Abstract
A large proportion of patients with mutations in the Wiskott-Aldrich syndrome (WAS) protein gene exhibit the milder phenotype termed X-linked thrombocytopenia (XLT). Whereas stem cell transplantation at an early age is the treatment of choice for patients with WAS, therapeutic options for patients with XLT are controversial. In a retrospective multicenter study we defined the clinical phenotype of XLT and determined the probability of severe disease-related complications in patients older than 2 years with documented WAS gene mutations and mild-to-moderate eczema or mild, infrequent infections. Enrolled were 173 patients (median age, 11.5 years) from 12 countries spanning 2830 patient-years. Serious bleeding episodes occurred in 13.9%, life-threatening infections in 6.9%, autoimmunity in 12.1%, and malignancy in 5.2% of patients. Overall and event-free survival probabilities were not significantly influenced by the type of mutation or intravenous immunoglobulin or antibiotic prophylaxis. Splenectomy resulted in increased risk of severe infections. This analysis of the clinical outcome and molecular basis of patients with XLT shows excellent long-term survival but also a high probability of severe disease-related complications. These observations will allow better decision making when considering treatment options for individual patients with XLT.
Introduction
In 1937 Wiskott described a clinical entity characterized by thrombocytopenia, eczema, bloody diarrhea, and recurrent otitis media in male infants. After rediscovery in 1954 by Aldrich as an X-linked recessive disorder, it was designated the Wiskott-Aldrich syndrome (WAS).1-3 X-linked thrombocytopenia (XLT), sometimes associated with mild eczema and/or infections, was recognized in the 1960s and was suspected to be a variant of WAS.4-6 This was confirmed when patients with XLT were shown to have mutations in the Wiskott-Aldrich syndrome protein gene (WAS).7-9
WAS gene mutations result in 3 distinct clinical phenotypes: classic WAS, XLT, and X-linked neutropenia,10,11 and a strong genotype phenotype correlation has been suggested.12-15 Mutations completely averting WAS protein (WASP) expression typically lead to the classic phenotype. Missense mutations resulting in expression of defective WASP, often in reduced quantity, most often result in the XLT phenotype, sometimes with only intermittent thrombocytopenia.16 X-linked neutropenia is caused by gain of function mutations resulting in constitutively activated WASP.17-19 There are however exceptions to these rules, making it difficult to predict the clinical course of a male infant solely based on the type of WAS gene mutation and its effect on WASP expression.
The classic WAS phenotype with microthrombocytopenia, severe eczema, increased susceptibility to pyogenic and opportunistic infections, and increased risk of autoimmune disease and cancer usually leads to death in early childhood or adolescence if left untreated.10,20,21 Curative treatment by allogeneic hematopoietic stem cell transplantation (HSCT) should be offered to all such patients. The outcome is excellent if performed early in life from a human leukocyte antigen–matched related or unrelated donor.10,22-24 Hematopoietic stem cell gene therapy might in the future offer an alternative approach in patients lacking a suitable donor.25-27
Generally accepted treatment policies do not exist for patients exhibiting the XLT phenotype, in whom HSCT would seem like an excessively risky procedure if they have thrombocytopenia and eczema only. Although it has been assumed that patients with XLT have a lower risk of cancer or autoimmunity than patients with WAS, this has never been formally examined. Therefore, the risk–benefit ratio for HSCT is not known in XLT.
In this multicenter study we assessed retrospectively the spectrum of clinical phenotypes, the associated genotypes, and the long-term outcome of the largest cohort of patients with XLT studied so far.
Methods
Data accrual
Questionnaires were sent worldwide to major centers treating patients with primary immunodeficiency diseases (PIDs), asking to enroll their patients with the XLT phenotype and to provide data on the following disease parameters: infections, eczema, thrombocytopenia, bleeding, malignancy, autoimmunity, WAS gene mutation, WASP expression, and type and extent of therapy. An alternative possibility was documentation online with the same questionnaire in the European Society for Immunodeficiencies registry (www.esid.org). Patient information was made anonymous by the submitting physician. The study was approved by the ethics committee of the University of Munich, Germany.
Patients
All submitted patient data were evaluated, and patients were included as study patients by consensual decision of a central review board (M.H.A., T.C.B., B.H.B., H.D.O.). To be enrolled into the final study, patients had to fulfill all of the following criteria: (1) confirmed mutation within the WAS gene; (2) classified by their treating physician as having XLT; (3) with or without mild-to-moderate eczema or mild, infrequent infections not resulting in sequelae; (4) age older than 2 years; and (5) no severe infection, autoimmunity, or malignancy within the first 2 years of life.
Bleeding events before the age of 2 years were no reason for exclusion from the study. Older than 2 years, severe infections, the development of autoimmunity, or malignancy was recorded and included in the analysis, but it was no reason for exclusion from the study.
If patients underwent allogeneic HSCT, the transplantation was recorded as the last date of follow-up; the resulting events/outcome were not part of this analysis.
Definitions
Life-threatening infections were defined as requiring hospitalization such as sepsis, meningitis, or pneumonia needing oxygen supply or mechanical ventilation. Serious bleeding was defined as a fatal or life-threatening bleeding episode resulting in hospitalization or red blood cell transfusion. Other serious complications were a diagnosis of autoimmunity, malignancy, or death. If a patient experienced more than 1 serious event, only the first event was registered for the analysis of event-free survival. Severity of thrombocytopenia was defined as follows: less than 20.0 × 109/L (20 000/μL) was severe, 20.0 to 50.0 × 109/L (20 000 to 50 000/μL) was moderate, and greater than 50.0 × 109/L (50 000/μL) or cyclic was mild. All patients with normal or reduced levels of WASP detectable by Western blot or fluorescence-activated cell sorting were designated as WASP positive; those with truncated (by Western blot) or undetectable protein were categorized as WASP negative. Intravenous immunoglobulin (IVIG) or antibiotic (AB) prophylaxes were defined as having had IVIG or prophylactic ABs more than once for any period of time.
Mutations are reported according to the current nomenclature of the Human Genome Variation Society (www.hgvs.org).28
Statistical analysis
Kaplan-Meier survival estimates and cumulative incidence rates were compared with the use of the log-rank test (Prism; GraphPad Software Inc). Cumulative incidence for different events adjusting for competing risks was estimated with the use of the statistics language R29 with the cmprsk package that used the method by Gray.30 Other analyses used the χ2 or Fisher exact test and were accepted as significantly different at a level of P less than .05.
Results
Study cohort
A total of 69 centers known to treat patients with PID were contacted and 50 responded (72%). Of 213 completed forms, representing 12 countries from 4 continents, 173 (171 male, 2 female) patients from 128 families and 21 centers with a median age of 11.5 years (range, 2.0-74.6 years) fulfilled the inclusion criteria, covering 2830 patient-years. The 2 female patients of our XLT cohort had been reported previously, 1 with a homozygous missense mutation and 1 with a heterozygous missense mutation and skewed X-inactivation in favor of the mutated allele.31,32
Mutations in patients with XLT
We identified 62 unique mutations (Table 1), including 3 mutational hotspots, defined as affecting 10 or more nonrelated families with either the identical mutation or a missense mutation affecting the same amino acid. Two hotspots were located in exon 2 affecting either a valine at position 75 (p.Val75Met or p.Val75Leu; 23 patients) or an arginine at position 86 (p.Arg86Gly, p.Arg86Cys, p.Arg86His, or p.Arg86Leu; 33 patients). The third hotspot mutation, located in intron 6 (c.559 + 5G>A) was found in 15 patients. Thus 41% of all patients had a hotspot mutation.
WAS gene mutations in patients with XLT
| Exon . | Coding DNA mutation . | Predicted protein change . | Mutation type . | Pt* . | Fam† . | Origin . | WASP expression (no. of pt) . | Score (no. of pt) . |
|---|---|---|---|---|---|---|---|---|
| 1 | c.G5C | p.Ser2Thr | Missense | 1 | 1 | Fr | ND | 2 |
| 1 | c.G18A | p.Met6Ile | Missense | 2 | 1 | JPN | Reduced (2) | 1, 2→5M |
| 1 | c.C71T | p.Ser24Phe | Missense | 2 | 2 | US (1), JPN (1) | Reduced (1), ND (1) | 1, 2→5A |
| 1 | c.C79T | p.Leu27Phe | Missense | 1 | 1 | US | Reduced | 1 |
| 1 | c.88_90delCAC | p.His30del | Deletion | 5 | 2 | UK (4), Ger (1) | Reduced (3), ND (2) | 1(4), 2 |
| 1 | c.G91A | p.Glu31Lys | Missense | 1 | 1 | Italy | Absent | 2→5A |
| 1 | c.T116C | p.Leu39Pro | Missense | 6 | 4 | US (3), Italy (2), Ger (1) | Reduced (5), absent (1) | 1, 1→5A/M, 2(4) |
| 2 | c.C134T | p.Thr45Met | Missense | 13 | 8 | JPN (4), US (2), Ger (1), UK (1), Sw (5) | Reduced (6), absent (1), ND (6) | 1(6), 1→5A, 2(4), 2→5A /B (2) |
| 2 | c.C140A | p.Ala47Asp | Missense | 1 | 1 | US | Reduced | 2 |
| 2 | c.A142G | p.Thr48Ala | Missense | 1 | 1 | JPN | Reduced | 2 |
| 2 | c.C143T | p.Thr48Ile | Missense | 1 | 1 | US | Reduced | 1→5M |
| 2 | c.C167T | p.Ala56Val | Missense | 5 | 4 | US (3), Italy (1), JPN (1) | Reduced (4), ND (1) | 1(3), 1→5A, 2 |
| 2 | c.C172A | p.Pro58Thr | Missense | 2 | 1 | US | Normal (2) | 1, 2 |
| 2 | c.C172G | p.Asp58Ala | Missense | 1 | 1 | US | Reduced | 2→5A/M |
| 2 | c.C173G | p.Pro58Arg | Missense | 3 | 1 | Italy | Reduced (2), ND (1) | 1, 1→5M, 2 |
| 2 | c.G199A | p.Glu67Lys | Missense | 1 | 1 | Fr | Reduced | 2 |
| 2 | c.G223A | p.Val75Met | Missense | 22 | 16 | Fr (6), UK (5), US (5), Ger (2), JPN (2), Sp (1), Italy (1) | Normal (1), reduced (10), absent (3), ND (8) | 1(6), 1→5A, 2(14), 2→5A |
| 2 | c.G223T | p.Val75Leu | Missense | 1 | 1 | US | ND | 2 |
| 2 | c.A227C | p.Lys76Thr | Missense | 2 | 2 | US | Reduced (1), ND (1) | 2(2) |
| 2 | c.G229C | p.Asp77His | Missense | 1 | 1 | Italy | Reduced | 1 |
| 2 | c.A230G | p.Asp77Gly | Missense | 2 | 1 | Italy | Reduced (2) | 1, 2 |
| 2 | c.A239G | p.Gln80Arg | Missense | 1 | 1 | Rus | Reduced | 2 |
| 2 | c.248insA | p.Tyr83X | Insertion | 1 | 1 | Fr | ND | 2 |
| 2 | c.C256G | p.Arg86Gly | Missense | 1 | 1 | US | Reduced | 2→5A |
| 2 | c.C256T | p.Arg86Cys | Missense | 24 | 18 | US (10), Ger (6), JPN (3), UK (3), Italy (1), Sw (1) | Normal (3), reduced (9), ND (12) | 1(10), 1→5M, 2(12), 2→5A |
| 2 | c.G257A | p.Arg86His | Missense | 7 | 7 | JPN (2), Fr (1), Ger (1), Isr (1), Rus (1), US (1) | Reduced (4), absent (1), ND (2) | 1→5A, 2(4), 2→5A(2) |
| 2 | c.G257T | p.Arg86Leu | Missense | 1 | 1 | US | Absent | 2 |
| 2 | c.A263G | p.Tyr88Cys | Missense | 1 | 1 | NL | ND | 2→5A |
| 2 | c.G266A | p.Gly89Asp | Missense | 1 | 1 | UK | Normal | 1 |
| 3 | c.A320G | p.Tyr107Cys | Missense | 1 | 1 | US | Reduced | 2 |
| 3 | c.326_330insC | p.Thr111HisfsX9 | Insertion | 1 | 1 | US | Absent | 2 |
| 3 | c.G355A | p.Gly119Arg | Missense | 1 | 1 | NL | ND | 1 |
| 4 | c.dup355_361 | p.Asp121insGD | Insertion | 1 | 1 | JPN | Absent | 2 |
| 4 | c.G399T | p.Glu133Asp | Missense | 1 | 1 | US | Reduced | 2 |
| 5 | c.G505T | p.Asn169X | Nonsense | 1 | 1 | JPN | Reduced | 2→5M |
| 6 | c.G538A | p.His180Asn | Missense | 1 | 1 | Italy | Reduced | 1 |
| 7 | c.C707G | p.Ala236Gly | Missense | 1 | 1 | Italy | Absent | 1 |
| 7 | c.A724T | p.Ser242Cys | Missense | 1 | 1 | NL | ND | 1 |
| 9 | c.854_855insG | p.Thr286AspfsX1 | Insertion | 2 | 1 | UK | Reduced and truncated (1), absent (1) | 1(2) |
| 9 | c.A919G | p.Met307Val | Missense | 1 | 1 | Ger | ND | 2 |
| 10 | c.C961T | p.Arg321X | Nonsense | 1 | 1 | JPN | Absent | 2→5M |
| 10 | c.983_984delC | Multiple products | Deletion | 1 | 1 | US | Reduced and truncated | 2 |
| 10 | c.991insA | p.Gly334X | Insertion | 1 | 1 | US | Absent | 2 |
| 10 | c.1073_1074delGA | p.Gly358AlafsX135 | Deletion | 1 | 1 | US | Reduced and truncated | 2 |
| 10 | c.1079delC | p.Pro360HisfsX84 | Deletion | 2 | 2 | Ger, JPN | Reduced (1), absent (1) | 2(2) |
| 10 | c.C1090T | p.Arg363X | Nonsense | 2 | 1 | Fr | ND (2) | 2(2) |
| 11 | c.G1430A | p.Arg477Lys | Missense | 1 | 1 | Sp | Reduced | 2 |
| 11 | c.T1442A | p.Ile481Asn | Missense | 2 | 1 | Italy | Normal (1), reduced (1) | 1(2) |
| 12 | c.G1453A | p.Asp485Asn | Missense | 1 | 1 | US | Reduced | 2→5A |
| 12 | c.A1454G | p.Asp485Gly | Missense | 3 | 1 | Sp | ND (3) | 1(3) |
| 12 | c.G1508C | p.X503SerextX76 | No-stop | 2 | 1 | US | Absent (1), ND (1) | 2(2) |
| Int 3 | c.360+1G>A | p.Ala92_Asp120del | Splice (donor site) | 1 | 1 | JPN | Reduced | 2 |
| Int 3 | c.361-1G>A | p.fsX201 | Splice (acceptor site) | 1 | 1 | US | Reduced | 2 |
| Int 4 | c.[463+1_463+8del;464-3_464-2insG] | p.fsX178/fsX251 | Splice (donor +acceptor site) | 1 | 1 | JPN | Reduced | 2 |
| Int 6 | c.559+5G>A | 70% fsX190/30% normal | Splice (donor site) | 15 | 11 | US (9), Ger (2), JPN (3), UK (1) | Reduced (12), absent (1), ND (2) | 1(6), 1→5M, 2(6), 2→5A(2) |
| Int 7 | c.734+5G>A | ND | Splice (donor site) | 4 | 1 | Ger | ND (4) | 2 (3), 2→5A |
| Int 7 | c.735-25A>C | ND | Splice (acceptor site) | 3 | 1 | UK | Reduced (3) | 1(3) |
| Int 8 | c.777+1G>A | p.fsX246 | Splice (donor site) | 2 | 2 | Australia, US | Absent (1), ND (1) | 1, 2 |
| Int 8 | c.777+3insT | ND | Splice (donor site) | 2 | 1 | Italy | Reduced (2) | 1, 2 |
| Int 8 | c.778-6G>A | ND | Splice (acceptor site) | 1 | 1 | UK | Reduced | 1 |
| Int 9 | c.(931_932)ins250 | ND | Splice site | 1 | 1 | JPN | Reduced | 1 |
| Int 11 | c.(1484_1485)ins118 | Normal and abnormal splice products | Splice site | 2 | 1 | JPN | Reduced (2) | 2→5A(2) |
| Exon . | Coding DNA mutation . | Predicted protein change . | Mutation type . | Pt* . | Fam† . | Origin . | WASP expression (no. of pt) . | Score (no. of pt) . |
|---|---|---|---|---|---|---|---|---|
| 1 | c.G5C | p.Ser2Thr | Missense | 1 | 1 | Fr | ND | 2 |
| 1 | c.G18A | p.Met6Ile | Missense | 2 | 1 | JPN | Reduced (2) | 1, 2→5M |
| 1 | c.C71T | p.Ser24Phe | Missense | 2 | 2 | US (1), JPN (1) | Reduced (1), ND (1) | 1, 2→5A |
| 1 | c.C79T | p.Leu27Phe | Missense | 1 | 1 | US | Reduced | 1 |
| 1 | c.88_90delCAC | p.His30del | Deletion | 5 | 2 | UK (4), Ger (1) | Reduced (3), ND (2) | 1(4), 2 |
| 1 | c.G91A | p.Glu31Lys | Missense | 1 | 1 | Italy | Absent | 2→5A |
| 1 | c.T116C | p.Leu39Pro | Missense | 6 | 4 | US (3), Italy (2), Ger (1) | Reduced (5), absent (1) | 1, 1→5A/M, 2(4) |
| 2 | c.C134T | p.Thr45Met | Missense | 13 | 8 | JPN (4), US (2), Ger (1), UK (1), Sw (5) | Reduced (6), absent (1), ND (6) | 1(6), 1→5A, 2(4), 2→5A /B (2) |
| 2 | c.C140A | p.Ala47Asp | Missense | 1 | 1 | US | Reduced | 2 |
| 2 | c.A142G | p.Thr48Ala | Missense | 1 | 1 | JPN | Reduced | 2 |
| 2 | c.C143T | p.Thr48Ile | Missense | 1 | 1 | US | Reduced | 1→5M |
| 2 | c.C167T | p.Ala56Val | Missense | 5 | 4 | US (3), Italy (1), JPN (1) | Reduced (4), ND (1) | 1(3), 1→5A, 2 |
| 2 | c.C172A | p.Pro58Thr | Missense | 2 | 1 | US | Normal (2) | 1, 2 |
| 2 | c.C172G | p.Asp58Ala | Missense | 1 | 1 | US | Reduced | 2→5A/M |
| 2 | c.C173G | p.Pro58Arg | Missense | 3 | 1 | Italy | Reduced (2), ND (1) | 1, 1→5M, 2 |
| 2 | c.G199A | p.Glu67Lys | Missense | 1 | 1 | Fr | Reduced | 2 |
| 2 | c.G223A | p.Val75Met | Missense | 22 | 16 | Fr (6), UK (5), US (5), Ger (2), JPN (2), Sp (1), Italy (1) | Normal (1), reduced (10), absent (3), ND (8) | 1(6), 1→5A, 2(14), 2→5A |
| 2 | c.G223T | p.Val75Leu | Missense | 1 | 1 | US | ND | 2 |
| 2 | c.A227C | p.Lys76Thr | Missense | 2 | 2 | US | Reduced (1), ND (1) | 2(2) |
| 2 | c.G229C | p.Asp77His | Missense | 1 | 1 | Italy | Reduced | 1 |
| 2 | c.A230G | p.Asp77Gly | Missense | 2 | 1 | Italy | Reduced (2) | 1, 2 |
| 2 | c.A239G | p.Gln80Arg | Missense | 1 | 1 | Rus | Reduced | 2 |
| 2 | c.248insA | p.Tyr83X | Insertion | 1 | 1 | Fr | ND | 2 |
| 2 | c.C256G | p.Arg86Gly | Missense | 1 | 1 | US | Reduced | 2→5A |
| 2 | c.C256T | p.Arg86Cys | Missense | 24 | 18 | US (10), Ger (6), JPN (3), UK (3), Italy (1), Sw (1) | Normal (3), reduced (9), ND (12) | 1(10), 1→5M, 2(12), 2→5A |
| 2 | c.G257A | p.Arg86His | Missense | 7 | 7 | JPN (2), Fr (1), Ger (1), Isr (1), Rus (1), US (1) | Reduced (4), absent (1), ND (2) | 1→5A, 2(4), 2→5A(2) |
| 2 | c.G257T | p.Arg86Leu | Missense | 1 | 1 | US | Absent | 2 |
| 2 | c.A263G | p.Tyr88Cys | Missense | 1 | 1 | NL | ND | 2→5A |
| 2 | c.G266A | p.Gly89Asp | Missense | 1 | 1 | UK | Normal | 1 |
| 3 | c.A320G | p.Tyr107Cys | Missense | 1 | 1 | US | Reduced | 2 |
| 3 | c.326_330insC | p.Thr111HisfsX9 | Insertion | 1 | 1 | US | Absent | 2 |
| 3 | c.G355A | p.Gly119Arg | Missense | 1 | 1 | NL | ND | 1 |
| 4 | c.dup355_361 | p.Asp121insGD | Insertion | 1 | 1 | JPN | Absent | 2 |
| 4 | c.G399T | p.Glu133Asp | Missense | 1 | 1 | US | Reduced | 2 |
| 5 | c.G505T | p.Asn169X | Nonsense | 1 | 1 | JPN | Reduced | 2→5M |
| 6 | c.G538A | p.His180Asn | Missense | 1 | 1 | Italy | Reduced | 1 |
| 7 | c.C707G | p.Ala236Gly | Missense | 1 | 1 | Italy | Absent | 1 |
| 7 | c.A724T | p.Ser242Cys | Missense | 1 | 1 | NL | ND | 1 |
| 9 | c.854_855insG | p.Thr286AspfsX1 | Insertion | 2 | 1 | UK | Reduced and truncated (1), absent (1) | 1(2) |
| 9 | c.A919G | p.Met307Val | Missense | 1 | 1 | Ger | ND | 2 |
| 10 | c.C961T | p.Arg321X | Nonsense | 1 | 1 | JPN | Absent | 2→5M |
| 10 | c.983_984delC | Multiple products | Deletion | 1 | 1 | US | Reduced and truncated | 2 |
| 10 | c.991insA | p.Gly334X | Insertion | 1 | 1 | US | Absent | 2 |
| 10 | c.1073_1074delGA | p.Gly358AlafsX135 | Deletion | 1 | 1 | US | Reduced and truncated | 2 |
| 10 | c.1079delC | p.Pro360HisfsX84 | Deletion | 2 | 2 | Ger, JPN | Reduced (1), absent (1) | 2(2) |
| 10 | c.C1090T | p.Arg363X | Nonsense | 2 | 1 | Fr | ND (2) | 2(2) |
| 11 | c.G1430A | p.Arg477Lys | Missense | 1 | 1 | Sp | Reduced | 2 |
| 11 | c.T1442A | p.Ile481Asn | Missense | 2 | 1 | Italy | Normal (1), reduced (1) | 1(2) |
| 12 | c.G1453A | p.Asp485Asn | Missense | 1 | 1 | US | Reduced | 2→5A |
| 12 | c.A1454G | p.Asp485Gly | Missense | 3 | 1 | Sp | ND (3) | 1(3) |
| 12 | c.G1508C | p.X503SerextX76 | No-stop | 2 | 1 | US | Absent (1), ND (1) | 2(2) |
| Int 3 | c.360+1G>A | p.Ala92_Asp120del | Splice (donor site) | 1 | 1 | JPN | Reduced | 2 |
| Int 3 | c.361-1G>A | p.fsX201 | Splice (acceptor site) | 1 | 1 | US | Reduced | 2 |
| Int 4 | c.[463+1_463+8del;464-3_464-2insG] | p.fsX178/fsX251 | Splice (donor +acceptor site) | 1 | 1 | JPN | Reduced | 2 |
| Int 6 | c.559+5G>A | 70% fsX190/30% normal | Splice (donor site) | 15 | 11 | US (9), Ger (2), JPN (3), UK (1) | Reduced (12), absent (1), ND (2) | 1(6), 1→5M, 2(6), 2→5A(2) |
| Int 7 | c.734+5G>A | ND | Splice (donor site) | 4 | 1 | Ger | ND (4) | 2 (3), 2→5A |
| Int 7 | c.735-25A>C | ND | Splice (acceptor site) | 3 | 1 | UK | Reduced (3) | 1(3) |
| Int 8 | c.777+1G>A | p.fsX246 | Splice (donor site) | 2 | 2 | Australia, US | Absent (1), ND (1) | 1, 2 |
| Int 8 | c.777+3insT | ND | Splice (donor site) | 2 | 1 | Italy | Reduced (2) | 1, 2 |
| Int 8 | c.778-6G>A | ND | Splice (acceptor site) | 1 | 1 | UK | Reduced | 1 |
| Int 9 | c.(931_932)ins250 | ND | Splice site | 1 | 1 | JPN | Reduced | 1 |
| Int 11 | c.(1484_1485)ins118 | Normal and abnormal splice products | Splice site | 2 | 1 | JPN | Reduced (2) | 2→5A(2) |
Pt indicates number of patients with the respective mutation; Fam, number of families with the respective mutation; 1→5, WAS score progressing from 1 to 5 because of either A, autoimmunity, or M, malignancy; Fr, France; ND, not done; JPN, Japan; US, United States of America; UK, United Kingdom; Ger, Germany; Sw, Sweden; Sp, Spain; Rus, Russia; Isr, Israel; and NL, The Netherlands.
There was a total of 173 patients.
There was a total of 128 families.
The majority of mutations was located in exon 1 (10% of all patients) and exon 2 (54%). Most mutations were missense (69% of all patients), followed by splice site mutations (19%), deletions (5%), insertions (3%), nonsense mutations (2%), and no-stop mutations (1%; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). With few exceptions, patients with missense and splice site mutations expressed WASP in reduced quantity or in truncated form (Table 1).
Survival
Without curative treatment classic WAS results in premature death, often during childhood.21,33 Patients with XLT are expected to have a better prognosis. To verify this perception, we defined the probability of survival in our cohort of patients with XLT.
Overall survival was excellent with 97% (95% confidence interval [95% CI], 95%-100%), 96% (95% CI, 91%-100%), 81% (95% CI, 66%-97%), and 81% (95% CI, 66%-97%) at 15, 30, 45, and 60 years, respectively, and only slightly reduced compared with the survival curve of the normal male German population34 (Figure 1A). However, survival probability without having experienced a severe disease-related event was less favorable with 74% (95% CI, 65%-82%), 56% (95% CI, 43%-70%), 36% (95% CI, 20%-53%), and 27% (95% CI, 10%-44%) at 15, 30, 45, and 60 years, respectively (Figure 1B).
Overall and event-free survival. (A) Kaplan-Meier estimate of overall survival probability of all study patients compared with survival of the normal German male population 2006.34 (B) Event-free survival probability. Event was defined as a severe or fatal infection, severe or fatal bleeding, autoimmunity, malignancy, or death. Each hash mark on a graph line indicates a censored event; # at risk, number of patients at risk at indicated time point.
Overall and event-free survival. (A) Kaplan-Meier estimate of overall survival probability of all study patients compared with survival of the normal German male population 2006.34 (B) Event-free survival probability. Event was defined as a severe or fatal infection, severe or fatal bleeding, autoimmunity, malignancy, or death. Each hash mark on a graph line indicates a censored event; # at risk, number of patients at risk at indicated time point.
Thus the excellent survival in patients with XLT is associated with a high rate of severe disease-related events throughout life.
Incidence of severe disease-related events
To better define the nature and occurrence of severe disease-related events, we analyzed the cumulative incidence rate of these events separately.
Median event-free survival was 10.2 years (range, 0.1-73.9 years). A total of 86 events in 47 patients were reported, some of them occurring in different event categories in the same patient (detailed in Table 2). Cumulative incidences for each event category are detailed separately in Figure 2. If events were analyzed honoring other events as competing, the cumulative incidences were slightly lower because later events in the same patient were ignored (data not shown).
Disease-related events
| . | Total events . | Fatal events . |
|---|---|---|
| Infections* | ||
| Pneumonia | 6 | 0 |
| Bacterial meningitis | 4 | 0 |
| Sepsis | 4 | 2 |
| Gastrointestinal (salmonellosis) | 1 | 1 |
| Orchitis | 1 | 0 |
| Tuberculosis | 1 | 0 |
| No. of events | 17† | 3‡ |
| No. of patients | 12 | 3 |
| Bleeding§ | ||
| ICH∥ | 18 | 3 |
| Gastrointestinal | 6 | 1 |
| Ear/nose/throat | 4 | 0 |
| Pulmonary | 2 | 1 |
| Traumatic, not ICH | 2 | 0 |
| Retinal | 1 | 0 |
| No. of events | 33 | 5 |
| No. of patients | 24 | 5 |
| Autoimmunity¶ | ||
| Nephropathy | 9 | 0 |
| AIHA | 6 | 0 |
| Vasculitis | 3 | 0 |
| ITP | 4 | 0 |
| Arthritis | 3 | 0 |
| Colitis | 1 | 0 |
| No. of events | 26 | 0 |
| No. of patients | 21 | 0 |
| Malignancy# | ||
| Lymphoma/EBV-LPD | 4 | 1 |
| MDS | 1 | 0 |
| Spinalioma | 2 | 0 |
| Seminoma | 1 | 0 |
| ALL | 1 | 0 |
| Pancreatic cancer | 1 | 1 |
| No. of events | 10 | 2 |
| No. of patients | 9 | 2 |
| . | Total events . | Fatal events . |
|---|---|---|
| Infections* | ||
| Pneumonia | 6 | 0 |
| Bacterial meningitis | 4 | 0 |
| Sepsis | 4 | 2 |
| Gastrointestinal (salmonellosis) | 1 | 1 |
| Orchitis | 1 | 0 |
| Tuberculosis | 1 | 0 |
| No. of events | 17† | 3‡ |
| No. of patients | 12 | 3 |
| Bleeding§ | ||
| ICH∥ | 18 | 3 |
| Gastrointestinal | 6 | 1 |
| Ear/nose/throat | 4 | 0 |
| Pulmonary | 2 | 1 |
| Traumatic, not ICH | 2 | 0 |
| Retinal | 1 | 0 |
| No. of events | 33 | 5 |
| No. of patients | 24 | 5 |
| Autoimmunity¶ | ||
| Nephropathy | 9 | 0 |
| AIHA | 6 | 0 |
| Vasculitis | 3 | 0 |
| ITP | 4 | 0 |
| Arthritis | 3 | 0 |
| Colitis | 1 | 0 |
| No. of events | 26 | 0 |
| No. of patients | 21 | 0 |
| Malignancy# | ||
| Lymphoma/EBV-LPD | 4 | 1 |
| MDS | 1 | 0 |
| Spinalioma | 2 | 0 |
| Seminoma | 1 | 0 |
| ALL | 1 | 0 |
| Pancreatic cancer | 1 | 1 |
| No. of events | 10 | 2 |
| No. of patients | 9 | 2 |
ICH indicates intracranial hemorrhage; AIHA, autoimmune hemolytic anemia; ITP, immune thrombocytopenic purpura; ALL, acute lymphoblastic leukemia; EBV-LPD, Epstein-Barr virus–associated lymphoproliferative disease; and MDS, myelodysplastic syndrome.
Three patients had more than 1 infectious event.
Eight events were in patients who had undergone a previous splenectomy.
Two events were in patients who had undergone a previous splenectomy.
Four patients had more than 1 bleeding episode.
Fifteen were spontaneous, 3 were traumatic.
Three patients had more than 1 autoimmune disease.
One patient had 2 malignancies.
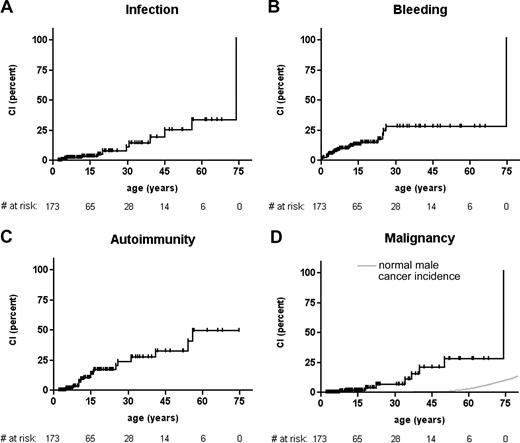
Cumulative incidence rate of severe events. Cumulative incidence of (A) severe or fatal infectious episodes in the study cohort, (B) severe or fatal bleeding episodes, (C) autoimmune disease, and (D) malignancy, compared with cancer incidence in the US male population.35 Each hash mark on a graph line indicates a censored event, # at risk, number of patients at risk at indicated time point.
Cumulative incidence rate of severe events. Cumulative incidence of (A) severe or fatal infectious episodes in the study cohort, (B) severe or fatal bleeding episodes, (C) autoimmune disease, and (D) malignancy, compared with cancer incidence in the US male population.35 Each hash mark on a graph line indicates a censored event, # at risk, number of patients at risk at indicated time point.
Life-threatening infections occurred at a median age of 24.8 years (range, 2.0-73.9 years), 3 of which were fatal. There was no discernible effect of patient age on the incidence of infectious events (Figure 2A). In contrast, all but 1 serious hemorrhage occurred before the age of 30 years, at a median age of 5.7 years (range, 0.1-74.6 years; Figure 2B). Most serious bleeding events (18 of 33) were intracranial hemorrhages. Five bleeding episodes were fatal at a median age of 4.9 years (range, 2.0-74.6 years). There was no correlation between the recorded platelet counts and the incidence of severe or fatal bleeding, which was 12.5% in mild, 9.7% in moderate, and 18.4% in severe thrombocytopenia (P = .31). Autoimmune nephropathy and hemolytic anemia were the most frequent autoimmune manifestations; the former occurring more frequently in Japanese patients than in patients from other countries (5 of 28 vs 4 of 145; P = .006). In general, autoimmune diseases were not significantly more frequent in Japanese patients (5 of 28 vs 16 of /145; P = .34). Autoimmunity was not restricted to adult patients but occurred at all ages with a median of 12.2 years (range, 4.9-56.0 years; Figure 2C). Malignancies developed at a median age of 34.0 years (range, 7.8-74.0 years; Figure 2D), half (5 of 10) of which were of lymphoid origin. Two patients died of their malignancies, 2 more went on to have HSCT and died of transplantation-related causes and 2 died of other complications.
In conclusion, with the exception of severe bleeding, which seems to be limited to the first 3 decades of life, a relatively high rate of life-threatening or fatal disease-related events was observed in XLT at all ages.
Influence of WAS gene mutation, protein expression, IVIG, or AB prophylaxis on overall and event-free survival
Because some patients with XLT have a largely uneventful course of disease and a normal life expectancy and others have severe or even fatal complications at any age, we asked whether individual WAS gene mutations, the presence or absence of WASP, or the prophylaxis with ABs and intravenous immunoglobulin had any influence on outcome.
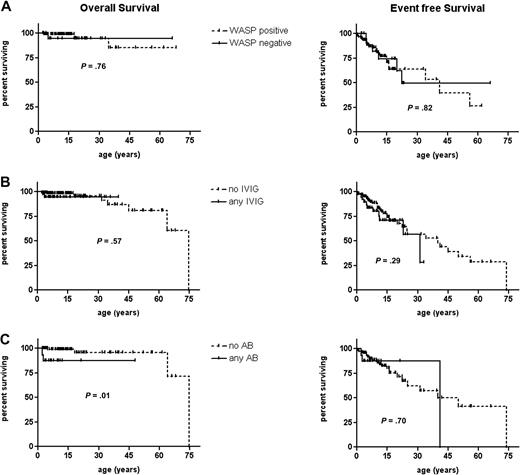
WASP expression, if assessed, was detectable in 98 patients and absent in 21. Presence or absence of WASP had no influence on overall and event-free survival in patients with the XLT phenotype (Figure 3A). Similarly, there was no significant effect on the incidence of disease-related events (data not shown). The same was true when the influence of IVIG prophylaxis (n = 39) was analyzed in comparison to patients having never received IVIG (n = 134; Figure 3B). AB prophylaxis had no positive influence on outcome (Figure 3C). Patients with hotspot mutations had no different overall and event-free survival and event incidences compared with others (data not shown).
Influence of WASP expression, IVIG, or AB prophylaxis on overall and event-free survival. Kaplan-Meier estimate of overall survival and event-free survival probability of (A) WASP-positive (n = 98, dotted line) and WASP-negative (n = 21, solid line) patients. (B) Patients receiving any IVIG prophylaxis (n = 39, solid line) or no IVIG prophylaxis (n = 134, dotted line) and (C) patients receiving any AB prophylaxis (n = 16, solid line) or no AB prophylaxis (n = 116, dotted line). Patients who underwent splenectomy were excluded from the analysis in panel C. Each hash mark on a graph line indicates a censored event.
Influence of WASP expression, IVIG, or AB prophylaxis on overall and event-free survival. Kaplan-Meier estimate of overall survival and event-free survival probability of (A) WASP-positive (n = 98, dotted line) and WASP-negative (n = 21, solid line) patients. (B) Patients receiving any IVIG prophylaxis (n = 39, solid line) or no IVIG prophylaxis (n = 134, dotted line) and (C) patients receiving any AB prophylaxis (n = 16, solid line) or no AB prophylaxis (n = 116, dotted line). Patients who underwent splenectomy were excluded from the analysis in panel C. Each hash mark on a graph line indicates a censored event.
In summary none of the tested outcome variables were of significance in this cohort of patients with XLT selected on the basis of their mild phenotype.
Influence of splenectomy on infections and bleeding episodes
Splenectomy in patients with XLT/WAS usually leads to a sustained increase in platelet counts and is considered an effective measure to control the bleeding predisposition. Therefore, splenectomy has been recommended by some investigators for patients with WAS and patients with XLT.36,37
A total of 41 patients (23.7%) underwent splenectomy at a median age of 7.02 years (range, 0.8-43.0 years). The indication for splenectomy was not reported, but 7 of these 41 patients had experienced a severe bleeding episode before splenectomy, and 28 of 41 patients had had severe thrombocytopenia. All 13 patients in whom postsplenectomy platelet counts were available had experienced an increase in platelet numbers, 7 having counts greater than 100.0 × 109/L (100 000/μL). In the 2 patients who experienced a severe bleeding event after splenectomy, platelet counts were not reported. Therefore, it cannot be excluded that these 2 patients may have had low counts despite splenectomy. The overall cumulative incidence rate of serious bleeding events in these patients after splenectomy compared with before splenectomy was reduced although not significantly (P = .15). However, there was a significantly higher incidence of severe infectious events after splenectomy than before (P = .005). This might possibly be due to negligent AB prophylaxis in some patients. Of the 9 patients who did not receive AB prophylaxis, 3 had a severe (1 fatal) infection up to 53 years after splenectomy. This compared unfavorably, however not statistically significant, to patients who underwent splenectomy with AB prophylaxis in whom only 5 of 32 (1 fatal) had such an event (P = .34). Overall survival in patients who underwent splenectomy was not significantly different from patients not undergoing splenectomy (data not shown).
These data indicate that patients with XLT who underwent splenectomy are at significant risk of severe infections and require life-long AB prophylaxis.
Discussion
WAS is a multifaceted disorder with a wide spectrum of disease severity. In contrast to classic WAS, patients with a mild clinical phenotype, termed XLT, require comprehensive assessment in deciding on the strategy to provide optimal treatment. This is true for children who often present with selective microthrombocytopenia and have an uncertain long-term prognosis at a time when they are excellent candidates for allogeneic HSCT.23,24 Similarly, adult patients with XLT who often are wrongly categorized as having chronic immune thrombocytopenic purpura and who may already have developed complications such as autoimmunity pose unique therapeutic challenges. This retrospective study was designed to better define the type of mutations and the clinical course of patients with XLT and to collect supportive evidence for optimal treatment choices.
The design of such a study requires a stringent definition of inclusion and exclusion criteria. The WAS scoring system has been used successfully in categorizing patients according to their disease severity.10,11 However, an individual patient is not expected to keep the same score throughout his or her life. Progression from a score of 1 to 4 to a score of 5 by developing cancer or autoimmunity can occur at any age, and patients with classic WAS often present with a relatively mild phenotype during infancy. We, therefore, chose inclusion criteria that best reflect the situation when patients with XLT/WAS present in an immunodeficiency clinic. In addition to the classification as XLT by physicians experienced in treating patients with PIDs, we deliberately chose stringent criteria to prevent the inclusion of patients with classic WAS with few disease symptoms as may be the case during the first 2 years of life. One possible drawback of this study could be its retrospective, cross-sectional design. It is probable that some events took place when medical care differed from that of today. Naturally, the study design might encompass a bias by some confounding factors such as patient compliance, physician preference, choice of prophylactic measures, and availability of HSCT. We can also not exclude some selection bias, missing very mild cases that are undiagnosed or misdiagnosed and not referred to an immunology center. But some older patients in this study had lived an uneventful life, before being diagnosed as XLT because their brothers, nephews, or grandsons were discovered to have a WAS gene mutation. Of note, the outcome of these older relatives did not differ from that of the rest of the cohort (data not shown). At this time the retrospective study design seems to be the only possible means to assess the clinical characteristics of a large cohort of patients with XLT. Having established this database of patients with XLT, we now have the opportunity to prospectively follow their course of disease.
Only 17.6% of evaluable patients with XLT from this cohort lacked WASP expression. In contrast, the proportion of WASP-negative patients from a multinational cohort of patients with WAS/XLT with known WAS mutations was 57% (104 of 184).15 Some patients may in fact express WASP because the methods used to assess expression, such as Western blot analysis, might not be sensitive enough to detect low protein levels. This possibility is supported by the fact that 10 patients who were WASP negative had mutations (missense and invariant splice site) expected to result in WASP expression. In this selected cohort of patients with XLT, the clinical outcome of patients who did not express WASP was not different from patients who expressed WASP. Similarly, we did not find any beneficial effect of IVIG or AB prophylaxis on overall and event-free survival or on the incidence of life-threatening infectious events. These results have to be interpreted with caution, and a possible beneficial effect of these measures cannot be ruled out because data on AB and IVIG prophylaxis were very heterogeneous about dose and duration of treatment. They might solely reflect the fact that, by definition, most patients with XLT can mount effective antibody responses and therefore do not need IVIG or AB prophylaxis. It is possible that the initiation of these prophylactic measures might have been triggered by slightly more severe disease symptoms.33
In this cohort of 173 patients, 108 (62%) had missense mutations in the first 4 WAS exons; the remaining 38% (including 11 patients with missense mutations in exons 6-12) were spread over the entire gene, including 19% in noncoding regions. This is in line with previous reports of XLT.13-15,33 We could not detect any influence of the type of mutation on survival or on the incidence of specific disease-related events. A mild phenotype despite a deleterious mutation might be due to other disease-modifying genes, pathogen exposure, or somatic mosaicism caused by in vivo reversion, leading to some WASP expression and thus a milder phenotype. Reversion is an event quite frequent in WAS,38,39 but it was not specifically analyzed in this cohort.
Forty-one patients (23.7%) had undergone splenectomy, reflecting the acceptance of splenectomy by some health care providers to reduce the risk of bleeding and thus improve quality of life in patients with XLT.37,40 Interestingly, there was only a nonsignificant reduction of severe bleeding episodes after splenectomy, possibly because of the low overall incidence that decreased with age. However, the incidence of severe infections was significantly increased, especially in patients not receiving AB prophylaxis. These data suggest that, before splenectomy in patients with XLT, one needs to carefully weigh the pros and cons of this procedure. If performed, that is, in patients with recurrent episodes of serious bleeding, the family must understand the risk of infections and be willing to accept the need for AB prophylaxis. In addition, vaccination against pneumococci and meningococci has to be considered, given the fact that most patients with XLT can be effectively immunized.33 The high incidence of severe infectious complications after splenectomy, including adult patients, highlights the importance of lifelong AB prophylaxis in patients with XLT who have undergone splenectomy.
The excellent overall survival rate that is close to that of the normal male population supports the perception that XLT is a mild, chronic disease and that, as a rule, patients with XLT do not require standard prophylactic interventions. Declining immune function has been observed in XLT, and defective antibody responses may require prophylactic measures such as IVIG in some patients. However, the reduced event-free survival shows substantial risks of severe, life-threatening or potentially debilitating disease-related complications. The cumulative incidence rate analysis of events showed that serious bleeding episodes were generally restricted to the first 30 years of life. In contrast, the risk of developing autoimmune disease, developing malignancy, or having a life-threatening infectious episode was rather constant throughout the patients' lifetime. The prevalence of autoimmunity is 12% in our cohort, suggesting that this complication is less common than in classic WAS whereby it was reported to be as high as 40% to 72%.20,41,42 Interestingly, we found a significantly higher incidence of autoimmune nephropathy in Japanese patients. Similarly, the prevalence of malignancy was less in our XLT cohort (5%) than in classic WAS (13%).20,43 Considering the higher mean age of patients with XLT compared with patients with classic WAS who have not received a transplant, these differences are even more significant.
The persistent morbidity associated with XLT might argue for HSCT as a treatment option for these patients. Given the excellent success in young children with classic WAS,23,24 HSCT might be considered a viable option for patients with XLT if an human leukocyte antigen–identical donor can be identified. However, when discussing HSCT, which requires full conditioning in patients with WAS and patients with XLT, one needs to carefully weigh the advantage of a possible cure against the acute risks and long-term consequences of this procedure, such as risk of secondary malignancy and infertility. Thus, HSCT in XLT has to be decided on an individual patient basis. In our cohort 25 of 173 patients underwent HSCT at a median age of 7.3 years (range, 2.1-38.0 years) and 22 (88%) are alive after a median follow-up of 2.2 years (range, 0.0-12.1 years). Of note, more than half of the patients received their transplant at an age older than 5 years, when matched unrelated transplants in WAS may have a less favorable outcome.23 Long-term studies of HSCT in patients with XLT, not available at present, are urgently needed.
Because patients with XLT may present to different medical specialists, it seems vital to raise awareness of this probably underdiagnosed or misdiagnosed condition. Although this study showed a high overall survival rate of patients with XLT, it also showed that they are at risk of life-threatening complications. By defining the natural course of XLT and recognizing the life-long medical problems that affect the prognosis and quality of life of these patients, it has become possible to select safe and effective individualized therapies for this unique set of patients with mutations of the WAS gene that are generally expected to be less devastating.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the following persons who contributed patient data to this study: M. Helbert, Manchester, United Kingdom; C. Bender-Götze, Munich, Germany; R. Buckley, Durham, NC; S. Choo, Victoria, Australia; W. Eberl, Braunschweig, Germany; A. Etzioni, Haifa, Israel; C. Kratz, Freiburg, Germany; A. Shcherbina, Moscow, Russia; and V. Wahn, Berlin, Germany. We also thank the staff of the European Society for Immunodeficiencies registry for their support.
This work was supported in part by a grant from Biotest AG, Dreieich, Germany (M.H.A.).
Authorship
Contribution: M.H.A., B.H.B., and H.D.O. designed the study; all authors except P.P. contributed data; M.H.A., T.C.B., P.P., and H.D.O. analyzed the data; and M.H.A., T.CB., B.H.B., and H.D.O. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael H. Albert, Dr von Haunersches Kinderspital der LMU, Lindwurmstr 4, 80337 Munich, Germany; e-mail: michael.albert@med.lmu.de.
References
Author notes
B.H.B. and H.D.O. contributed equally to this study.