Abstract
Because of its potent immunosuppressive yet stem cell–sparing activity, high-dose cyclophosphamide was tested as sole prophylaxis of graft-versus-host disease (GVHD) after myeloablative allogeneic bone marrow transplantation (alloBMT). We treated 117 patients (median age, 50 years; range, 21-66 years) with advanced hematologic malignancies; 78 had human leukocyte antigen (HLA)–matched related donors and 39 had HLA-matched unrelated donors. All patients received conventional myeloablation with busulfan/cyclophosphamide (BuCy) and T cell–replete bone marrow followed by 50 mg/kg/d of cyclophosphamide on days 3 and 4 after transplantation. The incidences of acute grades II through IV and grades III through IV GVHD for all patients were 43% and 10%, respectively. The nonrelapse mortality at day 100 and 2 years after transplantation were 9% and 17%, respectively. The actuarial overall survival and event-free survivals at 2 years after transplantation were 55% and 39%, respectively, for all patients and 63% and 54%, respectively, for patients who underwent transplantation while in remission. With a median follow-up of 26.3 months among surviving patients, the cumulative incidence of chronic GVHD is 10%. These results suggest that high-dose posttransplantation cyclophosphamide is an effective single-agent prophylaxis of acute and chronic GVHD after BuCy conditioning and HLA-matched BMT (clinicaltrials.gov no. NCT00134017).
Introduction
Graft-versus-host disease (GVHD) remains a major complication of allogeneic blood or marrow transplantation (alloBMT).1 The optimal approach for GVHD prophylaxis remains uncertain. Standard regimens of GVHD prophylaxis comprise the combination of a calcineurin inhibitor (CNI), cyclosporine or tacrolimus, and either methotrexate, mycophenolate mofetil (MMF), or sirolimus.2-6 However, acute GVHD still occurs in 35% to 55% of BMT patients who receive transplants from human leukocyte antigen (HLA)–matched siblings, and more frequently in unrelated donor transplant recipients.7-9 Although CNIs inhibit acute GVHD, they are not as effective in reducing the incidence of chronic GVHD, even if administered for 24 months after transplantation.10 Moreover, CNIs may impair immune reconstitution by inhibiting T-cell development and increasing the risk of disease relapse.11-13 Thus, patients with hematologic malignancies undergoing alloBMT might benefit from GVHD prophylaxis that would minimize the use of CNIs, prevent GVHD, and retain a graft-versus-tumor effect.
Cyclophosphamide can safely be administered in high doses after alloBMT because of its favorable safety profile, including lack of toxicity to primitive hematopoietic stem cells.14 When given in high doses after alloBMT, cyclophosphamide targets proliferating alloreactive T cells and successfully prevents GVHD in mouse models.15-20 The safety and efficacy of high-dose, posttransplantation cyclophosphamide when combined with tacrolimus and MMF was previously demonstrated after nonmyeloablative conditioning and transplantation of T cell–replete bone marrow from partially HLA-mismatched related donors.21,22 With this background, a phase 1/2 study was conducted to assess high-dose cyclophosphamide as sole GVHD prophylaxis after myeloablative conditioning and T cell–replete, HLA-matched alloBMT.
Methods
Study design and patients
The primary endpoint of this phase 1/2 study (registered with clinicaltrials.gov; no. NCT00134017) was the cumulative incidence of grades II through IV acute GVHD. The goal was to determine an optimal regimen of posttransplantation immunosuppression yielding an incidence of acute GVHD of approximately 25% plus or minus 20%. The phase 1 portion of the study was run according to a Bayesian algorithm to select among predefined regimens of immunosuppression23 that included posttransplantation cyclophosphamide with or without MMF or tacrolimus. Death due to regimen-related toxicity within first 60 days after transplantation and graft rejection were 2 additional safety endpoints. The boundaries were set as more than 3 deaths in the first 10 patients, more than 5 deaths in the first 20 patients, or nonengraftment of 1, 2, or 3 patients among the first 5, 10, or 20 patients, respectively. In the dose-finding phase (phase 1), the plan was to recruit 5 patients receiving related and 5 patients receiving unrelated BM transplants to select a regimen, starting with the least immunosuppressive one (cyclophosphamide alone). According to a preplanned algorithm, the first regimen that produced the target rate of GVHD would be selected for the phase 2 portion of the trial.
The initial dose level, comprising cyclophosphamide alone on days 3 and 4 after transplantation, met the protocol-defined criteria for an acceptable rate of GVHD, and the study proceeded to the phase 2 stage. The initial plan was to recruit an additional 20 related and unrelated patients to achieve the precision of plus or minus 20% (95% confidence interval [CI]) in the estimate of GVHD. Since outcomes continued to result in an acceptable target rate of GVHD, and no boundaries were met, recruitment was extended to more precisely estimate the incidence of GVHD at a precision of plus or minus 15% (95% CI) in both the related and unrelated cohorts, as well as to collect further safety data. A total of 96 patients received treatment on a phase 1/2 clinical trial (J0373) approved by the Johns Hopkins Institutional Review Board (IRB) after informed consent was granted in accordance with the Declaration of Helsinki. An additional 21 patients consented to the study, but received identical treatment outside of the study because their health insurance did not include a clinical trial benefit. Permission to include these patients in the analysis of outcomes was granted by the Johns Hopkins IRB. The patients were treated consecutively between July 2004 and March 2008; analysis was locked on June 5, 2009.
Eligibility criteria included the following: age younger than 66 years and the availability of a genotypically HLA-identical sibling, a phenotypically HLA-matched first-degree relative, or an unrelated donor matched for HLA-A, HLA-B, HLA-Cw, HLA-DRB1, and HLA-DQB1 alleles. All unrelated donor/recipient pairs were typed at the allelic level for HLA-A, HLA-B, HLA-Cw, HLA-DRB1, and HLA-DQB1. Patients with relapsed, refractory, or high-risk first-remission hematologic malignancies were eligible (Table 1). Exclusion criteria included pregnancy, positive serology for HIV, active serious infections, refractory central nervous system (CNS) disease, Karnofsky performance status less than 70%, or serious organ dysfunction (eg, left ventricular ejection fraction < 45%, pulmonary forced vital capacity < 50% of predicted, liver transaminases > 3× the upper limit of normal, or creatinine > 221μmol/L [2.5 mg/dL]).
Patient and graft characteristics
| Demographics . | Related (n = 78) . | Unrelated (n = 39) . | Total (n = 117) . |
|---|---|---|---|
| Median age, y (range) | 48 (21-66) | 52 (22-66) | 50 (21-66) |
| Female sex, no. (%) | 35 (45) | 23 (59) | 58 (50) |
| Disease, no. | |||
| De novo AML* | 20 | 13 | 33 |
| CR1*/CR ≥ 2 | 5/4 | 5/4 | 10/8 |
| Not in CR | 11 | 4 | 15 |
| Secondary AML | 16 | 9 | 25 |
| CR1*/CR ≥ 2 | 8/1 | 7/1 | 15/2 |
| Not in CR | 7 | 1 | 8 |
| MDS | 6 | 8 | 14 |
| Secondary | 3 | 3 | 6 |
| Not in CR | 5 | 8 | 13 |
| ALL† | 7 | 3 | 10 |
| Not in CR | 1 | 0 | 1 |
| CML | 3 | 5 | 8 |
| Accelerated phase/active disease | 1 | 1 | 2 |
| Chronic phase/active disease | 1 | 4 | 5 |
| Chronic phase in CR2 | 1 | 0 | 1 |
| CLL, all refractory | 2 | 1 | 3 |
| Multiple myeloma | 5 | 0 | 5 |
| Not in CR | 4 | 0 | 4 |
| NHL | 8 | 0 | 8 |
| Not in CR | 6 | 0 | 6 |
| Hodgkin lymphoma | 11 | 0 | 11 |
| Not in CR | 11 | 0 | 11 |
| Cytomegalovirus serology‡ | |||
| CMV D−R− | 27 | 12 | 39 |
| CMV D+R− | 15 | 5 | 20 |
| CMV D+R+ | 16 | 5 | 21 |
| CMV D−R+ | 18 | 17 | 35 |
| Donor/recipient sex, no.‡ | |||
| Female → male | 21 | 4 | 25 |
| Other | 57 | 34 | 91 |
| Infused cell doses, median (IQR) | |||
| Total nucleated cells, × 108/kg | 4.1 (3.4-4.8) | 4.0 (3.0-4.7) | 4.1 (3.2-4.7) |
| CD34+ cells, × 106/kg | 3.6 (2.8-4.8) | 3.9 (2.8-5.5) | 3.7 (2.8-4.9) |
| Demographics . | Related (n = 78) . | Unrelated (n = 39) . | Total (n = 117) . |
|---|---|---|---|
| Median age, y (range) | 48 (21-66) | 52 (22-66) | 50 (21-66) |
| Female sex, no. (%) | 35 (45) | 23 (59) | 58 (50) |
| Disease, no. | |||
| De novo AML* | 20 | 13 | 33 |
| CR1*/CR ≥ 2 | 5/4 | 5/4 | 10/8 |
| Not in CR | 11 | 4 | 15 |
| Secondary AML | 16 | 9 | 25 |
| CR1*/CR ≥ 2 | 8/1 | 7/1 | 15/2 |
| Not in CR | 7 | 1 | 8 |
| MDS | 6 | 8 | 14 |
| Secondary | 3 | 3 | 6 |
| Not in CR | 5 | 8 | 13 |
| ALL† | 7 | 3 | 10 |
| Not in CR | 1 | 0 | 1 |
| CML | 3 | 5 | 8 |
| Accelerated phase/active disease | 1 | 1 | 2 |
| Chronic phase/active disease | 1 | 4 | 5 |
| Chronic phase in CR2 | 1 | 0 | 1 |
| CLL, all refractory | 2 | 1 | 3 |
| Multiple myeloma | 5 | 0 | 5 |
| Not in CR | 4 | 0 | 4 |
| NHL | 8 | 0 | 8 |
| Not in CR | 6 | 0 | 6 |
| Hodgkin lymphoma | 11 | 0 | 11 |
| Not in CR | 11 | 0 | 11 |
| Cytomegalovirus serology‡ | |||
| CMV D−R− | 27 | 12 | 39 |
| CMV D+R− | 15 | 5 | 20 |
| CMV D+R+ | 16 | 5 | 21 |
| CMV D−R+ | 18 | 17 | 35 |
| Donor/recipient sex, no.‡ | |||
| Female → male | 21 | 4 | 25 |
| Other | 57 | 34 | 91 |
| Infused cell doses, median (IQR) | |||
| Total nucleated cells, × 108/kg | 4.1 (3.4-4.8) | 4.0 (3.0-4.7) | 4.1 (3.2-4.7) |
| CD34+ cells, × 106/kg | 3.6 (2.8-4.8) | 3.9 (2.8-5.5) | 3.7 (2.8-4.9) |
IQR indicates interquartile range.
A total of 10 patients with de novo AML were in CR1 (5 unrelated, 5 related) with following high-risk disease: Flt-3+ mutation (4), monosomy 7 (1), more than 3 cytogenetic abnormalities (1), t(11;19) (1), t(9;11) plus trisomy 8 and leukemia cutis (1), primary refractory but with CR following reinduction (1), and morphologic CR with aberrant blast phenotype by flow cytometry (1).
A total of 7 patients with ALL who underwent transplantation in CR1 had high-risk disease: Ph+ ALL (4), t(4;11) (1), natural killer ALL (1), T-cell lymphoblastic lymphoma (1).
Data are available for 115 donor/recipient pairs.
Regimen and supportive care
Conditioning consisted of busulfan at 4 mg/kg/d orally (n = 72) or 3.2 mg/kg/d intravenously (n = 45) given in 4 daily divided doses for 4 consecutive days, followed by cyclophosphamide at 50 mg/kg intravenously for 2 consecutive days. The fifth and subsequent doses of busulfan were adjusted according to first-dose pharmacokinetic measurements to achieve a target area under the curve of 800 to 1400μmol/L × min. Patients received donor marrow obtained in a targeted collection of 4 × 108 nucleated marrow cells/kg. Cyclophosphamide at 50 mg/kg/d was given intravenously on days 3 and 4 after transplantation. Mesna (80% of cyclophosphamide dose) was administered in 4 divided doses on all days of cyclophosphamide administration. All dosing was based on ideal body weight. Phenytoin at 300 mg orally was administered on days −7 to −3. Colony-stimulating factors were not given. All supportive care measures were administered according to institutional protocols and included prophylaxis against Pneumocystis jirovecii, Candida albicans, and herpes zoster/simplex infections. All blood products except for the allograft were irradiated before transfusion. Cytomegalovirus (CMV)–seronegative patients were given transfusions from CMV-seronegative donors or leukoreduced blood products if CMV− products were unavailable. Supportive care measures were identical for recipients of related and unrelated allografts. Patients had weekly measurements of CMV copy number by polymerase chain reaction (PCR) until discharge from intensive transplantation follow-up (usually around day 60). Preemptive therapy was initiated when more than 500 copies CMV/mL were detected. The incidence of CMV viremia after transplantation was categorized according to pretransplantation donor and recipient serologic status: donor and recipient serologically negative (D−R−), donor serologically positive and recipient negative (D+R−), and seropositive recipients (D+R+ or D−R+).
Regimen-related toxicity
Toxicities were graded using National Cancer Institute (NCI) criteria (Common Toxicity Criteria version 3) with the exception of veno-occlusive disease (VOD), which was graded using Baltimore criteria,24 and hemorrhagic cystitis, which was considered significant if accompanied by macroscopic hematuria or pain on voiding.
Diagnosis and treatment of GVHD
A clinical diagnosis of GVHD was confirmed pathologically. Acute GVHD was graded according to the Keystone Criteria.25 First-line therapy of clinically significant acute cutaneous GVHD consisted of methylprednisolone (MP) 1 to 2.5 mg/kg/d orally or intravenously, while patients with acute GVHD of the viscera received tacrolimus and MP or were enrolled into a clinical trial of GVHD therapy.26 Chronic GVHD was graded according to both National Institutes of Health and Seattle guidelines.27,28
Engraftment and donor chimerism
Neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) greater than 0.5 × 109/L. Platelet recovery was defined as a platelet count greater than 20 × 109/L without platelet transfusion in the preceding 7 days. Donor chimerism was assessed on days 30, 60, 180, and 360 after transplantation, and then on a yearly basis. Primary graft failure was defined as a lack of neutrophil recovery in the absence of progressive malignancy affecting the marrow. Secondary graft failure was defined as loss of donor engraftment (< 5% donor chimerism) in the absence of progressive malignancy affecting the marrow.
Statistical analysis
Event time distributions for overall survival (OS) and event-free survival (EFS) were estimated with the method of Kaplan and Meier,29 and compared using the log-rank statistic,30 or the Cox proportional hazards regression model.31 Probabilities of acute and chronic GVHD, donor stem cell engraftment, nonrelapse mortality (NRM), and relapse were calculated using cumulative incidence estimates.32 The model-based approach of Fine and Gray33 was used to compare cumulative incidence functions between 2 or more groups. Patients were considered to have died of NRM if there was no evidence of disease persistence or progression before death. NRM and relapse were treated as competing risk events. Death was the competing risk for engraftment. The cumulative incidences of acute and chronic GVHD were calculated with primary graft failure, death, or relapse as competing risk. The cumulative incidence of acute GVHD was also computed with death and graft failure as competing events. Factors tested in regression analyses influencing the kinetics of engraftment included total nucleated bone marrow cell (TNC) dose infused, CD34+ cell dose, and disease status before transplantation. Factors tested for an association with acute GVHD, NRM, relapse, OS, and EFS included the following: donor relatedness, recipient age, donor age, donor/recipient sex mismatch, CMV serologic status of the donor, CMV serologic status of the recipient, TNC dose, and disease status before transplantation. The TNC dose was analyzed as a 3-group categoric variable representing interquartile range values with TNC less than or equal to 3.2 × 108/kg (low dose; n = 29) as the reference; more than 3.2 to less than 4.7 × 108/kg (intermediate dose; n = 59); and more than 4.7 × 108/kg (high dose; n = 29) as described previously.34,35 Multivariate models were obtained with backward stepwise elimination using a P value of .15 as the criterion for entry and retention in the models. Computations were performed using Statistical Analysis Software, version 9.1 (SAS Institute) and R software (www.r-project.org).
Results
Patient, donor, and graft characteristics
Patient, disease, and transplantation characteristics are summarized in Table 1. A total of 14 (12%) patients were 60 years of age or older at the time of transplantation. A total of 68 (58%) patients were not in remission at the initiation of transplantation conditioning. The most common diagnosis was acute myeloid leukemia (AML; n = 58, 50%); 33 patients presented with disease de novo, and 25 patients developed AML from an antecedent hematologic disorder or after prior cancer therapy (secondary AML). A total of 10 patients with de novo AML were in first complete remission (CR1), and 8 patients were in second complete remission (CR2). A total of 15 patients with de novo AML had either refractory disease that showed no response to prior chemotherapy (7 patients were primary refractory and 4 had refractory relapse) or had AML in relapse (n = 4) at the beginning of the conditioning regimen. There were 14 patients with myelodysplastic syndrome (MDS), of whom 6 had secondary MDS. A total of 10 patients with MDS had more than 5% blasts in the bone marrow or circulating blasts in the peripheral blood. Of the 10 patients with acute lymphoblastic leukemia (ALL), 7 were in CR1. All patients with chronic myelogenous leukemia (CML) were unresponsive to at least one tyrosine kinase inhibitor (TKI) before transplantation, with 4 patients unresponsive to 2 or more TKIs. Of 19 patients with non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL), 17 had active disease and 11 did not respond to prior chemotherapy. All patients with lymphoma received related donor allografts. The median donor age was 43 years (range, 19-69 years). One donor was a genotypically HLA-identical parent. The median numbers of TNCs and CD34+ cells infused into recipients were 4.0 × 108/kg (interquartile range, 3.2-4.7 × 108/kg) and 3.6 × 106/kg (interquartile range, 2.8-4.9 × 106/kg), respectively (Table 1). The target dose of 4 × 108/kg TNC was not achieved in 37 (48%) of 78 recipients of marrow from related donors and in 19 (49%) of 39 of marrow from unrelated donors.
Engraftment
The median times to neutrophil recovery for recipients of related and unrelated grafts were 23 and 25 days (range, 2-100 days), respectively, and the median times to platelet recovery were 31 and 35 days (range, 5-354 days), respectively (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Neutrophil and platelet recovery were faster in patients who received higher than the median TNC and CD34+ doses, respectively (supplemental Figure 2).
At day 30 after BMT, the median donor chimerism in the peripheral blood of the 116 evaluable patients (1 patient died before day 30) was 100% (range, 0%-100%). Of these 116 patients, 99 (85%) achieved full donor chimerism by day 30. Of the remaining 17 patients, 6 had more than 80% donor chimerism by day 30; 2 of these 6 patients converted to full donor chimerism by day 60. Primary graft failure occurred in one recipient of a sibling graft and 2 recipients of unrelated donor grafts. Of the 2 unrelated patients, 1 received a graft containing a low TNC count (1.1 × 108 cells/kg), and the other had a history of a previously failed HLA-haploidentical BMT. Two patients with primary graft failure engrafted successfully after conditioning and transplantation of filgrastim-mobilized peripheral blood from the original donor.36 Of the 12 remaining patients with mixed donor chimerism at day 30, 9 were found to have early disease relapse, while 3 had a further decline in donor chimerism by day 60. Of those 3 patients, 2 developed secondary graft failure and 1 received prophylactic donor lymphocyte infusion (DLI) at day 90 resulting in rapid conversion to full donor chimerism accompanied by mild grade II GVHD. Only one patient with initial full donor chimerism subsequently lost donor chimerism. Two of the 3 patients with secondary graft failure had high-grade MDS; however, they received pretransplantation intensive chemotherapy. All 3 patients were rescued with a second allograft.36
Toxicity and NRM
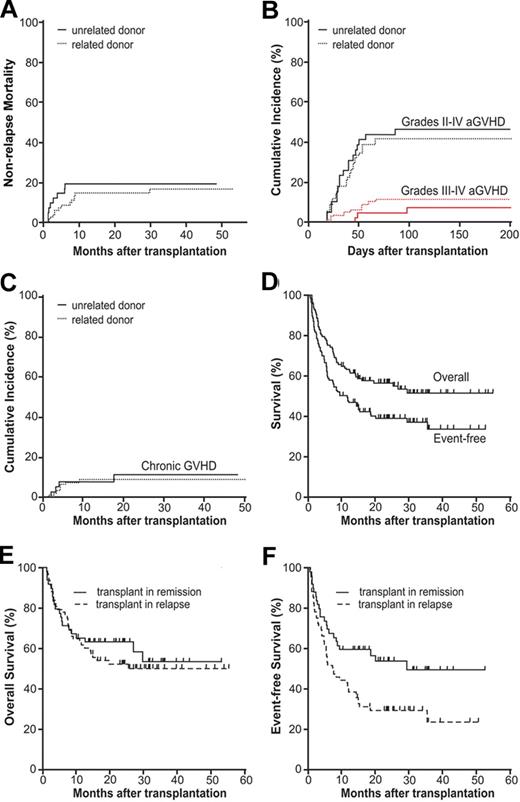
Nonfatal toxicities occurring in the first 100 days after transplantation are listed in Table 2. The most common toxicities were reversible elevations in serum transaminases or creatinine. Hepatic VOD developed in 10 (9%) patients and resolved without treatment in 8 patients. Hemorrhagic cystitis occurred in 12 (10%) patients. A total of 3 patients had evidence of pericarditis, and 1 patient required drainage of an associated effusion. Causes of death are listed in Table 2. The cumulative incidences of NRM at 100 days and 2 years after transplantation were 6% (95% CI, 2%-13%) and 15% (95% CI, 8%-24%), respectively. The cumulative NRM at 2 years was 13% (95% CI, 5%-25%) for related recipients and 21% (95% CI, 9%-34%) for unrelated recipients (hazard ratio [HR] 0.74; 95% CI, 0.31-1.78, P = .49; Figure 1A). In the univariate analysis, recipient age greater than 60 years was associated with a significantly increased risk of NRM (supplemental Table 1). There was also a trend for age greater than 60 years to be associated with increased NRM in the multivariate analysis (HR 2.23; 95% CI, 0.75-6.64, P = .15; Table 3).
Morbidity and mortality after transplantation
| . | Related, no. . | Unrelated, no. . | Combined no. (%) . |
|---|---|---|---|
| Nonfatal toxicity up to day 100* | |||
| ALT elevation, grade IV | 8 | 5 | 13 (11) |
| AST elevation, grade IV | 4 | 2 | 6 (5) |
| Bilirubin elevation, grade IV | 4 | 4 | 8 (7) |
| Serum creatinine, grade III | 3 | 3 | 6 (5) |
| Reversible VOD | 7 | 1 | 8 (7) |
| Hemorrhagic cystitis | 1 | 11 | 12 (10) |
| Pericardial effusion | 3 | 0 | 3 (3) |
| Subdural hematoma | 1 | 0 | 1 (1) |
| Cause of death | |||
| Related to relapse or progression | 21 | 10 | 31 (26) |
| Related to transplantation | |||
| Graft failure | 0 | 1 | 1 (1) |
| VOD | 2 | 0 | 2 (2) |
| Noninfectious pneumonia | 1 | 1 | 2 (2) |
| Sepsis/bacterial infection | 2 | 1 | 3 (3) |
| Hemorrhage | 2 | 2 | 4 (3) |
| Refractory GVHD | 2 | 1 | 3 (3) |
| Multisystem organ failure | 3 | 2 | 5 (4) |
| . | Related, no. . | Unrelated, no. . | Combined no. (%) . |
|---|---|---|---|
| Nonfatal toxicity up to day 100* | |||
| ALT elevation, grade IV | 8 | 5 | 13 (11) |
| AST elevation, grade IV | 4 | 2 | 6 (5) |
| Bilirubin elevation, grade IV | 4 | 4 | 8 (7) |
| Serum creatinine, grade III | 3 | 3 | 6 (5) |
| Reversible VOD | 7 | 1 | 8 (7) |
| Hemorrhagic cystitis | 1 | 11 | 12 (10) |
| Pericardial effusion | 3 | 0 | 3 (3) |
| Subdural hematoma | 1 | 0 | 1 (1) |
| Cause of death | |||
| Related to relapse or progression | 21 | 10 | 31 (26) |
| Related to transplantation | |||
| Graft failure | 0 | 1 | 1 (1) |
| VOD | 2 | 0 | 2 (2) |
| Noninfectious pneumonia | 1 | 1 | 2 (2) |
| Sepsis/bacterial infection | 2 | 1 | 3 (3) |
| Hemorrhage | 2 | 2 | 4 (3) |
| Refractory GVHD | 2 | 1 | 3 (3) |
| Multisystem organ failure | 3 | 2 | 5 (4) |
ALT indicates alanine aminotransferase; and AST, aspartate aminotransferase.
Only highest-grade toxicities are presented.
Transplantation outcomes. (A) Cumulative incidence of nonrelapse mortality. (B) Results for acute GVHD. (C) Results for chronic GVHD. (D) OS and EFS of all patients. (E) OS and (F) EFS according to disease status before transplantation.
Transplantation outcomes. (A) Cumulative incidence of nonrelapse mortality. (B) Results for acute GVHD. (C) Results for chronic GVHD. (D) OS and EFS of all patients. (E) OS and (F) EFS according to disease status before transplantation.
Multivariate analysis of transplantation outcomes
| Variable . | NRM . | Relapse . | EFS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Recipient age, y | ||||||||||||
| < 50 | 1 | — | — | — | — | — | — | — | — | 1 | — | — |
| 50-59 | 0.82 | 0.28-2.42 | .72 | — | — | — | — | — | — | 1.01 | 0.51-1.99 | .98 |
| ≥ 60 | 2.23 | 0.75-6.64 | .15 | — | — | — | — | — | — | 1.95 | 0.93-4.10 | .08 |
| Donor age, y | ||||||||||||
| < 43 | 1 | — | — | — | — | — | — | — | — | 1 | — | — |
| ≥ 43 | 2.29 | 0.87-6.01 | .09 | — | — | — | — | — | — | 1.66 | 0.90-3.08 | .1 |
| TNC dose, × 108/kg | ||||||||||||
| < 3.2 | — | — | — | 1 | — | — | 1 | — | — | 1 | — | — |
| 3.2-4.7 | — | — | — | 0.47 | 0.24-0.87 | .017 | 0.62 | 0.36-1.07 | .09 | 0.53 | 0.28-1.02 | .06 |
| > 4.7 | — | — | — | 0.44 | 0.20-0.93 | .031 | 0.59 | 0.31-1.11 | .10 | 0.53 | 0.25-1.14 | .10 |
| Disease status at transplantation | ||||||||||||
| CR | — | — | — | 1 | — | 1 | — | — | — | — | — | |
| Not in CR before alloBMT | — | — | — | 2.94 | 1.53-5.67 | .001 | 1.88 | 1.14-3.09 | .01 | — | — | — |
| Recipient CMV serology | ||||||||||||
| Negative | 1 | — | — | — | — | — | — | — | — | — | — | — |
| Positive | 2.02 | 0.78-5.21 | .14 | — | — | — | — | — | — | — | — | — |
| Variable . | NRM . | Relapse . | EFS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Recipient age, y | ||||||||||||
| < 50 | 1 | — | — | — | — | — | — | — | — | 1 | — | — |
| 50-59 | 0.82 | 0.28-2.42 | .72 | — | — | — | — | — | — | 1.01 | 0.51-1.99 | .98 |
| ≥ 60 | 2.23 | 0.75-6.64 | .15 | — | — | — | — | — | — | 1.95 | 0.93-4.10 | .08 |
| Donor age, y | ||||||||||||
| < 43 | 1 | — | — | — | — | — | — | — | — | 1 | — | — |
| ≥ 43 | 2.29 | 0.87-6.01 | .09 | — | — | — | — | — | — | 1.66 | 0.90-3.08 | .1 |
| TNC dose, × 108/kg | ||||||||||||
| < 3.2 | — | — | — | 1 | — | — | 1 | — | — | 1 | — | — |
| 3.2-4.7 | — | — | — | 0.47 | 0.24-0.87 | .017 | 0.62 | 0.36-1.07 | .09 | 0.53 | 0.28-1.02 | .06 |
| > 4.7 | — | — | — | 0.44 | 0.20-0.93 | .031 | 0.59 | 0.31-1.11 | .10 | 0.53 | 0.25-1.14 | .10 |
| Disease status at transplantation | ||||||||||||
| CR | — | — | — | 1 | — | 1 | — | — | — | — | — | |
| Not in CR before alloBMT | — | — | — | 2.94 | 1.53-5.67 | .001 | 1.88 | 1.14-3.09 | .01 | — | — | — |
| Recipient CMV serology | ||||||||||||
| Negative | 1 | — | — | — | — | — | — | — | — | — | — | — |
| Positive | 2.02 | 0.78-5.21 | .14 | — | — | — | — | — | — | — | — | — |
Table reflects only those variables that remained in the final multivariate model.
—indicates not applicable.
No patient died of CMV or invasive fungal infections. There were 2 documented cases of CMV disease. CMV viremia was detected by PCR in 28 (24%) patients through day +100. CMV was detected in 1 (3%) of 39 D−R− patients, in 3 (15%) of 20 D+R− patients, 5 (24%) of 21 D+R+ patients, and 19 (54%) of 35 D−R+ patients. The median time to CMV reactivation was 50 days (range, 37-90 days). Acute GVHD was diagnosed in 18 patients on or about the time of CMV reactivation. One patient developed human herpesvirus 6 viremia. There were no cases of Epstein-Barr virus–associated lymphoproliferative disease. One patient developed de novo chronic myelomonocytic leukemia (CMML) of donor origin with chromosome 6 deletion; in retrospect, the donor had unsuspected CMML at the time of bone marrow harvest.
GVHD
The day-100 cumulative incidences of grades II through IV and III through IV acute GVHD for all patients were 43% (95% CI, 34%-52%) and 10% (95% CI, 6%-17%), respectively (Figure 1A). The median times to onset of grades II through IV and III through IV acute GVHD were 38 days (range, 19-86 days) and 48 days (range, 20-97 days), respectively. Among recipients of grafts from related donors, the cumulative incidence of grades II through IV acute GVHD at day 100 was 42% (95% CI, 30%-52%), and for recipients of unrelated donor grafts the incidence was 46% (95% CI, 30%-61%). This difference was not significant (HR 0.87, 95% CI, 0.50-1.54, P = .64; Figure 1B). The cumulative incidences of grades III through IV acute GVHD at day 100 were 12% (95% CI, 6%-20%) and 8% (95% CI, 2%-19%) for recipients of related and unrelated donor grafts, respectively (HR 1.56, 95% CI, 0.43-5.62, P = .50; Figure 1B). No patient developed acute GVHD beyond day 100 after transplantation (Figure 1B).
A total of 3 additional patients developed GVHD after primary disease relapse. Of these, 2 had grade III GVHD and one had grade II GVHD, all requiring treatment. Two of the patients had refractory AML and recovered counts with persistent malignant hematopoiesis. An additional patient with ALL in CR2 relapsed early after alloBMT with extramedullary disease followed by systemic involvement. Although the development of GVHD resulted in a transient disease response, all 3 patients subsequently died of progressive disease. In a competing risk model in which the occurrence of relapse was not considered a competing event, the cumulative incidences of grades II through IV and III through IV acute GVHD for all patients were 46% and 12%, respectively.
In a multivariate analysis, the male recipients of a graft from a female donor had an increased risk of developing grades II through IV acute GVHD (HR 2.11, 95% CI, 1.78-3.80, P = .012) and grades III through IV acute GVHD (HR 3.88; 95% CI, 1.27-11.82, P = .017). CMV-seropositive recipients showed a trend toward an increased risk of developing grades II through IV GVHD (Table 4; supplemental Table 1).
Multivariate analysis of acute GVHD
| Variable . | GVHD II-IV . | GVHD III-IV . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Recipient CMV serology | ||||||
| Negative | 1 | — | — | 1 | — | — |
| Positive | 1.67 | 0.96-2.91 | .072 | 3.13 | 0.85-11.52 | .087 |
| Donor-recipient pairs | ||||||
| Others | 1 | — | — | 1 | — | — |
| Female → male | 2.11 | 1.78-3.80 | .012 | 3.89 | 1.27-11.82 | .017 |
| Variable . | GVHD II-IV . | GVHD III-IV . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Recipient CMV serology | ||||||
| Negative | 1 | — | — | 1 | — | — |
| Positive | 1.67 | 0.96-2.91 | .072 | 3.13 | 0.85-11.52 | .087 |
| Donor-recipient pairs | ||||||
| Others | 1 | — | — | 1 | — | — |
| Female → male | 2.11 | 1.78-3.80 | .012 | 3.89 | 1.27-11.82 | .017 |
Table reflects only those variables that remained in the final multivariate model. — indicates not applicable.
With a median follow-up of surviving patients of 26.3 months (range, 10-55 months), the cumulative incidence of chronic GVHD for all patients was 10% (95% CI, 5%-16%; Figure 1C). At 2 years after transplantation, the cumulative incidences of chronic GVHD for recipients of related and unrelated donor grafts were 9% (95% CI, 4%-17%) and 11% (95% CI, 3%-25%), respectively (HR 0.83, 95% CI, 0.25-2.88, P = .79; Figure 1C). The median time to occurrence of chronic GVHD was 4.1 months (range, 45-536 days), with only 2 new cases beyond 6 months and only 1 new case beyond 1 year after transplantation. Of 11 patients with chronic GVHD, 7 had classic limited and 3 had classic extensive forms of the disease. An overlap syndrome (limited) was diagnosed in 1 patient. According to the National Institutes of Health severity scale, 3 had severe disease (2 with obliterative bronchiolitis). Only 1 patient developed chronic GVHD without preceding acute GVHD. Acute grades II through IV GVHD was treated with steroids alone in 10 (20%) patients, steroids plus a CNI in 31 (61%) patients, and steroids plus non-CNI–based agents in 7 (14%) patients; 3 (6%) patients were left untreated. A total of 3 patients, all with chronic GVHD, remained on systemic immunosuppression at the time of last follow-up.
Relapse
The cumulative incidence of relapse at 2 years after transplantation was 44% (95% CI, 34%-53%) and did not differ significantly between recipients of related versus unrelated donor grafts (HR 1.4, 95% CI, 0.78-2.60, P = .25). The 2-year cumulative incidence of relapse for patients undergoing transplantation in CR was 26% (95% CI, 14%-40%). In both univariate and multivariate analyses, patients who were not in remission at the initiation of conditioning had a higher risk of relapse, whereas patients who received a bone marrow cell dose above the median value of more than 3.2 × 108/kg had a lower risk of disease progression after transplantation (Table 3; supplemental Table 1).
Survival
The median follow-up is 29 months (range, 10-55 months) for surviving recipients of related donor grafts and 24 months (range, 14-48 months) for surviving recipients of unrelated donor grafts. The actuarial OS and EFS for all patients at 1 year after transplantation are 63% (95% CI, 54%-71%) and 48% (95% CI, 39%-57%), and at 2 years after transplantation are 55% (95% CI, 45%-64%) and 39% (95% CI, 30%-48%), respectively (Figure 1D). There was no significant difference in the OS of patients receiving related versus unrelated donor grafts (HR 0.85, 95% CI, 0.49-1.50, P = .58). EFS also did not differ significantly according to the donor type (HR 1.12, 95% CI 0.68-1.86, P = .65). A total of 15 patients with disease progression after transplantation are alive, and 4 are in CR: 2 patients with AML treated with salvage chemotherapy and DLI, 1 patient with CML treated with nilotinib, and 1 patient with NHL treated with nonmyeloablative, HLA-haploidentical BMT. There were no significant factors influencing OS in the multivariate analysis (Table 3).
OS and EFS according to pretransplantation disease status are shown in Figure 1E-F. The 1- and 2-year EFS for patients who underwent transplantation in CR were 59% (95% CI, 44%-71%) and 54% (95% CI, 38%-67%), and for patients not in remission at the time of transplantation were 40% (95% CI, 28%-51%) and 29% (95% CI, 19%-40%), respectively; (HR 1.88, 95% CI, 1.14-3.08, P = .01; Figure 1F). Among patients with AML/MDS in remission at the time of transplantation, the 1- and 2-year EFSs were 61% (95% CI, 43%-75%) and 52% (95% CI, 34%-68%), respectively. The cumulative incidence of relapse for patients with AML/MDS in CR was 28% (95% CI, 13%-45%) at 2 years after transplantation. Active disease at the time of transplantation was associated with poorer EFS in univariate analysis (supplemental Table 1). In the multivariate analysis, patients who were in CR before transplantation had a significantly improved EFS (Table 3).
Discussion
The results presented here suggest that high-dose, posttransplantation cyclophosphamide is effective as a single-agent GVHD prophylaxis after myeloablative conditioning and HLA-matched BMT. In our study, the incidences of acute grades II through IV and grades III through IV GVHD were 45% and 10%, respectively, and did not differ significantly between recipients of related or unrelated allografts. With a median follow-up of surviving patients beyond 2 years, the cumulative incidence of chronic GVHD for all patients was 10%, and was not different between related and unrelated cohorts.
Cyclophosphamide was one of the first agents shown to be effective in controlling acute GVHD in animal models.16,17 Subsequent mouse studies demonstrated that tolerance to minor histocompatibility antigens could be induced if cyclophosphamide was given in a high dose 2 to 3 days after alloantigen exposure; tolerance was not induced if the same dose of cyclophosphamide was given 4 or more days after transplantation.37,38 However, a randomized clinical trial demonstrated that a lower dose of cyclophosphamide was inferior to cyclosporine in the prophylaxis of acute GVHD after HLA-matched sibling alloBMT.39 In this earlier clinical trial of posttransplantation cyclophosphamide, the drug may have been given at the wrong time or at too low a dose to be maximally effective at suppressing GVHD. The selection of low, intermittent doses of cyclophosphamide in that trial was motivated by concerns that higher doses of the drug might be toxic to donor stem cells, impairing engraftment. Newer data that high-dose cyclophosphamide is not toxic to hematopoietic stem cells because of their high expression of the detoxifying enzyme aldehyde dehydrogenase14,40 provided support for the use of high doses after BMT. The time to hematopoietic reconstitution was slower than reported with peripheral blood stem cell allografts or methotrexate-free GVHD prophylaxis regimens,5,6 but this did not appear to be associated with higher infection rates after transplantation.
High-dose posttransplantation cyclophosphamide appears to differ from GVHD prophylaxis strategies that incorporate CNIs in its ability to prevent chronic, as well as acute, GVHD. Since their introduction nearly 3 decades ago, the CNIs cyclosporine and tacrolimus have become integral components of GVHD prophylaxis regimens.2-4 CNIs specifically inhibit intracellular signals generated by ligation of the clonotypic T-cell antigen receptor, which are required for T-cell activation.41 By inhibiting the activation of alloreactive T cells, CNIs have reduced the incidence of acute GVHD and NRM after allogeneic BMT.42 However, CNIs also block T-cell development11 and the induction of T-cell43 and transplantation tolerance,44 leading some investigators to hypothesize that cyclosporine treatment and/or withdrawal itself could play a role in the development of chronic GVHD.45,46 Accordingly, chronic GVHD occurs in more than 50% of patients treated with CNIs after alloBMT.4-8 In contrast, high-dose posttransplantation cyclophosphamide induces tolerance to minor histocompatibility antigens,37 an example of the phenomenon of drug-induced immunologic tolerance.47 This difference in the effect of high-dose, posttransplantation cyclophosphamide and CNI-containing regimens on the induction of T-cell tolerance may be responsible for the apparent difference in the incidence of chronic GVHD between these prophylaxis strategies.
Another advantage of high-dose posttransplantation cyclophosphamide is that the elimination of CNIs permits reconstitution of the immune system in an environment free of ongoing pharmacologic immunosuppression. A preliminary analysis of blood concentrations of CD4+ and CD8+ T lymphocytes compares favorably with levels seen after T cell–replete bone marrow transplantation and GVHD prophylaxis with cyclosporine and methotrexate (data not shown).48,49 The low overall incidence of infection-related complications is consistent with favorable immunologic recovery after posttransplantation cyclophosphamide. It is possible that the timing of the high-dose, posttransplantation cyclophosphamide results in selective killing of activated alloreactive T cells while sparing resting T cells specific for infectious agents. Studies are ongoing to characterize the effects of posttransplantation cyclophosphamide on immune reconstitution and the graft-versus-leukemia effect of allogeneic T cells.
In conclusion, high-dose posttransplantation cyclophosphamide is effective as a single-agent strategy for prophylaxis of acute and chronic GVHD after conventional myeloablative busulfan/cyclophosphamide conditioning and HLA-matched related or unrelated bone marrow transplantation. The low incidence of chronic GVHD makes this strategy attractive for patients with nonmalignant disorders and those with acute leukemias in CR1 undergoing alloBMT. Furthermore, the potential ability to limit posttransplantation immunosuppression provides an excellent platform for early posttransplantation administration of novel immunologic approaches, with the goal of improving outcomes in patients with advanced hematologic malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grant P01 CA015396. L.L. was the recipient of the Amy Strelzer Manasevit Scholars Program funded by the Marrow Foundation in cooperation with the National Marrow Donor Program.
National Institutes of Health
Authorship
Contribution: L.L., R.J.J., and E.J.F. designed research, treated patients, analyzed data, and wrote the paper; J.B-M. and R.F.A. recruited and treated patients, analyzed data, and wrote the paper; M.Z. and S.N.G. designed research and analyzed data; A.R.C. designed research; R.B. designed research and recruited and treated patients; B.D.S, C.A.H., I.B., W.M., J.D.P., and Y.K. recruited and treated patients; A.H. and H.I.L. provided administrative and logistic support; and all authors approved the manuscript submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leo Luznik, Cancer Research Building I, Room 2M88, 1650 Orleans St, Baltimore, MD 21231; e-mail: luznile@jhmi.edu.