated β2-integrin activation and slow rolling has to be addressed in further studies.
The integrin-mediated outside-in signaling pathway has similarities to the PSGL-1–Fgr-DAP12/FcRγ pathway, but there are also some differences.7 It is still unknown whether slow rolling is only mediated by inside-out signaling. Using Itgb2−/− neutrophils in the selectin engagement assay, we could demonstrate that p38 MAPK phosphorylation is independent of integrin-mediated outside-in signaling. However, we cannot fully exclude that slow rolling in the flow chamber and intravital microscopy experiments is independent of integrin-mediated outside-in signaling.
PI3Kδ and PI3Kγ exhibit partially redundant functions in leukocyte recruitment, and it has been shown that both isoforms are regulated in a time- and stimuli-dependent manner.32 In this study, we demonstrate that PI3Kγ, but not PI3Kδ, is downstream of Btk. However, our data do not exclude that other PI3K isoforms could also be involved in PIP3 production and subsequent recruitment of Btk to the plasma membrane after E-selectin engagement. This would be in agreement with a previously published study that demonstrated that binding of P-selectin to PSGL-1 induces the activation of PI3Kδ, which is involved in P-selectin–induced integrin activation.40 Because membrane-bound Btk is involved in phosphoinositide synthesis,49 it remains unclear whether its kinase function leads to the activation of downstream molecules and/or if its contribution to PIP2 production is necessary for second messenger generation by activated PI3Kγ and PLCγ2 (supplemental Figure 3). Liu et al32 demonstrated that elimination of PI3Kγ did not influence leukocyte rolling velocity in postcapillary venules of the cremaster muscle after TNF-α injection.32 In this model, leukocyte rolling velocity is regulated by E-selectin, P-selectin, and Gαi signaling.8 As Liu et al32 did not block P-selectin and Gαi signaling, these data are consistent with our flow chamber data showing that only E-selectin–triggered slow rolling is PI3Kγ-dependent. After TNF-α injection, Pik3cg−/− mice and WT mice show the same number of adherent cells in the postcapillary venules of the cremaster muscle, but the number of transmigrated cells is significantly reduced in Pi3kcg−/− mice (Figure 4A). These data are consistent with published data,32 and suggest that PI3Kγ is also involved in transmigration. Taken together, the role and versatility of the different PI3K isoforms in leukocyte recruitment is very complex and requires further studies to elucidate their different functions.
Our in vitro and in vivo experiments provide evidence for a relevant role of Btk in regulating E-selectin–mediated slow rolling. In addition, we demonstrate that downstream of Btk the signaling pathway divides into PLCγ2- and PI3Kγ-dependent pathways, which both regulate β2-integrin–mediated slow rolling. Eliminating either Btk or PLCγ2 blocked Gαi-independent neutrophil recruitment into the peritoneal cavity, demonstrating the physiologic relevance of our findings. Further studies are required to clarify how PLCγ2 and PI3Kγ regulate E-selectin–induced β2-integrin–mediated slow rolling on ICAM-1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Cornelia Brunner for the Btk−/− mice and Dr Barry Wolitzky for providing the mouse E-selectin–blocking antibody 9A9.
This study was supported by grants from the German Research Foundation (AZ 428/3-1 to A.Z.), the Interdisciplinary Clinical Research Center (IZKF; Münster, Germany), and from the National Institutes of Health (NIH; R01 AI079087 to D.W.).
National Institutes of Health
Authorship
Contribution: H.M. performed experiments and helped analyze the data; A.S. performed experiments; H.V.A., E.H., and D.W. helped design the study and interpret the results; K.L. helped design the study and interpret the results, and revised the manuscript; and A.Z. designed the study, performed many of the experiments, analyzed the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Zarbock, Department of Anesthesiology and Critical Care Medicine, University of Muenster, Albert-Schweitzer Str 33, 48149 Muenster, Germany; e-mail: zarbock@uni-muenster.de.



![Figure 4. Gαi-independent neutrophil recruitment is defective in Btk−/− and Plcg2−/− mice. (A) Number of extravasated leukocytes in cremasteric venules of TNF-α–treated chimeric mice reconstituted with bone marrow from WT mice (n = 4), Btk−/− mice (n = 4), Pik3cg−/− mice (n = 3), or Plcg2−/− mice (n = 4) per 1.5 × 104 μm2 tissue area. The measurements were performed 2 hours after intrascrotal TNF-α injection. The same groups were also analyzed after pretreatment with 4 μg PTx intravenously (+PTx; WT mice + PTx [n = 4], Btk−/− mice + PTx [n = 4], Pik3cg−/− mice + PTx [n = 3], Plcg2−/− mice + PTx [n = 4]). In addition to this, we analyzed WT mice after pretreatment with a blocking E-selectin antibody alone (+ anti–E-sel. ab [n = 3]) and in combination with PTx (WT mice + anti–E-sel. ab + PTx [n = 4]). (B) Neutrophil influx into the peritoneal cavity 8 hours after 1 mL injection of 3% thioglycollate into chimeric mice reconstituted with bone marrow from WT mice (n = 5), Btk−/− mice (n = 5), or Plcg2−/− mice (n = 5). The same groups were also analyzed after pretreatment with 4 μg PTx intravenously (+PTx; WT mice + PTx [n = 5], Btk−/− mice + PTx [n = 4], and Plcg2−/− mice + PTx [n = 5]). Total numbers of neutrophils in the peritoneal lavage fluid were determined by flow cytometry and hemocytometer count. #P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/15/10.1182_blood-2009-11-254185/4/m_zh89991051370004.jpeg?Expires=1765002265&Signature=CdXeMx-tzw-99EUn23SW79YHxlHyfCkrWNsb3qU7TlrldT62cGeimjRL17T5~gYudv7KNIIhGyLYGjAk0UC8x9k1J6rCVXdMW6xZKKWQ3OiP3fW6-7LnkNXb5iSsCKenMGPowazI3eSiwrWrUnoyLK1mqpiBhSHhm7sIcXwDBZ~1E1V8HrYA1WCGUP95r57NmsoFGOC00A6gfxMPqmVS9H-99X3EWkIJsCqJKi9U6vXOs83HMLS4PkDK0CGbEhIjy5QA4oxreiVLLu5MPhIgtog3kIjuFmuP8I30MeQSwYMbbEr6RU5czou3xTeqjZGrNgEPzxu2CBwMlFkZf7qmzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. E-selectin engagement induces phosphorylation of Btk, PLCγ2, and PI3K. Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared. (A) Lysates were immunoprecipitated with anti-Btk, followed by immunoblotting (IB) with a general phosphotyrosine (PY; 4G10) antibody. (B) Lysates were immunoblotted with antibody to phosphorylated PLCγ2 (phospho-PLCγ2 [Tyr1217]), total PLCγ2 (n = 3), phosphorylated Akt (n = 3), total Akt (n = 3), phosphorylated p38 MAPK (phospho-p38), or total p38 (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/15/10.1182_blood-2009-11-254185/4/m_zh89991051370005.jpeg?Expires=1765002265&Signature=a5wYXqeTYcubcxVrgIefnfh8eE9cemHdSDvoG5a6k4uuY~ROJcB4~GFwkL5d2Rp4O9LNFuj03g6LnuP-SwPjbxrYosYnXEN0YVCSBZrOOsw7zSkfvfVDKB1pyQOMmHt-g5erw1hi~msndZtFrANUvG2itPGJfWzw9-UWPyK46VKzktKbTws2U7cmhqXm8-0q0sniLhX2yJMfzEaOos6FKFuj4oBMyws8xk0Bt-kgaFMRB4UQ0nVGbIUvHSGlYYxIqVVtPNA5ZpwylwhWTgPM3jzROx8sMEw55R3t-UyQ9kSDFPER2yDyP3TFlcjtpFzN8sMkqMheCQmnhQmHXM-q6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Btk is required for the activation of the PLCγ2- and PI3Kγ-dependent pathways. Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then lysates were prepared. (A) Lysates were immunoblotted with antibodies to phosphorylated PLCγ2 (phospho-PLCγ2 [Tyr1217]), total PLCγ2 (n = 3), phosphorylated Akt (n = 3), total Akt (n = 3), phosphorylated MAPK (phospho-p38), or total p38 (n = 3). (B) Lysates were immunoprecipitated with anti-Syk, followed by immunoblotting (IB) with a general phosphotyrosine (PY; 4G10) antibody. (C) Bone marrow–derived neutrophils were plated on uncoated (unstimulated) or E-selectin–coated wells for 10 minutes, and then intracellular IP3 levels were determined using a competitive binding assay. *P < .05 vs other groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/15/10.1182_blood-2009-11-254185/4/m_zh89991051370006.jpeg?Expires=1765002265&Signature=Ru77Bhp7oskJuP9TYZ-qYPpw22-WtiNETWxNhWsM35YqQQ0tQOvWgKRCQqsOKit72FI6ptx0~VALy5kJ7FGEN-M1PGLqlh8~NMPHMVDnyeyaRBN9H2qtxlN5JNRCQG6LM4XHQiOzx-gXwQGQZfgtUTlJsceFSLuUUu5rBrJLPfk0QA7F~taIFKqvoGinW9yGgG6yLYkCSVf8WZJbSBbV4L~pfRNW1LrY7mauCYlibrUHj3TRaL65J-pUJ8df08dis5qdnt067XF6f9vTKrqIz4E6z8YqYjmDC11xUZhERzlWxT8EYs3FpoPoA5c-bD3DXiq2SH0Lt0Ogk~X98pjEkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal