Abstract
Growth arrest-specific gene 6 (Gas6) is expressed in antigen-presenting cells and endothelial cells (ECs) but not in T cells. When wild-type (WT) or Gas6−/− mice received allogeneic non–T cell–depleted bone marrow cells, hepatic graft-versus-host disease (GVHD) was alleviated in Gas6−/− recipients regardless of donor genotype, but not in WT recipients. T-cell infiltration was more prominent and diffuse in WT than in Gas6−/− recipients' liver. When mice received 0.5 × 106 allogeneic T cells with T cell–depleted allogeneic bone marrow, clinical signs indicated that GVHD was less severe in Gas6−/− than in WT recipients, as shown by a significant improvement of the survival and reduced liver GVHD. These data demonstrate that donor cells were not involved in the protection mechanism. In addition, lack of Gas6 in antigen-presenting cells did not affect WT or Gas6−/− T-cell proliferation. We therefore assessed the response of WT or Gas6−/− ECs to tumor necrosis factor-α. Lymphocyte transmigration was less extensive through Gas6−/− than WT ECs and was not accompanied by increases in adhesion molecule levels. Thus, the lack of Gas6 in ECs impaired donor T-cell transmigration into the liver, providing a rationale for considering Gas6 pathway as a potential nonimmunosuppressive target to minimize GVHD in patients receiving allogeneic hematopoietic stem cell transplantation.
Introduction
Allogeneic hematopoietic stem cell transplantation is an effective treatment for malignant and nonmalignant hematologic disorders, but its wide applicability is limited by graft-versus-host disease (GVHD). GVHD is a multiorgan disorder combining autoimmunity and immunodeficiency1,2 that develops in 3 phases: (1) epithelial injury caused by the conditioning regimen; (2) activation of donor T lymphocytes by antigen-presenting cells (APCs) and rapid burst of T-lymphocyte proliferation; and (3) cell death induced by activated T lymphocytes, cytokines (eg, tumor necrosis factor-α [TNF-α]), and cells of the innate immune system. GVHD is currently treated by immunosuppressive drugs favoring secondary infections and preventing the expected graft-versus-leukemia effect. Consequently, understanding the mechanisms leading to GVHD is critical for optimizing therapy with drugs not directly targeting the immune system.
We therefore explored whether novel factors might be involved in GVHD. We considered Growth arrest-specific gene 6 (Gas6) product, acting via its receptor tyrosine kinases Tyro3, Axl, and Mer, as a probable candidate because Gas6 and its receptors are expressed in hematopoietic tissue,3-6 APCs,7,8 and endothelium.9,10 Gas6 is a secreted vitamin K-dependent protein that interacts with phospholipid membranes via its γ-carboxyglutamic acid–containing domain and binds to its receptors via its carboxy-terminal globular domain.11-14 This then leads to further intracellular signaling.15,16 Gas6 has been implicated in reversible cell growth arrest, survival, proliferation, cell adhesion, and hemostasis (reviewed by Saller et al17 ).
In this study, we showed in 2 different models that Gas6 deficiency in recipient mice from allogeneic bone marrow transplantation (BMT) alleviated GVHD by dampening T lymphocytes transmigration in the liver, providing a rationale for considering Gas6 as a potential nonimmunosuppressive target to minimize GVHD.
Methods
Mice
Wild-type (WT) and Gas6−/− mouse progenies of the original colony (50% 129/Sv × 50% Swiss)18 were backcrossed onto C57BL/6 (B6), FVB, or Balb/C backgrounds for more than 10 generations. Animal experiments were approved by the Veterinary Services of Geneva and Lausanne (Switzerland).
Allogeneic non–T cell–depleted BMT in mice
WT and Gas6−/− B6 (H-2b) recipients received 9 Gy total body irradiation (137Cs source, 129 cGy/minute). Bone marrow cells (BMCs) were isolated from a pool of 10 to 15 WT or Gas6−/− FVB (H-2q) donors. BMCs from the WT pool were injected into recipients from either WT or Gas6−/− genotype, and BMCs from the Gas6−/− pool were injected into recipients from either WT or Gas6−/− genotype. Experiments were performed in multiple sets, and the data were pooled. Thus, in each series, each recipient from either WT or Gas6−/− genotype received the 107 BMCs isolated from the same pool, indicating that each recipient from any genotype received an identical number of T cells within the same set of experiments.
Depletion and isolation of T cells
T-cell depletion has been performed by a first incubation of BMCs with anti-Thy1.2 antibodies (BD Biosciences), followed by the incubation for 40 minutes with Low-Tox-M rabbit complement in Cytotoxicity Medium (Cedarlane). T cell–depleted bone marrow hematopoietic potential was confirmed by 12-day cultures in methylcellulose (MethoCult; StemCell Technologies).
T-cell isolation was performed by negative magnetic selection from spleen cells (Mouse T-Cell Negative Isolation Kit; Invitrogen) following the manufacturer's instructions.
Allogeneic T cell–depleted BMT and T-cell infusion in mice
WT and Gas6−/− Balb/C mice (H-2d) received 9 Gy total body irradiation (137Cs source) and were then injected intravenously with 5 × 106 T cell–depleted BMCs and simultaneously with 0.5 × 106 T cells, all donor cells originating from WT C57BL/6 (H2b). Body weight and clinical signs of GVHD were scored daily after transplantation using a standard method.19 Because repeated measurement statistics cannot handle missing values, we assigned a score of 3 to all studied parameters for mice that died in the course of the experiment. For control experiments, we performed syngeneic T cell–depleted BMT and syngeneic T-cell infusion. Control recipients were WT and Gas6−/− Balb/C mice and donor cells originated from WT Balb/C mice.
Flow cytometric analysis
BMCs were stained with anti–mouse fluorescein isothiocyanate (FITC)–conjugated H-2q and phycoerythrin (PE)–conjugated anti–mouse H-2b (BD Biosciences). Cell-surface expression was analyzed using a BD Biosciences FACScan instrument, CellQuest, and WinMDI 2.9 software.
Blood was drawn from retro-orbital puncture into heparinized tubes, and cells were incubated with labeled anti–CD4-PE-Cy5 (clone RM4-5), CD8a-allophycocyanin (clone 53-6.7), B220-PE-Cy5 (clone RA3-6B2), H-2Kd-FITC (clone SF1-1.1), and H-2Kb-PE (clone AF6-88.5) antibodies (all antibodies from BD Biosciences, except for B220-Cy5 from eBioscience). Red blood cells were lysed and white blood cells were washed in phosphate-buffered saline/1% bovine serum albumin and analyzed on a FACSCalibur cytometer (BD Biosciences) and CellQuest software.
Spleens were isolated from mice and red blood cells lysed. Splenocytes were incubated with labeled anti–CD4-allophycocyanin (clone RM4-5), CD8-PE-Cy7 (clone 53-6.7), H-2Kd-FITC (clone SF1-1.1), and H-2Kb-PE (clone AF6-88.5) antibodies (all antibodies from BD Biosciences), and analyzed on a FACSCalibur cytometer (BD Biosciences), CellQuest, and WinMDI 2.9 software.
Immunohistochemistry
The skin, liver, and large bowel were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained by hematoxylin and eosin and examined under microscopy. An observer blinded to the mouse genotype evaluated the histopathologic changes19,20 as follows: skin (inflammatory infiltrates, dyskeratotic cells, ulceration, and loss of hair follicles), large bowel (lamina propria lymphocytic infiltrate, mucosal ulceration, and outright crypt destruction), and liver (infiltrates in bile ducts and portal tracts, vascular endothelialitis, and hepatocellular damage).21 The severity of pathologic changes was graded as follows: 0 = normal; 0.5 = rare/scattered; 1 = minimal or focal; 2 = mild and more diffuse; 3 = moderate damage; and 4 = severe damage. Scores were cumulated into a single value per organ and then per animal.
Apoptosis was evaluated by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end (TUNEL) labeling. The ABC Vectastain kit (Vector) with DAB-cobalt-nickel staining was used to localize TUNEL+ cells.
Sections were viewed on an Axiophot microscope (Zeiss) equipped with a 40×/0.75 dry objective. Images were captured with an AxioCam MRc5 camera (Zeiss) and AxioVision 4.5 software (Zeiss) and processed with Adobe Photoshop CS3 software (Adobe Systems).
Mixed lymphocyte reaction
Responder lymphocytes from FVB mouse bone marrow were mixed with irradiated (30 Gy)–stimulator B6 spleen cells. Proliferation of responder lymphocytes was measured by a chromogenic substrate (MTS to Formazan production, CellTiter 96 Aqueous One Solution; Promega) after 3 days of culture.
Primary murine ECs
Mouse capillary endothelial cells (ECs) were obtained as described.22 Mice were injected subcutaneously with 500 μL of growth-factor reduced Matrigel (BD Biosciences) supplemented with vascular endothelial growth factor (0.1 μg/mL; BD Biosciences), basic fibroblast growth factor (1 μg/mL; Invitrogen), and Liquemin (20 UI/mL; Roche Diagnostics). After 7 days, the Matrigel pellet was enzymatically dispersed and ECs were cultured in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered Dulbecco modified Eagle medium supplemented with 50 μg/mL EC growth factor (50 μg/mL; Sigma-Aldrich), 20% fetal calf serum (HyClone Laboratories), Liquemin (20 IE/mL; Roche), and penicillin/streptomycin (Invitrogen) on gelatin (Sigma-Aldrich)–coated dishes.
Quantitative real-time PCR
RNA was extracted from murine primary ECs (RNeasy Mini or Micro Kit) and quantified. Reverse transcription was performed with Improm-II enzyme (Promega) in the presence of ribonuclease inhibitors. After genomic DNA removal with DNase I (GE Healthcare Bio-Sciences), quantitative real-time polymerase chain reaction (PCR) was performed on ABI Prism 7500 (Applied Biosystems) with SYBR Green PCR Master Mix (Applied Biosystems) and the following primers (Operon Biotechnologies) were used: Gas6 5′-CGAGTCTTCTCACACTGTGCTGTT-3′ and 5′-GCACTCTTGATATCGTGGATAGAAATAC-3′; Axl 5′-TCATGTGAAGCCCACAATGC-3′ and 5′-GGAGCACTGTGATGGTGGCT-3′; Tyro3 5′-GTGAAGCCCGCAACATAAAAG-3′ and 5′-GGTGCTTGAAGGCG-AACAAT-3′; Mer 5′-CGCCAAGGCCGCATT-3′ and 5′-TCGGTCCGCCAGGCT-3′, GAPDH 5′-CAACGGGAAGCCCATCAC-3′ and 5′-CGGCCTCACCCCATTTG-3′. Melt-curve was performed together with sample where the reverse transcriptase was absent to control that neither genomic DNA nor unspecific products were amplified.
E-selectin expression and transmigration assay
Primary murine ECs were stimulated by murine TNF-α (100 ng/mL; R&D Systems) in 96-well plates. E-selectin expression was determined at the cell surface by enzyme-linked immunosorbent assay. Transmigration experiments were performed in 24-well plates with transwell inserts (8-μm pore size; BD Biosciences). Plastic membranes coated with gelatin (Sigma-Aldrich) were covered with ECs. ECs were then stimulated or not for 4 hours with murine TNF-α (100 ng/mL). WT splenic mononuclear cells were placed in the upper transwell chamber in the presence of 15 ng/mL TNF-α. The number of cells in the upper and lower transwell chambers was assessed by a cell counter.
Statistical analysis
Liver infiltration, mixed lymphocyte reaction (MLR), E-selectin expression, and transmigration data were analyzed by 1-way analysis of variance followed by Bonferroni post test to compare differences among the groups; histopathology score and relative expression of Gas6 receptors by PCR were compared using the unpaired 2-tailed t test. GVHD clinical scores were analyzed by a 2-way analysis of variance for repeated measures with Bonferroni post tests; Kaplan-Meier curves were analyzed by a log-rank test. All analyzes were carried out using GraphPad Prism, Version 5.01 (GraphPad Software). P less than .05 was considered statistically significant.
Results
Loss of Gas6 protected recipient mice from non–T cell–depleted allogeneic BMT against hepatic GVHD
We compared the ability of WT and Gas6−/− non–T cell–depleted BMCs from FVB mice (H-2q) to induce GVHD in major histocompatibility complex (MHC) completely mismatched WT and Gas6−/− B6 mice (H-2b). WT and Gas6−/− B6 mice received BMCs from either WT or Gas6−/− FVB mice (H-2q). On day 28, recipients did not present any weight loss, hair loss, skin lesions, or decreased motor activity. They were killed, and chimerism was demonstrated in both groups of mice by the expression of the donor's H-2q haplotype in recipient BMCs (Figure 1A).
Loss of Gas6 protected recipient mice from non–T cell–depleted allogeneic BMT against hepatic GVHD. C57BL/6 mice received total body irradiation and bone marrow cells (BMCs) from FVB mice; recipients were killed at day 28. (A) Full bone marrow chimerism of recipient. Expression of H-2q (left panel) and H-2b (right panel) in a recipient 28 days after bone marrow transplantation (BMT) was determined by flow cytometry (in red). A C57BL/6 mouse that was not transplanted is shown (black line) as control. (B) Liver histology: hematoxylin and eosin staining. Arrow indicates infiltration of portal spaces by mononuclear cells. Scale bar represents 100 μm. (C) Percentage of infiltrated portal spaces are mean ± SEM (n = 5-10; analysis of variance, *P < .05, **P < .01, ***P < .001); WT (■) and Gas6−/− recipients (□). (D) Apoptotic cells in portal spaces were TUNEL+ (brown dark precipitate). Scale bar represents 100 μm.
Loss of Gas6 protected recipient mice from non–T cell–depleted allogeneic BMT against hepatic GVHD. C57BL/6 mice received total body irradiation and bone marrow cells (BMCs) from FVB mice; recipients were killed at day 28. (A) Full bone marrow chimerism of recipient. Expression of H-2q (left panel) and H-2b (right panel) in a recipient 28 days after bone marrow transplantation (BMT) was determined by flow cytometry (in red). A C57BL/6 mouse that was not transplanted is shown (black line) as control. (B) Liver histology: hematoxylin and eosin staining. Arrow indicates infiltration of portal spaces by mononuclear cells. Scale bar represents 100 μm. (C) Percentage of infiltrated portal spaces are mean ± SEM (n = 5-10; analysis of variance, *P < .05, **P < .01, ***P < .001); WT (■) and Gas6−/− recipients (□). (D) Apoptotic cells in portal spaces were TUNEL+ (brown dark precipitate). Scale bar represents 100 μm.
Histologic studies indicated that hepatic lesions in Gas6−/− recipients were less severe than those in WT recipients, as documented by a considerable reduction in number and size of portal space leukocyte infiltrates in Gas6−/− recipients (n = 5-12, P < .01, Figure 1B-C). Skin and intestine were free of GVHD lesions (data not shown). Moreover, the reduced lesions in Gas6−/− recipients were comparable regardless of the donor cell genotype (Figure 1B-C). Apoptosis of liver cells in portal spaces was also substantially reduced in Gas6−/− mice (Figure 1D), indicating that lack of Gas6 in the recipient protected from liver damage. Thus, Gas6 deficiency in recipients of non–T cell–depleted allogeneic transplantation alleviated GVHD in the liver.
Loss of Gas6 conferred protection from lethal acute GVHD
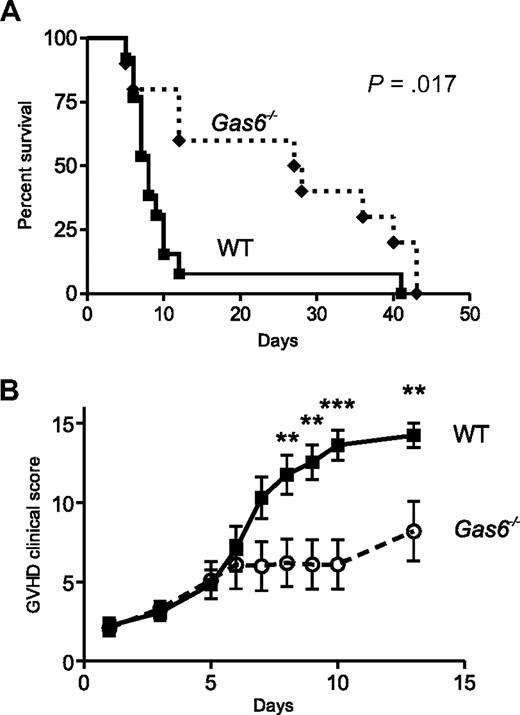
To confirm that the loss of Gas6-protected recipients from allogeneic BMT against GVHD, we used a model of lethal acute GVHD, in which a fixed number of donor T cells was transplanted. Bone marrow isolated from donor mice was T cell–depleted. Because the donor genotype was not responsible for the protection in the model of non–T cell–depleted allogeneic BMT, only WT donors were used for the lethal acute GVHD model. Each lethally irradiated recipient mouse (Balb/C, H-2d) with a WT or Gas6−/− genotype received 5 × 106 T cell–depleted BMCs and 0.5 × 106 WT splenic T cells from a pool of full MHC-mismatched WT donors (B6, H-2b). As represented in Figure 2A, 92% of the WT versus 20% of the Gas6−/− recipients given allogeneic spleen cells died within 10 days after transplantation. Overall survival was significantly improved in Gas6−/− than WT recipients (n = 10 and 13, respectively; P = .017; Figure 2A). In this model of GVHD, clinical signs of acute GVHD became apparent within 6 days and were more prominent in WT than Gas6−/− recipients (P = .011, Figure 2B). Syngeneic controls were WT and Gas6−/− Balb/C mice that received 5 × 106 WT T cell–depleted BMCs and 0.5 × 106 WT splenic T cells from a pool of WT syngeneic donors (Balb/C). All these mice survived after BMT and did not develop any clinical signs of acute GVHD (data not shown).
Loss of Gas6 conferred protection from lethal acute GVHD. Bone marrow isolated from donor mice was T cell–depleted. Each lethally irradiated recipient mouse (Balb/C, H-2d) with a wild-type (WT) or Gas6−/− genotype received 5 × 106 T cell–depleted BMCs from a pool of full MHC-mismatched WT donors (C57BL/6, H-2b). We infused 0.5 × 106 WT splenic donor T cells to WT or Gas6−/− recipient mice. (A) Kaplan-Meier plots of the survival of WT and Gas6−/− mice. Survival after lethal acute graft-versus-host disease (GVHD) was improved in Gas6−/− mice compared with WT mice (log-rank test, n = 10 and 13, respectively, P > .017). (B) Clinical signs of GVHD scored daily after transplantation according to Cooke et al.19 A score of 3 was assigned to mice that died in the course of the experiment. Two-way analysis of variance for repeated measures; n = 13 for WT and 10 for Gas6−/−, P = .012; followed by Bonferroni post tests for specific time points, **P < .01, ***P < .001.
Loss of Gas6 conferred protection from lethal acute GVHD. Bone marrow isolated from donor mice was T cell–depleted. Each lethally irradiated recipient mouse (Balb/C, H-2d) with a wild-type (WT) or Gas6−/− genotype received 5 × 106 T cell–depleted BMCs from a pool of full MHC-mismatched WT donors (C57BL/6, H-2b). We infused 0.5 × 106 WT splenic donor T cells to WT or Gas6−/− recipient mice. (A) Kaplan-Meier plots of the survival of WT and Gas6−/− mice. Survival after lethal acute graft-versus-host disease (GVHD) was improved in Gas6−/− mice compared with WT mice (log-rank test, n = 10 and 13, respectively, P > .017). (B) Clinical signs of GVHD scored daily after transplantation according to Cooke et al.19 A score of 3 was assigned to mice that died in the course of the experiment. Two-way analysis of variance for repeated measures; n = 13 for WT and 10 for Gas6−/−, P = .012; followed by Bonferroni post tests for specific time points, **P < .01, ***P < .001.
Effects of Gas6 deficiency on acute lethal GVHD-associated histopathology
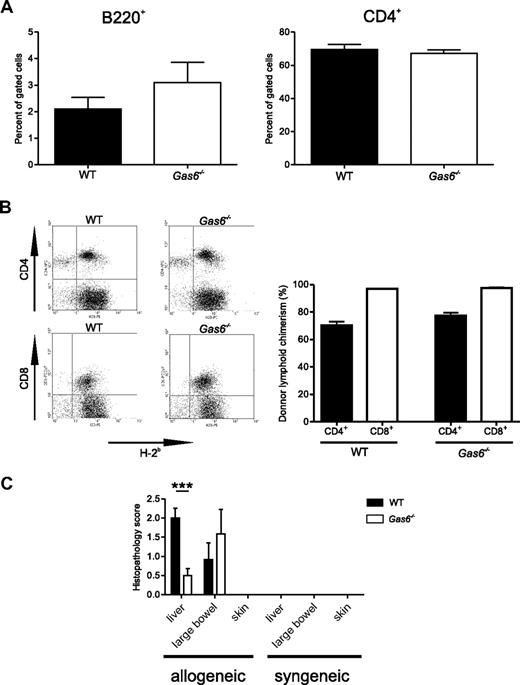
To evaluate the effect of Gas6 on histopathology of GVHD, tissue sections of target organs (skin, liver, and large bowel) were prepared on day 6, a time point corresponding to the peak time of donor T-cell expansion23 and preceded the death phase of WT mice. Engraftment of the lymphoid compartment was not different in WT and Gas6−/− recipients as all mice displayed similar amounts of circulating donor's B cells (B220+/H-2b+) and donor's CD4+ T cells (CD4+/H-2b+; Figure 3A). Donor's CD8+ T cells (CD8+/H-2b+) were nearly not detected in peripheral blood either from WT or Gas6−/− recipients, indicating that these cells already entered secondary lymphoid organs and target organs. In the spleen, flow cytometric analysis revealed that approximately 75% of CD4+ cells were from donor origin and that almost all the CD8+ cells were from donor origin (H-2b+; Figure 3B). The chimerism was comparable in WT and Gas6−/− for both the CD4 and CD8 spleen compartments (n = 6 or 7, P > .05, Figure 3B). In the liver, leukocyte infiltrates in the portal spaces were larger and more diffuse in the WT than in the Gas6−/− recipients with acute lethal GVHD (n = 6, P < .001, Figure 3C). The large bowel from WT and Gas6−/− recipients exhibited comparable dilatation, flattening of the villi, elevation and atrophy of the crypts, and leukocyte infiltrates. Consequently, histopathologic scores for the large bowel were comparable in both genotypes (Figure 3C). No sign of GVHD was observed in the skin in both genotypes. Syngeneic controls from either WT or Gas6−/− genotype did not display any liver, skin, or bowel damage on day 6 (histopathologic score = 0 for both genotypes, Figure 3C). Thus, Gas6 deficiency in mice undergoing acute lethal GVHD limited leukocyte infiltration in the liver.
Effects of Gas6 deficiency on acute lethal GVHD-associated histopathology. Lethally irradiated recipients from allogeneic T cell–depleted BMT that received 0.5 × 106 WT splenic donor T cells were killed at day 6. (A) Flow cytometric analysis of recipient circulating cells on day 6. Left panel: B220-H-2b double-positive cells gated to the mononuclear compartment; right panel: CD4+ cells gated to the H-2b mononuclear compartment. No significant difference is shown between WT and Gas6−/−. (B) Left panel: representative flow cytometric analysis of spleen cells on day 6. Right panel: donor lymphoid chimerism in the CD4+ or CD8+ splenic compartment of WT and Gas6−/− recipient mice (n = 6 or 7; Student t test, P > .05). (C) Semiquantitative analysis of tissue pathology at day 6 after BMT according to Cooke et al19 and Hill et al20 (Student t test, n = 6, ***P < .001).
Effects of Gas6 deficiency on acute lethal GVHD-associated histopathology. Lethally irradiated recipients from allogeneic T cell–depleted BMT that received 0.5 × 106 WT splenic donor T cells were killed at day 6. (A) Flow cytometric analysis of recipient circulating cells on day 6. Left panel: B220-H-2b double-positive cells gated to the mononuclear compartment; right panel: CD4+ cells gated to the H-2b mononuclear compartment. No significant difference is shown between WT and Gas6−/−. (B) Left panel: representative flow cytometric analysis of spleen cells on day 6. Right panel: donor lymphoid chimerism in the CD4+ or CD8+ splenic compartment of WT and Gas6−/− recipient mice (n = 6 or 7; Student t test, P > .05). (C) Semiquantitative analysis of tissue pathology at day 6 after BMT according to Cooke et al19 and Hill et al20 (Student t test, n = 6, ***P < .001).
Gas6 deficiency did not affect donor T-cell proliferation and stimulating capacities of APCs
The fact that GVHD was dampened in the Gas6−/− recipient, independently of the Gas6 genotype of the donor, indicated that donor cells were not implicated in the protective mechanism. An additional argument against a role for donor's T cells is that they did not express Gas6 and its receptors (not shown). This is in agreement with a previous study.7
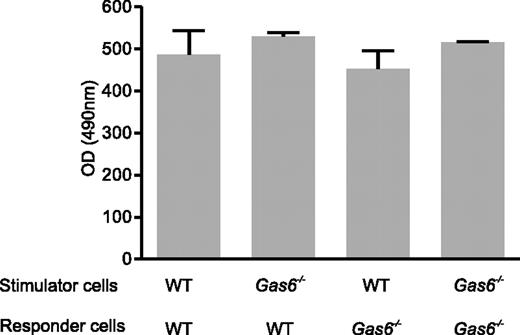
Because T cells proliferated when stimulated by APCs, which expressed Gas6 and its receptors,8 we assessed the T-cell proliferative response to allogeneic APC stimulation in WT and Gas6−/− mice by performing MLR. Stimulator cells from WT and Gas6−/− B6 (H-2b) mice induced comparable proliferation responses of responder cells from WT and Gas6−/− FVB (H-2q) mice (Figure 4). Thus, Gas6 deficiency did not affect donor T-cell proliferation and stimulating capabilities of APCs.
Gas6 deficiency did not affect donor T-cell proliferation and stimulating capacities of APCs in MLR. WT or Gas6−/− responder lymphocytes isolated from FVB mice bone marrow were mixed with irradiated (30 Gy) stimulator spleen cells (WT or Gas6−/−) with a different H-2D locus. Proliferation of responder lymphocytes was measured by a chromogenic substrate after 3 days of culture. Data are mean ± SEM; n = 9. P > .05 (analysis of variance).
Gas6 deficiency did not affect donor T-cell proliferation and stimulating capacities of APCs in MLR. WT or Gas6−/− responder lymphocytes isolated from FVB mice bone marrow were mixed with irradiated (30 Gy) stimulator spleen cells (WT or Gas6−/−) with a different H-2D locus. Proliferation of responder lymphocytes was measured by a chromogenic substrate after 3 days of culture. Data are mean ± SEM; n = 9. P > .05 (analysis of variance).
Gas6 receptors were expressed by murine ECs
Primary ECs were isolated from Matrigel plugs inserted subcutaneously in WT and Gas6−/− mice. Gene expression of Tyro3, Axl, and Mer was quantified by quantitative real-time PCR, relative to the expression of GAPDH levels. Transcripts of the 3 Gas6 receptors (Tyro3, Axl, and Mer) were expressed in ECs of both WT and Gas6−/− mice. Accumulation of Axl mRNA did not differ between the 2 genotypes, whereas Tyro3 and Mer mRNA levels were higher in Gas6−/− than in WT mice (Figure 5).
Gas6 and its receptors were expressed by murine endothelial cells. Gene expression by primary endothelial cells of the 3 Gas6 receptors (Axl, Tyro3, and Mer) by relative quantitative real-time PCR relative to the expression of GAPDH levels is represented (mean ± SEM, n = 3). *P < .05 (Student t test).
Gas6 and its receptors were expressed by murine endothelial cells. Gene expression by primary endothelial cells of the 3 Gas6 receptors (Axl, Tyro3, and Mer) by relative quantitative real-time PCR relative to the expression of GAPDH levels is represented (mean ± SEM, n = 3). *P < .05 (Student t test).
Resistance of Gas6−/− ECs to TNF-α activation prevented donor T-lymphocyte transmigration
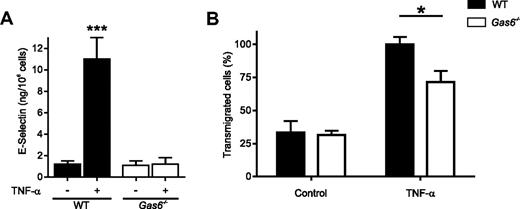
We hypothesized that the protection of hepatic GVHD might be the result of defects in cytokine-induced expression of adhesion molecules by Gas6−/− ECs, thereby impairing T-lymphocyte transmigration into the liver. We therefore assessed the response of primary ECs isolated from WT and Gas6−/− mice to TNF-α because TNF-α is produced by alloreactive T lymphocytes in vivo.24 Gas6−/− ECs were resistant to TNF-α by failing to up-regulate the expression of the adhesion molecules VCAM-1 and ICAM-1, to produce interleukin-1β (IL-1β) and IL-6 and to secrete the content of Weibel-Palade bodies25 (A.A.-S., unpublished observations, February 2001). These data were extended here by assessing E-selectin expression (Figure 6A). Moreover, lymphocytes transmigrated less efficiently through TNF-α–treated Gas6−/− ECs compared with WT (Figure 6B). This resistance to TNF-α suggested that the effect of Gas6 on vascular endothelial function was critical for normal leukocyte trafficking in GVHD.
Resistance of Gas6−/− endothelial cells to TNF-α activation prevented donor T-lymphocyte transmigration. (A) Expression of E-selectin by activated endothelial cells (ECs). Primary ECs isolated from WT and Gas6−/− mice were stimulated by murine TNF-α (100 ng/mL) in 96-well plates. E-selectin expresion was determined at the cell surface by enzyme-linked immunosorbent assay. Data are mean ± SEM; n = 10. ***P < .001 (analysis of variance). (B) Transmigration experiments were performed in transwells with gelatin-coated membrane inserts covered with ECs isolated from WT or Gas6−/− mice. ECs were then stimulated or not for 4 hours with murine TNF-α (100 ng/mL). WT splenic mononuclear cells were placed in the upper transwell chamber in the presence of 15 ng/mL TNF-α. The number of cells in the upper and lower transwell chambers was assessed by a cell counter. Data are percentage of transmigrated cells (mean ± SEM, n = 3). *P < .05 (analysis of variance).
Resistance of Gas6−/− endothelial cells to TNF-α activation prevented donor T-lymphocyte transmigration. (A) Expression of E-selectin by activated endothelial cells (ECs). Primary ECs isolated from WT and Gas6−/− mice were stimulated by murine TNF-α (100 ng/mL) in 96-well plates. E-selectin expresion was determined at the cell surface by enzyme-linked immunosorbent assay. Data are mean ± SEM; n = 10. ***P < .001 (analysis of variance). (B) Transmigration experiments were performed in transwells with gelatin-coated membrane inserts covered with ECs isolated from WT or Gas6−/− mice. ECs were then stimulated or not for 4 hours with murine TNF-α (100 ng/mL). WT splenic mononuclear cells were placed in the upper transwell chamber in the presence of 15 ng/mL TNF-α. The number of cells in the upper and lower transwell chambers was assessed by a cell counter. Data are percentage of transmigrated cells (mean ± SEM, n = 3). *P < .05 (analysis of variance).
Discussion
The initiation of GVHD depends on the capability of allogeneic T cells to traffic to target organs.26 This traffic is dependent on T-cell activation state but also on the activation state of target organs. The conditioning regimen and the GVH reaction up-regulate inflammatory cytokines, enhance expression of MHC and costimulatory molecules by APCs, and increase expression of adhesion molecules, key molecules for T-cell diapedesis. The release of inflammatory cytokines, including TNF-α and IL-1, can accelerate acute GVHD onset.27,28 Inhibition of these cytokines does not affect acute GVHD initiation but the acute GVHD effector phase, resulting in reduced bowel and liver alloreactive T-cell infiltration.29 Therefore, TNF-α and IL-1, together with effects further downstream, are pivotal in rendering acute GVHD target organs permissible for alloreactive T-cell infiltration as opposed to non-GVHD targets.24,27,30-33
Here we provided evidence for a novel role of Gas6 in acute GVHD. Gas6−/− mice were protected against acute GVHD in 2 different experimental models. In the first model, where WT or Gas6−/− mice received allogeneic non–T cell–depleted BMCs, hepatic GVHD was alleviated in Gas6−/− recipients regardless of the donor genotype, compared with WT recipients. These data were corroborated using a model of acute lethal GVHD, in which the number of transplanted donor T cells was determined. In this model, clinical signs indicated that GVHD was less severe in Gas6−/− than in WT recipients and the overall survival was significantly improved in Gas6−/− recipients. In both models, T-cell infiltration was more prominent and diffuse in the liver of Gas6−/− than WT recipients. In the model of transplantation of allogeneic non–T cell–depleted BMCs, involvement of organs other than the liver was not observed. In the model of acute lethal GVHD, both large bowel and liver displayed histologic signs of GVHD. However, histologic damages in large bowel were comparable in WT and Gas6−/− recipients.
The fact that GVHD was dampened in Gas6−/− recipient, independently of the Gas6 genotype of the donor, indicated that donor cells were not implicated in the protective mechanism. An additional argument against a role for donor's T cells is that they do not express Gas6 and its receptors.7
Which cell type of the recipient is involved in the GVHD mediated by Gas6? Because T cells proliferate when stimulated by APCs, expressing Gas6 and its receptors,8 we assessed the T-cell proliferative response to allogeneic APC stimulation in WT and Gas6−/− mice by performing MLR. Stimulator cells from WT and Gas6−/− B6 (H-2b) mice induced comparable proliferation responses of responder cells from WT and Gas6−/− FVB (H-2q) mice. These data indicated that Gas6 deficiency did not affect donor T-cell proliferation and stimulating capabilities of APCs.
We therefore considered recipient ECs as a potential cell candidate for Gas6-mediated GVHD. We found that Gas6 (data not shown) and its 3 receptors (Tyro3, Axl, and Mer) were expressed by murine ECs. These data corroborate the recent publication by Tjwa et al where Gas6 has been shown to be secreted by murine ECs and to play an important role in the regulation of the inflammatory response of the vessel wall.25 We observed that Mer and Tyro3 but not Axl were constitutively overexpressed in Gas6−/− ECs. WT and Gas6−/− erythroblasts express comparable amounts of Axl, Mer, and Tyro3.34 Axl but not Mer and Tyro3 expression increases in dendritic cells subsequent to activation of Toll-like receptors.35 Previous work has revealed that Gas6 and Axl are expressed by human ECs.9,10 However, to our knowledge, there is no previous report of Mer and Tyro3 overexpression in Gas6−/− ECs in steady-state conditions. Based on this observation and recent work highlighting the central role of Gas6 receptor signaling in the inflammatory response to pathogens by APCs,35 we can speculate that the lack of Gas6 in ECs drives the up-regulation of expression of Mer and Tyro3. Engagement of Mer and Tyro3 signaling pathways may result in activation of the negative regulators of inflammation by ECs given the interaction of those proteins with suppressor of cytokine signaling 1 (SOCS1) and SOCS3 proteins. Thus, SOCS1 and SOCS3 may inhibit both Toll-like receptors and cytokine receptor cascades in ECs, thereby ending the inflammatory response by these cells.
Recently, Shao et al have demonstrated that mice lacking Mer expression are almost completely protected from the development of an autoimmune chronic graft-versus-host reaction.36 Mer expression was evidenced on B cells and overexpressed during autoimmune chronic graft-versus-host reaction, indicating an important role of Mer in B-cell autoreactivity and in the pathogenesis of systemic lupus erythematosus.36 We (A.A.-S., unpublished observations, February 2001) and others25 have demonstrated that Gas6−/− ECs fail to up-regulate the expression of the adhesion molecules VCAM-1 and ICAM-1, to produce IL-1β and IL-6 and to secrete the content of Weibel-Palade bodies when exposed to TNF-α. These data were extended here by showing that E-selectin expression was significantly reduced in Gas6−/− mice compared with WT in the same conditions.
We showed here that lymphocytes transmigrated less efficiently through TNF-α–treated Gas6−/− ECs compared with WT. This resistance to TNF-α suggests that the effect of Gas6 on vascular endothelial function is critical for normal leukocyte trafficking in GVHD. Transmigration is dependent on lymphocyte integrins, junctional adhesion molecules, and other intercellular junctions.37 Integrins and selectins are important for cell arrest on ECs. Treating mice before transplantation with both anti-CD62L (L-selectin) and anti-α4 (anti-integrin) delays GVHD, predominantly by retarding homing of donor T cells to mesenteric lymph nodes.38 However, inhibition of E-selectin has no effect on GVHD induced by CD8+ T-cell–endothelium interactions in the large bowel.39 It has been shown that ICAM-1 and VCAM-1 are up-regulated on host conditioning, facilitating T-cell entry into acute GVHD targets. Induced adhesion molecules can also enhance retention of alloreactive effector T cells in target organs. Early up-regulation of ICAM-1 is associated with an increase of infiltrating cells after BMT. A combination of antibodies against leukocyte function antigen-1 (heterodimer formed by CD11b and CD18, binding ICAM) and ICAM-1 prevents GVHD.40 However, ICAM-1−/− mice, used as recipient, are not protected from liver and bowel inflammation in a GVHD model. The mortality is even exaggerated in these mice.41 Antibodies directed against VLA-4 prevent T-cell infiltration of the liver, indicating a possible novel therapeutic role of these antibodies for GVHD.42
Our data give further support to strategies that target recipient ECs by blocking their activation and thereby alloreactive T lymphocyte transmigration and consecutive target GVHD organ damage. By targeting the activation of ECs rather than activation of T lymphocytes and APCs to treat GVHD, immune response would be preserved to protect recipients against infections and to promote graft-versus-leukemia effect.
In conclusion, the lack of Gas6 in recipient mice alleviated hepatic GVHD. These findings correlated with differences in the migration of T cells across TNF-α–treated ECs in vitro. Our data thus provide a rationale for considering Gas6 pathway as a potential nonimmunosuppressive target to minimize hepatic GVHD in patients receiving allogeneic hematopoietic stem cell transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Monica Azevedo, Nathalie Flores, and Raphaël Miguet for technical assistance.
This work was supported by the Dinu Lipatti-Dr Henri Dubois-Ferrière Foundation, the Swiss National Foundation for Scientific Research (grants 3100-064027.00, 3234-066307.01, Marie-Heim-Vögtlin; 3232-066350.01, SCORE; 3200-066351.01, PP00B-106690/1, PP00P3-123430, and 3100A0-105872), the Roche Research Foundation, and the Swiss League for Cancer Research (OCS 01775-08-2005).
National Institutes of Health
Authorship
Contribution: L. Burnier designed and performed the experiments and wrote the paper; F.S., L.K., A.C.B., R.S., and L. Baudino performed research; F.B., J.-M.H., P.C., M.S., and S.I. participated in writing the paper; and A.A.-S. designed and organized the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Angelillo-Scherrer, Service and Central Laboratory of Hematology, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Rue du Bugnon 46, CH-1011 Lausanne, Switzerland; e-mail: Anne.Angelillo-Scherrer@chuv.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal