Abstract
Patients with Wiskott-Aldrich syndrome (WAS) have numerous immune cell deficiencies, but it remains unclear how abnormalities in individual cell types contribute to the pathologies of WAS. In T cells, the WAS protein (WASp) regulates actin polymerization and transcription, and plays a role in the dynamics of the immunologic synapse. To examine how these events influence CD4 function, we isolated the WASp deficiency to CD4+ T cells by adoptive transfer into wild-type mice to study T-cell priming and effector function. WAS−/− CD4+ T cells mediated protective T-helper 1 (Th1) responses to Leishmania major in vivo, but were unable to support Th2 immunity to Nippostrongylus brasiliensis or L major. Mechanistically, WASp was not required for Th2 programming but was required for Th2 effector function. WAS−/− CD4+ T cells up-regulated IL-4 and GATA3 mRNA and secreted IL-4 protein during Th2 differentiation. In contrast, cytokine transcription was uncoupled from protein production in WAS−/− Th2-primed effectors. WAS−/− Th2s failed to produce IL-4 protein on restimulation despite elevated IL-4/GATA3 mRNA. Moreover, dominant-negative WASp expression in WT effector T cells blocked IL-4 production, but had no effect on IFNγ. Thus WASp plays a selective, posttranscriptional role in Th2 effector function.
Introduction
The Wiskott-Aldrich syndrome protein (WASp) is expressed in all hematopoietic cells and is a key regulator of the actin cytoskeleton via activation of the Arp2/3 complex.1 In humans, mutations that cause WASp deficiency or truncation lead to Wiskott-Aldrich syndrome (WAS), a rare X-linked recessive disease characterized by the triad of eczema, thrombocytopenia, and immune deficiency.2,3 Patients suffer from both humoral and cellular immune deficiencies and are susceptible to recurrent pyogenic and viral infections. Functional defects in both innate and adaptive arms of the immune response likely conspire to disrupt protective immunity.
In CD4+ T cells, WASp plays a role in actin polymerization and T-cell receptor (TCR)–mediated transcriptional activation. Lymphocytes deficient in WASp proliferate poorly to TCR signals with defects in IL-2 production.4-8 NFAT transcription after TCR activation is potentiated in WASp mutants that disrupt actin polymerization, suggesting that WASp-mediated transcriptional activation is independent of WASp's role in actin polymerization.7 Despite a critical role for actin polymerization in T-cell activation, the function of WASp is still controversial. Although defects in synapse formation have been demonstrated,9 WASp-deficient T cells can establish and sustain synapse formation in some settings,6 indicating that a WASp requirement for synapse formation is not absolute. Recently, a requirement for WASp not in the formation of the synapse but in the dynamics of synapse reformation after migration has been proposed.10 How such defects in proximal signaling affect subsequent T-cell function is not well understood. T-cell lines from patients with WAS exhibit impaired IFNγ and to a lesser extent IL-4 responses at the transcriptional level.11 We previously reported a role for WASp in posttranscriptional regulation of effector function through a defect in TCR signals for CD4+ T-cell IFNγ secretion that correlated with the loss of IFNγ localization at the immunologic synapse.12 Similar defects in lytic granule polarization and natural killer (NK)–cell cytotoxicity have been noted in the absence of the WAS interacting protein (WIP).13 Therefore, WASp may regulate multiple downstream signal pathways that affect T-cell function at different stages in the development of an effector response.
To provide insight into the pathology of WAS, we examined the role of WASp in CD4+ T-cell effector function. Adoptive transfer of CD4+ T cells from WASp-deficient mice into WASp-sufficient T cell–deficient hosts showed that T-helper 1 (Th1) and Th2 development was differentially sensitive to the loss of WASp. Whereas WASp-deficient CD4+ T cells mounted a protective Th1 response in vivo, protective Th2 responses in vivo were severely impaired. Similarly, disruption of WASp activity in Th2-primed wild-type (WT) cells using retroviral expression of a dominant-negative WASp abrogated IL-4 production, demonstrating a nonredundant role for WASp in Th2 function. In contrast, IL-4 production from basophils and γδ T cells remained intact in the absence of WASp. We reveal a posttranscriptional requirement for WASp in CD4 Th2 cytokine production.
Methods
Mice
WASp-deficient mice were backcrossed 10 generations to C57BL/6 and BALB/c. Rag-2−/− mice were purchased from The Jackson Laboratory. Mice were age- and sex-matched and 6 to 8 weeks old. Mice were maintained in the pathogen-free animal facility at the University of Rochester School of Medicine and Dentistry. All mice studies were performed under University of Rochester Committee on Animal Resources–approved protocols.
Cell purification
CD4+ T cells were enriched by antibody/complement lysis12 and sorted for naive CD62LhighCD44low CD4+ T cells (BD FACSAria). T cell–depleted splenocytes (antigen-presenting cells [APCs]) were generated by complement lysis of Thy1+ cells and exposed to 25 Gy (2500 rad) of irradiation.
In vitro T-cell priming
Naive CD4+ T cells (106 cells/well) were cultured in plates coated with anti-TCRβ (H57.597; 0.5 μg/mL) and anti-CD28 (37N51.1; 2 μg/mL) mAbs and with addition of 10 U/mL IL-2 (recombinant human [rh] IL-2): neutral priming. Th1 polarizing: rhIL-2, 10 ng/mL recombinant murine (rm) IL-12, and 20 μg/mL anti–IL-4 mAb (11B11). Th2 polarizing: rhIL-2, 50 ng/mL rmIL-4, and 50 μg/mL anti-IFNγ mAb (XMG1.2). Th17 priming: 1 ng/mL TGFβ and 10 μg/mL IL-6. On day 5 after priming, cells were restimulated with plate-bound anti-TCRβ mAb with or without rhIL-2. Alternatively, naive CD4+ T cells were cultured with WT APCs (1:10 T cell/APC) and 1μM OVA323-339 peptide.
Flow cytometry
Innate cells.
Cells from the lungs of Nippostrongylus brasiliensis (Nb)–infected mice were isolated by physical disruption. By 10-color fluorescence-activated cell sorter (FACS) analysis cells were gated on: basophils, CD4−, B220−, GR-1−, FcϵRIα+, SSClow, FSClow; mast cells, CD4−, B220−, GR-1low, FcϵRIα+, SSChigh, FSChigh; eosinophils, CD4−, B220−, GR-1low, FcϵRIα−, SSChigh, FSClow. Innate cell types were confirmed by FACS sorting on size and the designated markers, followed by histology.
Phospho-flow.
BD Pharmingen phospho-STAT6 reagents (J71-773.58.11) or phospho-ERK (phosphorylated Y641, T2002/Y204) were used.
Phalloidin.
Th2-primed cells were stimulated with plate-bound anti-TCRβ for 15 minutes, fixed with paraformaldehyde, treated with 0.1% Triton X-100, and incubated with biotinylated phalloidin and streptavidin-PE before FACS analysis.
Cytokines
Secreted cytokines were measured using standard enzyme-linked immunosorbent assay (ELISA). Intracellular staining was performed using the BD Pharmingen Kit. Golgi Plug was added in the final 4 hours of culture. CD4+ cytokine secreting cells were analyzed by Miltenyi Biotec magnetic-activated cell sorter (MACS) Mouse Cytokine Secretion Assay as described.14 In vivo capture for IL-4 was performed using the BD mouse IL-4 in vivo capture assay: capture Ab (10 μg/mouse) was injected intravenously 24 hours before the day 10 harvest of Nb-infected mice. Free serum IL-13 and sIL-13Rα2 and the IL-13/IL-13Rα2 complex were measured, and the percentage saturation of serum sIL-13Rα2 with IL-13 was calculated as described.15
qRT-PCR
Quantitative real-time reverse transcriptase–polymerase chain reaction (qRT-PCR) was performed using real-time fluorogenic 5′-nuclease PCR using an ABI Prism 7700 Sequence BioDetector. Total RNA was extracted using TRIzol and reversed-transcribed using the Advantage RT-for PCR Kit. CD3δ was used as an endogenous control and unstimulated CD4+ T cells were used as the calibrator. TaqMan gene expression assays were as follows: IFN-γ, Mm00801778_m1; IL-4, Mm00445259_ml; IL-5, Mm00439646_ml; IL-13, Mm00434204_ml; Tbet (Tbx21), Mm00450960_ml; and GATA3, Mm00484683_ml.
Retroviral transfections
OTII+CD4+ T cells were cultured under Th2 and Th1 polarizing conditions in the presence of peptide (OVA323-339 1μM), WT APCs, and rhIL-2. On day 4, cells were infected with bicistronic retrovirus DN-WASp (WASΔVCA-IRES-GFP) or empty vector (-IRES-GFP) and cultured at 37°C for 36 hours total (virus was removed after 24 hours). CD4+GFP+ and CD4+GFP− populations were isolated by FACS.
Nippostrongylus brasiliensis infection
A total of 107 CD4+ T cells were transferred intravenously to TCR Ca−/− mice and infected 24 hours later subcutaneously with 500 infective N brasiliensis third-stage larvae. On infection day 10, the frequency of cytokine producers in lung and mesenteric lymph nodes (LNs) was measured by 18-hour enzyme-linked immunospot assay (ELISPOT) in the presence of Nb antigens. Live adult worms in the small intestine were counted using a dissecting microscope.16
Leishmania major infection
A total of 107 CD4+ T cells were transferred intravenously into Rag−/− or TCR Ca−/− mice and infected 24 hours later with 2.0 × 105L major promastigotes subcutaneously into the hind footpads or ear dermis. Infectious L major promastigotes were isolated from stationary cultures by negative selection using peanut agglutinin. Footpad swelling was measured with metric calipers. Popliteal LN cells were cultured ex vivo with soluble Leishmania antigen (SLA) for cytokine ELISPOT analysis at 6 hours.17 Parasite burden was determined by limiting dilution analysis.
Statistical analysis
A P value of less than .05 was considered statistically significant. The statistical tests used are documented in each figure legend.
Results
Nonredundant role for WASp in Th2 but not Th1 effector function
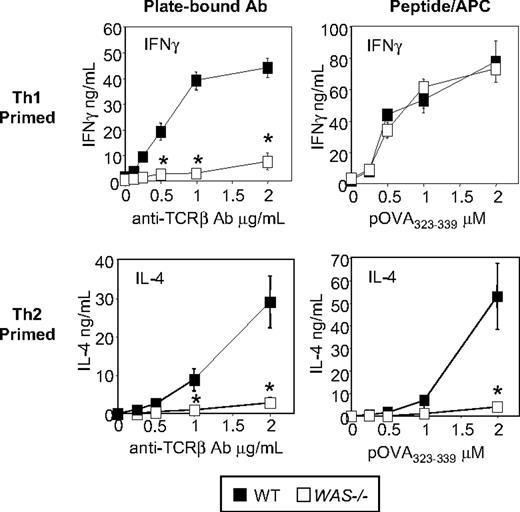
Our previous studies described a deficiency in IFNγ secretion in Th1-primed WAS−/− CD4+ T cells after TCR/CD28 activation.12 To investigate the role of WASp in CD4+ T-cell differentiation we generated Th1- and Th2-primed effector cells in vitro using naive OTII TCR transgenic CD4+ T cells. Primed cells were restimulated with plate-bound anti-TCRβ mAb or pOVA323-339/APCs (Figure 1). Activation through the TCR only failed to support IFNγ or IL-4 in the absence of WASp (Figure 1 left panels). However, the defect in IFNγ secretion observed on TCR stimulation of WAS−/− Th1 effectors was bypassed by additional signals provided from APC (Figure 1 right panel). In vitro priming and pOVA/APC restimulation of Th17 cells was similarly WASp-independent (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In contrast, activation by pOVA/APC failed to restore IL-4 production by Th2-primed WASp-deficient cells (Figure 1; right panel), indicating WASp plays a nonredundant role in IL-4 production by Th2 effectors.
Nonredundant role for WASp in IL-4 but not IFNγ production. WT and WAS−/− Th1- and Th2-primed OTII TCR Tg+ CD4 T cells were restimulated with plate-bound anti-TCRβ (left panels) or pOVA/APC (WT T-depleted splenocytes; right panels) for 24 hours, and the supernatants collected for cytokine detection by ELISA. Results represent 1 of at least 4 comparable experiments. *P ≤ .01 for differences between WT and WAS−/− T-cell cytokine production, Student t test. Error bars indicate mean and SEM.
Nonredundant role for WASp in IL-4 but not IFNγ production. WT and WAS−/− Th1- and Th2-primed OTII TCR Tg+ CD4 T cells were restimulated with plate-bound anti-TCRβ (left panels) or pOVA/APC (WT T-depleted splenocytes; right panels) for 24 hours, and the supernatants collected for cytokine detection by ELISA. Results represent 1 of at least 4 comparable experiments. *P ≤ .01 for differences between WT and WAS−/− T-cell cytokine production, Student t test. Error bars indicate mean and SEM.
Early events in Th2 differentiation are WASp-independent
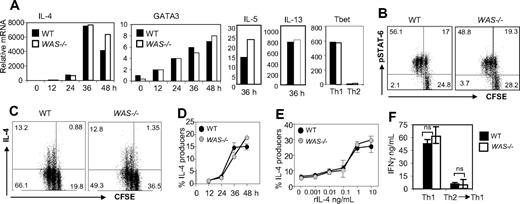
To examine early events in Th2 differentiation, naive C57BL/6 WAS−/− and WT T cells (CD4+CD62Lhigh CD44low) were primed under Th2 conditions (rIL-4 and anti-IFNγ). Exogenous IL-2 was provided to bypass WASp requirements for IL-2 production. As shown in Figure 2A, WAS−/− CD4+ T cells up-regulated IL-4, IL-5, IL-13, and GATA3 mRNA similarly to WT CD4+ T cells during the first 48 hours of Th2 priming and failed to induce Tbet expression (Figure 2A). In addition, WAS−/− and WT CD4+ T cells showed comparable IL-4-R signaling via phosphorylated STAT6 (Figure 2B). Early IL-4 mRNA was also detected under neutral priming conditions (IL-2 only), albeit 2 orders of magnitude lower, in both WT and WAS−/− CD4+ T cells (data not shown). There was also no aberrant expression of GATA3 mRNA in Th1-primed cultures (data not shown).
Early events in the Th2 differentiation program are WASp-independent. (A) WT and WAS−/− naive CD4+ T cells (CD4+CD62LhighCD44low) were loaded with CFSE and primed under Th2 (anti-IFNγ mAb and rIL-4) conditions with plate-bound anti-TCRβ and anti-CD28 Ab for 5 days. Cells were harvested at given time points, and mRNA was extracted for qRT-PCR. mRNA was normalized to CD3δ and expressed as fold change over unstimulated naive CD4+ cells. mRNA from WT and WAS−/− Th1 (anti–IL-4 mAb and rIL-12)–primed cells were collected at 48 hours as controls. Data from 1 representative experiment of 3. Results were not statistically different for all cytokine mRNAs tested over 3 independent experiments, by paired Student t test. (B) CFSE-labeled CD4+ T cells were harvested after 48 hours of primary Th2 stimulation and analyzed for phospho-STAT6 by FACS: dot plots are gated on CD4+ T cells, and quadrant numbers equal the percentage of CD4 T cells. Percentage of phospho-STAT6+ cells in negative control (Th1-primed) was less than 5%. (C) At 48 hours, IL-4 production was analyzed by the cytokine secretion assay (CSA) on gated live CD4+ T cells. Quadrant gates were set on “no catch” controls (< 0.5% IL-4+ for WT and WAS−/−); numbers in quadrants equal the percentage of CD4+ T cells. (D) Kinetic analysis of IL-4 production by CD4+ T cells during Th2 differentiation by CSA. (E) IL-4 dose response during initial cell culture; IL-4 effects determined by the induction of IL-4 production by CD4+ T cells by CSA 48 hours after primary stimulation. Results represent one of at least 4 comparable experiments. (F) WT and WAS−/− naive CD4+ T cells were primed under Th1 or Th2 conditions with pOVA/APC for 5 days. Th2 cells were primed a subsequent time under opposing Th1 conditions (anti–IL-4 mAb and rIL-12) with pOVA/APC. At 5 days later, cells were restimulated for 24 hours in the absence of exogenous cytokines with pOVA/APC. IFNγ ELISA analysis of collected supernatants 24 hours after restimulation, nonsignificant by Student t test. Error bars indicate mean and SEM.
Early events in the Th2 differentiation program are WASp-independent. (A) WT and WAS−/− naive CD4+ T cells (CD4+CD62LhighCD44low) were loaded with CFSE and primed under Th2 (anti-IFNγ mAb and rIL-4) conditions with plate-bound anti-TCRβ and anti-CD28 Ab for 5 days. Cells were harvested at given time points, and mRNA was extracted for qRT-PCR. mRNA was normalized to CD3δ and expressed as fold change over unstimulated naive CD4+ cells. mRNA from WT and WAS−/− Th1 (anti–IL-4 mAb and rIL-12)–primed cells were collected at 48 hours as controls. Data from 1 representative experiment of 3. Results were not statistically different for all cytokine mRNAs tested over 3 independent experiments, by paired Student t test. (B) CFSE-labeled CD4+ T cells were harvested after 48 hours of primary Th2 stimulation and analyzed for phospho-STAT6 by FACS: dot plots are gated on CD4+ T cells, and quadrant numbers equal the percentage of CD4 T cells. Percentage of phospho-STAT6+ cells in negative control (Th1-primed) was less than 5%. (C) At 48 hours, IL-4 production was analyzed by the cytokine secretion assay (CSA) on gated live CD4+ T cells. Quadrant gates were set on “no catch” controls (< 0.5% IL-4+ for WT and WAS−/−); numbers in quadrants equal the percentage of CD4+ T cells. (D) Kinetic analysis of IL-4 production by CD4+ T cells during Th2 differentiation by CSA. (E) IL-4 dose response during initial cell culture; IL-4 effects determined by the induction of IL-4 production by CD4+ T cells by CSA 48 hours after primary stimulation. Results represent one of at least 4 comparable experiments. (F) WT and WAS−/− naive CD4+ T cells were primed under Th1 or Th2 conditions with pOVA/APC for 5 days. Th2 cells were primed a subsequent time under opposing Th1 conditions (anti–IL-4 mAb and rIL-12) with pOVA/APC. At 5 days later, cells were restimulated for 24 hours in the absence of exogenous cytokines with pOVA/APC. IFNγ ELISA analysis of collected supernatants 24 hours after restimulation, nonsignificant by Student t test. Error bars indicate mean and SEM.
The Miltenyi cytokine secretion assay provides quantitative analysis of the frequency of live CD4+ IL-4–producing cells during differentiation (Figure 2C). Both WT and WAS−/− CD4+ T-cell cultures contained the same frequency of IL-4–secreting cells (13% of CD4+ T cells) 48 hours into the primary stimulation and similar kinetics (Figure 2D-E). Therefore, induction of Th2 gene transcription and early IL-4 protein production appears WASp-independent. Moreover, WAS−/− Th2-primed cells appeared committed to the Th2 lineage as secondary stimulation in Th1 conditions failed to reveal the potential for further differentiation (Figure 2F).
Defective Th2 cytokine production in WASp-deficient effector Th2 cells
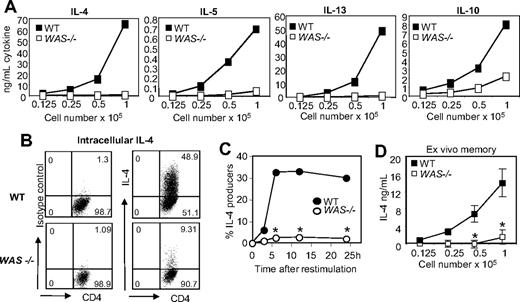
Despite no obvious defects in Th2 priming (Figure 2), WASp-deficient Th2 cells failed to produce IL-4 during restimulation (Figure 1). We found no evidence of differences in CD4+ T-cell death: WT and WAS−/− CD4+ T cells expanded 30.08-fold plus or minus 5.7-fold and 36.92-fold plus or minus 1.6-fold, respectively. IL-5 and IL-13, and to a lesser extent IL-10, were also impaired in the absence of WASp (Figure 3A). Similar defects were observed on C57BL/6 and BALB/c backgrounds (data not shown). At the single-cell level, there was a striking reduction in the frequency of IL-4 cells in WAS−/− Th2-primed CD4+ T cells compared with WT (Figure 3B-C) and reduced IL-4 per cell (mean fluorescence intensity [MFI] of the IL-4+ population: 149 for WT, 80 for WAS−/−). IL-4 production was not rescued by provision of IL-2 or CD28 costimulation (data not shown). WASp-deficient ex vivo memory CD4+ T cells (CD62LlowCD44high) also exhibited poor IL-4 responses to restimulation (Figure 3D), suggesting recall IL-4 responses to environmental antigen are similarly impaired.
Impaired cytokine production by WASp-deficient Th2-primed effectors. WT and WAS−/− naive CD4+ T cells were primed under Th2 conditions with plate-bound anti-TCRβ and anti-CD28 mAbs for 5 days. Cells were harvested and restimulated with plate-bound anti-TCRβ mAb. (A) ELISA from supernatants 24 hours after restimulation. (B) Th2-primed cells were restimulated with anti-TCRβ mAb for 24 hours, followed by intracellular cytokine staining. Numbers in quadrants equal the percentage of Th2-primed cells. (C) Kinetic analysis of CD4+ IL-4+ cells after restimulation using the CSA. *P ≤ .05 for differences between WT and WAS−/− Th2-primed effectors across 3 independent experiments; paired Student t test. (D) Ex vivo memory cells (CD4+CD62LlowCD44high) were isolated from the spleens (SPNs) and LNs of unimmunized mice by FACS and restimulated using plate-bound anti-TCRβ mAb for 24 hours; cytokines in supernatants were assayed by ELISA. Results represent 1 of at least 3 comparable experiments. *P ≤ .05 for differences between WT and WAS−/− Th2-primed effectors across 3 independent experiments; paired Student t test. Error bars indicate mean and SEM.
Impaired cytokine production by WASp-deficient Th2-primed effectors. WT and WAS−/− naive CD4+ T cells were primed under Th2 conditions with plate-bound anti-TCRβ and anti-CD28 mAbs for 5 days. Cells were harvested and restimulated with plate-bound anti-TCRβ mAb. (A) ELISA from supernatants 24 hours after restimulation. (B) Th2-primed cells were restimulated with anti-TCRβ mAb for 24 hours, followed by intracellular cytokine staining. Numbers in quadrants equal the percentage of Th2-primed cells. (C) Kinetic analysis of CD4+ IL-4+ cells after restimulation using the CSA. *P ≤ .05 for differences between WT and WAS−/− Th2-primed effectors across 3 independent experiments; paired Student t test. (D) Ex vivo memory cells (CD4+CD62LlowCD44high) were isolated from the spleens (SPNs) and LNs of unimmunized mice by FACS and restimulated using plate-bound anti-TCRβ mAb for 24 hours; cytokines in supernatants were assayed by ELISA. Results represent 1 of at least 3 comparable experiments. *P ≤ .05 for differences between WT and WAS−/− Th2-primed effectors across 3 independent experiments; paired Student t test. Error bars indicate mean and SEM.
WASp-deficiency uncouples IL-4 mRNA expression from IL-4 protein production
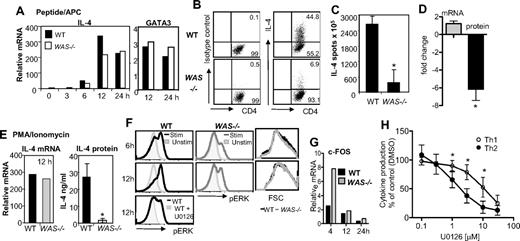
Transcriptional up-regulation of cytokine genes appears to be essential for high-level IL-4 protein production and Th2 effector function,18 and a likely WASp-dependent control point. However, we found comparable transcriptional up-regulation in IL-4 and GATA3 expression in Th2-primed WAS−/− and WT CD4+ T-cell effectors restimulated with pOVA/APC (Figure 4A). Despite robust mRNA induction, there remained a severe defect in IL-4 protein production in the absence of WASp (Figure 4B-C). Similar results were obtained for IL-13 (data not shown). Direct analysis of the fold change in IL-4 mRNA and protein production highlights the uncoupling of transcription and protein production in the absence of WASp (Figure 4D). Thus, we found no role for WASp in transcriptional activation of cytokine genes in Th2 effectors. Indeed, bypassing TCR proximal signaling through stimulation with PMA/ionomycin failed to restore cytokine production in WAS−/− Th2 cells despite strong induction of IL-4 mRNA (Figure 4E). These results suggest an additional WASp-dependent posttranscriptional mechanism for IL-4 production.
IL-4 transcriptional enhancement but no IL-4 protein production in the absence of WASp. (A) Th2-primed WT and WAS−/− CD4+ T cells were restimulated with pOVA/APC (WT T-depleted splenocytes). Cells were collected at given times after stimulation, and mRNA was extracted for qRT-PCR. mRNA was normalized to CD3δ and expressed as fold change over Th2-primed unstimulated (resting) cells. Results were not statistically different for IL-4 mRNA tested over 3 independent experiments by paired Student t test. (B) Intracellular staining on Th2-primed cells stimulated with pOVA/APC. (C) ELISPOT analysis of Th2-primed cells for frequency of IL-4 secretors 24 hours after restimulation with pOVA/APC. Data are from 1 of 3 comparable experiments. *P ≤ .05; paired Student t test. (D) Fold change in mRNA versus protein in WAS−/− Th2 cells compared with WT Th2 cells. *P = .03, paired Student t test, for relative differences in IL-4 protein in WAS−/− and WT Th2 cells. Differences in absolute IL-4 mRNA between WAS−/− and WT Th2 cells was not significant. Average and SEM of data from 3 independent experiments. (E) Th2-primed WT and WAS−/− CD4+ T cells were restimulated with phorbol myristyl acetate (PMA) and ionomycin: IL-4 mRNA analysis (left) and protein secretion by ELISA (right) 12 hours after restimulation. Data are from 1 of 3 comparable experiments. *P ≤ .05; paired Student t test. Error bars indicate mean and SEM. (F) ERK phosphorylation after pOVA/APC restimulation of Th2-primed WT and WAS−/− CD4+ T cells using an anti–phospho-specific ERK Ab. Specificity of binding of the phospho-specific Ab was confirmed by treatment with the ERK inhibitor U0126 (bottom panel). Representative plots from 1 of 4 experiments. (G) qRT-PCR analysis of ERK transcriptional target gene c-FOS in Th2-primed WT and WAS−/− effectors restimulated with pOVA/APC. Representative data from 1 of 2 similar experiments. (H) Cytokine production after restimulation of WT Th1- and Th2-primed cells in the presence of the ERK inhibitor U0126. IFNγ and IL-4 were measured by ELISA and normalized to effector cell stimulation in the absence of the ERK inhibitor (DMSO vehicle control = 100%). Mean and standard error from 4 independent experiments. *P ≤ .05; Student t test. Error bars indicate mean and SEM.
IL-4 transcriptional enhancement but no IL-4 protein production in the absence of WASp. (A) Th2-primed WT and WAS−/− CD4+ T cells were restimulated with pOVA/APC (WT T-depleted splenocytes). Cells were collected at given times after stimulation, and mRNA was extracted for qRT-PCR. mRNA was normalized to CD3δ and expressed as fold change over Th2-primed unstimulated (resting) cells. Results were not statistically different for IL-4 mRNA tested over 3 independent experiments by paired Student t test. (B) Intracellular staining on Th2-primed cells stimulated with pOVA/APC. (C) ELISPOT analysis of Th2-primed cells for frequency of IL-4 secretors 24 hours after restimulation with pOVA/APC. Data are from 1 of 3 comparable experiments. *P ≤ .05; paired Student t test. (D) Fold change in mRNA versus protein in WAS−/− Th2 cells compared with WT Th2 cells. *P = .03, paired Student t test, for relative differences in IL-4 protein in WAS−/− and WT Th2 cells. Differences in absolute IL-4 mRNA between WAS−/− and WT Th2 cells was not significant. Average and SEM of data from 3 independent experiments. (E) Th2-primed WT and WAS−/− CD4+ T cells were restimulated with phorbol myristyl acetate (PMA) and ionomycin: IL-4 mRNA analysis (left) and protein secretion by ELISA (right) 12 hours after restimulation. Data are from 1 of 3 comparable experiments. *P ≤ .05; paired Student t test. Error bars indicate mean and SEM. (F) ERK phosphorylation after pOVA/APC restimulation of Th2-primed WT and WAS−/− CD4+ T cells using an anti–phospho-specific ERK Ab. Specificity of binding of the phospho-specific Ab was confirmed by treatment with the ERK inhibitor U0126 (bottom panel). Representative plots from 1 of 4 experiments. (G) qRT-PCR analysis of ERK transcriptional target gene c-FOS in Th2-primed WT and WAS−/− effectors restimulated with pOVA/APC. Representative data from 1 of 2 similar experiments. (H) Cytokine production after restimulation of WT Th1- and Th2-primed cells in the presence of the ERK inhibitor U0126. IFNγ and IL-4 were measured by ELISA and normalized to effector cell stimulation in the absence of the ERK inhibitor (DMSO vehicle control = 100%). Mean and standard error from 4 independent experiments. *P ≤ .05; Student t test. Error bars indicate mean and SEM.
Extracellular signal–regulated kinase (ERK) signaling pathways have been implicated in regulating translational efficiencies and protein stability in several cell types. WASp-deficient signaling led to a marked decrease in TCR-induced phosphorylation of ERK (Figure 4F) in the absence of general reduction in activation signals as WAS−/− and WT Th2-primed cells blasted with similar kinetics (Figure 4F right panels). The reduced ERK phosphorylation did not alter the induction of its transcriptional target c-FOS (Figure 4G). Thus, WASp-deficient changes in ERK activity likely affect a transcription-independent role in translation and/or protein stability. Reductions in ERK activation were also seen during initial neutral or Th2 priming of WAS−/− CD4 cells (data not shown), although the defect was more modest than seen in Th2-primed cultures. How might these defects in ERK phophorylation, a common TCR signaling component, explain the differential requirement for WASp in Th1 and Th2 function? To address selectivity, we added an ERK inhibitor to WT Th1 and Th2 T-cell cultures at the time of restimulation and measured the secretion of IFNγ and IL-4 protein, respectively (Figure 4H). IL-4 production was 5-fold more sensitive to reduced ERK activity than IFNγ production (50% inhibition: IL-4 at 2.23μM, IFNγ at 10.98μM). These results suggest WASp, possibly via ERK, may in part regulate a common posttranscriptional control point for which IL-4 and IFNγ are differentially sensitive.
WASp-mediated signaling is necessary for IL-4 cytokine production by Th2 effectors
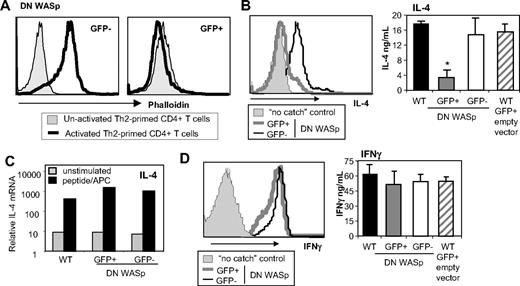
To formally test WASp requirements in Th2 effector function, we modulated WASp expression in WT CD4+ T cells by retroviral expression of a dominant-negative WASp (WASpΔVCA).19 Deletion of the verprolin homology, cofilin homology, and acidic region VCA domain results in a dominant-negative form of WASp (DN-WASp) and has been used extensively in the study of N-WASp activity.20,21 The WASpΔVCA is thought to compete for binding of upstream effectors (such as WIP22 ) that provide stimulatory signals for actin polymerization. At 4 days into Th2 priming, WT CD4+ T cells were infected using a bicistronic retrovirus expressing the WASpΔVCA-IRES-eGFP or “empty vector” (-IRES-eGFP). Cells expressing the WASpΔVCA (eGFP+) showed marked inhibition of TCR-inducible actin polymerization by phalloidin staining, confirming the dominant-negative function (Figure 5A).19 There was also a marked reduction in the secretion of IL-4 cytokine in WT CD4+ T cells transduced with DN-WASp (Figure 5B; FACS and ELISA). Importantly, induction of IL-4 mRNA was not blocked by expression of the DN-WASp (Figure 5C), supporting a posttranscriptional role for WASp. Moreover, the DN-WASp construct did not interfere with IFNγ production in Th1 cells (Figure 5D; FACS and ELISA). Thus, inducible WASp activity is critical for Th2 but not Th1 function.
WT Th2 cells require WASp signaling for IL-4 production. WT naive CD4+ T cells were primed under Th2 (A-C) or Th1 (D) conditions with peptide and WT APCs. Cells were retrovirally transduced with DN WASp (WASpΔVCA-IRES-GFP) or empty vector (-IRES-GFP) for 36 hours late during priming (day 4). On day 6, retrovirally transduced cells were sorted based on GFP expression and restimulated. (A) GFP− and GFP+ cells were not activated or activated with anti-TCRβ mAb for 15 minutes. Cells were fixed and stained with phalloidin and analyzed by FACS. (B-C) Cells were restimulated with pOVA/APC for 24 hours. (B) The frequency of cytokine producers was determined using CSA (left panel) and cytokine produced in the supernatant by ELISA (right panel). Mean ± SEM; n = 3. *P ≤ .05 between GFP+ and GFP− cells in 3 independent experiments; paired Student t test. (C) mRNA analysis 24 hours after restimulation. mRNA was normalized to CD3δ and expressed as fold change over naive unstimulated CD4+ T cells. (D) Th1-primed cells were restimulated with pOVA/APC for 24 hours. The frequency of cytokine producers was determined using CSA (left panel) and cytokine produced in the supernatant by ELISA (right panel). Mean ± SEM; n = 3. Data are from 1 of 3 comparable experiments.
WT Th2 cells require WASp signaling for IL-4 production. WT naive CD4+ T cells were primed under Th2 (A-C) or Th1 (D) conditions with peptide and WT APCs. Cells were retrovirally transduced with DN WASp (WASpΔVCA-IRES-GFP) or empty vector (-IRES-GFP) for 36 hours late during priming (day 4). On day 6, retrovirally transduced cells were sorted based on GFP expression and restimulated. (A) GFP− and GFP+ cells were not activated or activated with anti-TCRβ mAb for 15 minutes. Cells were fixed and stained with phalloidin and analyzed by FACS. (B-C) Cells were restimulated with pOVA/APC for 24 hours. (B) The frequency of cytokine producers was determined using CSA (left panel) and cytokine produced in the supernatant by ELISA (right panel). Mean ± SEM; n = 3. *P ≤ .05 between GFP+ and GFP− cells in 3 independent experiments; paired Student t test. (C) mRNA analysis 24 hours after restimulation. mRNA was normalized to CD3δ and expressed as fold change over naive unstimulated CD4+ T cells. (D) Th1-primed cells were restimulated with pOVA/APC for 24 hours. The frequency of cytokine producers was determined using CSA (left panel) and cytokine produced in the supernatant by ELISA (right panel). Mean ± SEM; n = 3. Data are from 1 of 3 comparable experiments.
WAS requirement for IL-4 production is restricted to CD4+ T cells
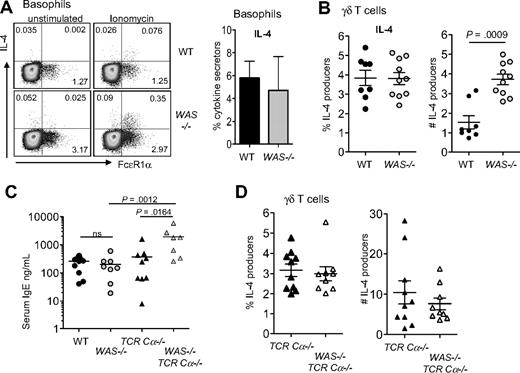
The defects in IL-4 production from WAS−/− CD4+ T cells in the mouse were at odds with the elevated serum IgE levels in patients with WAS.23 In recent years, several cell types have been shown to produce IL-4 in addition to CD4+ αβ T cells, raising the possibility that other cell types may be less dependent on WASp for IL-4 production. In contrast to CD4+ T cells, IL-4 production from basophils (CD4−Gr1−FcϵR1α+) and from γδ T cells were intact in the absence of WASp (Figure 6A-B); indeed, the total numbers of IL-4–producing γδ T cells was elevated in WAS−/− mice compared with WT (Figure 6B). Thus, IL-4 production from these non-αβ T cells is not compromised in the absence of WASp, revealing a cell type–restricted requirement for WASp production of IL-4 by CD4+ αβ T cells. IgE levels were not measured in the original reports of WASp knockout mice, and our initial measure of serum IgE in WAS−/− and WT mice found no significant difference in IgE levels in mice 6 to 8 weeks of age (Figure 6C). However, analysis of WAS−/− mice in the absence of T cells (WAS−/−TCR-Cα−/−) revealed striking increases in serum IgE (Figure 6C). This elevated IgE was accompanied by increased number of IL-4–producing γδ T cells in T cell–deficient WAS−/− mice compared with T cell–sufficient WAS−/− mice (Figure 6B,D) (WAS−/− vs WAS−/− TCR-Cα−/−, P = .002; WT vs WAS+/+ TCR-Cα−/−, P < .001). However, we did not see a significant difference in the frequency or number of IL-4–producing γδ T cells between WASp-sufficient and WASp-deficient T cell–deficient mice (Figure 6D) despite higher IgE in the latter group (Figure 6C). Similarly, we found no increases in number or frequency of IL-4–producing basophils in WAS−/− TCR-Cα−/− mice compared with WAS+/+ TCR-Cα−/− mice (data not shown). Therefore, the cellular requirements driving high IgE in WAS−/− TCRCα−/− mice requires further investigation, but these data do raise the possibility that the IgE and eczema in humans with WAS may not be αβ T cell–driven. Given the distinct requirement for WASp in CD4+ αβ T cells, we focused on WAS−/− CD4+ T cell–functional properties in vivo.
IL-4 production from non-αβ T cells in the absence of WASp. (A) Left panel shows intracellular staining for IL-4 from CD4−Gr1−FcϵR1α+ cells in peripheral blood after 6 hours of ionomycin stimulation, with brefeldin added for the final 4 hours of culture. Cells were gated on negative staining for CD19, CD4, and GR-1 from red cell–depleted peripheral blood. Representative plots are from 3 independent experiments. Numbers in quadrants equal the percentage of gated (isotype control for IL-4, 0.0048%). Right panel shows frequency of IL-4 producers within the FcϵR1α+ compartment. Mean ± SEM from 3 independent experiments. (B) Frequency and total number (×104 per mouse) of IL-4 producers within the γδ T-cell compartment from LNs by intracellular staining after 6 hours of PMA/ionomycin stimulation as in panel A. Symbols represent individual mice from 2 independent experiments; P value by Mann-Whitney. Mean isotype control staining of activated cells is 1.460%. (C) Total serum IgE from 6- to 8-week-old mice was measured by ELISA. Symbols represent individual mice from 2 independent experiments. P values by Mann-Whitney. (D) Frequency and number of IL-4–producing γδ T cells from WAS−/− TCR-Cα−/− and WAS+/+ TCR-Cα−/− mice analyzed as in panel B. Symbols represent individual mice from 2 to 3 independent experiments.
IL-4 production from non-αβ T cells in the absence of WASp. (A) Left panel shows intracellular staining for IL-4 from CD4−Gr1−FcϵR1α+ cells in peripheral blood after 6 hours of ionomycin stimulation, with brefeldin added for the final 4 hours of culture. Cells were gated on negative staining for CD19, CD4, and GR-1 from red cell–depleted peripheral blood. Representative plots are from 3 independent experiments. Numbers in quadrants equal the percentage of gated (isotype control for IL-4, 0.0048%). Right panel shows frequency of IL-4 producers within the FcϵR1α+ compartment. Mean ± SEM from 3 independent experiments. (B) Frequency and total number (×104 per mouse) of IL-4 producers within the γδ T-cell compartment from LNs by intracellular staining after 6 hours of PMA/ionomycin stimulation as in panel A. Symbols represent individual mice from 2 independent experiments; P value by Mann-Whitney. Mean isotype control staining of activated cells is 1.460%. (C) Total serum IgE from 6- to 8-week-old mice was measured by ELISA. Symbols represent individual mice from 2 independent experiments. P values by Mann-Whitney. (D) Frequency and number of IL-4–producing γδ T cells from WAS−/− TCR-Cα−/− and WAS+/+ TCR-Cα−/− mice analyzed as in panel B. Symbols represent individual mice from 2 to 3 independent experiments.
WASp-deficient CD4+ T cells elicit a protective Th1 immune response in vivo
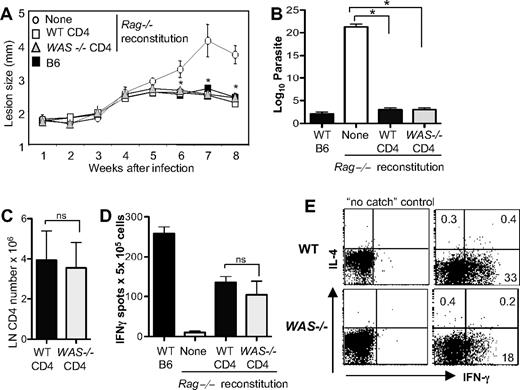
Studies in both mouse and human suggest that WAS-deficient T cells might fail to mount effective Type 1 immunity. To directly determine the capacity of WAS-deficient CD4+ T cells to mount a productive Th1 response in vivo, we reconstituted T cell–deficient mice (Rag−/−) with C57BL/6 WT or WAS−/− CD4+ T cells and infected them with L major. Clearance of L major is strictly dependent on an effective IFNγ-producing Th1 effector response.24 Surprisingly, pathogen clearance was similar after reconstitution with WT or WAS−/− cells (Figure 7A-B). Total numbers of CD4+ T cells (Figure 7C), frequency of L major–specific IFNγ-producing T cells, and amount of cytokine per cell (MFI: WT, 124 ± 27; WAS−/−, 105 ± 35; n = 3 experiments, P = ns), was comparable in the LNs of WT and WAS−/−-reconstituted mice (Figure 7D). Defects in cell migration with WASp deficiency have been described25 ; therefore, we analyzed CD4+ T-cell accumulation in the infected dermis (Figure 7E). We found a reduction in the frequency of L major–specific IFNγ-producing WAS−/− CD4+ T cells at the infection site compared with WT CD4+ T cells (Figure 7E), suggesting that WASp-dependent events after Th differentiation, such as appropriate tissue localization, may affect the efficiency of the immune response. Nonetheless, the reduction in IFNγ producers at the L major infection site did not alter the kinetics of parasite clearance (Figure 7A-B). Thus, we find no evidence of an absolute requirement for WASp in Th1 immunity: WASp-deficient CD4+ T cells acquire the ability to produce IFNγ, home (albeit less efficiently) to the infection site, and mount a protective Th1 response to L major in vivo.
WAS−/− CD4+ T cells exert Th1 effector function in vivo. WT and WAS−/− CD4+ T cells (C57BL/6) were adoptively transferred into Rag-deficient mice. At 24 hours later, mice were infected with 2.0 × 105L major promastigotes. (A) Progression of disease measured by footpad (lesion) size, mean of 4 to 6 mice per group. *P ≤ .05 (Mann-Whitney) for differences between Rag−/− with no reconstitution and either WT or WAS−/− CD4 reconstitution. (B) Limiting dilution analysis of parasite load in the footpad. *P ≤ .05 by paired Student t test across 3 independent experiments. (C) Draining LN CD4+ T-cell numbers 8 weeks after reconstitution of RAG−/− mice and L major infection. ns indicates not significant; paired Student t test. (D) ELISPOT analysis of antigen-specific IFNγ-producing cells upon 6 hours of restimulation with SLA. ns indicates P = .5 between WT and WAS−/− IFNγ production across 3 independent experiments; paired Student t test. Error bars indicate mean and SEM. (E) CSA for IL-4 and IFNγ production by WT and WAS−/− CD4+ T cells in the L major–infected dermis at 8 weeks. Cytokines were measured after 6 hours of restimulation with SLA. Dot plots were gated on CD4+ T cells. Numbers in quadrants equal the percentage of CD4+ T cells. Data are from 1 experiment representative of at least 3 comparable independent experiments.
WAS−/− CD4+ T cells exert Th1 effector function in vivo. WT and WAS−/− CD4+ T cells (C57BL/6) were adoptively transferred into Rag-deficient mice. At 24 hours later, mice were infected with 2.0 × 105L major promastigotes. (A) Progression of disease measured by footpad (lesion) size, mean of 4 to 6 mice per group. *P ≤ .05 (Mann-Whitney) for differences between Rag−/− with no reconstitution and either WT or WAS−/− CD4 reconstitution. (B) Limiting dilution analysis of parasite load in the footpad. *P ≤ .05 by paired Student t test across 3 independent experiments. (C) Draining LN CD4+ T-cell numbers 8 weeks after reconstitution of RAG−/− mice and L major infection. ns indicates not significant; paired Student t test. (D) ELISPOT analysis of antigen-specific IFNγ-producing cells upon 6 hours of restimulation with SLA. ns indicates P = .5 between WT and WAS−/− IFNγ production across 3 independent experiments; paired Student t test. Error bars indicate mean and SEM. (E) CSA for IL-4 and IFNγ production by WT and WAS−/− CD4+ T cells in the L major–infected dermis at 8 weeks. Cytokines were measured after 6 hours of restimulation with SLA. Dot plots were gated on CD4+ T cells. Numbers in quadrants equal the percentage of CD4+ T cells. Data are from 1 experiment representative of at least 3 comparable independent experiments.
WASp is required for Th2 immune responses in vivo
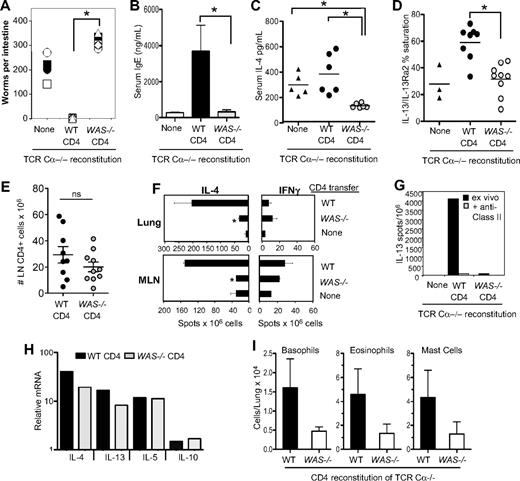
To study the role of WASp in Th2 immune function in vivo, we investigated the clearance of the prototypic Th2-inducing nematode Nippostrongylus brasiliensis.26 BALB/c WT or WAS−/− CD4+ T cells were transferred to T cell–deficient mice (BALB/c TCR Cα−/−) 24 hours before N brasiliensis. Mice reconstituted with WT CD4+ T cells expelled all adult worms by day 10, whereas mice reconstituted with WAS−/− CD4+ T cells had worm burdens similar, if not higher, than TCR Ca−/− mice receiving no cells (Figure 8A). Worms were still present in WAS−/− CD4 recipients at day 17 after infection, but were reduced by 50% from day 10 levels, suggesting a marked delay in parasite clearance (data not shown). N brasiliensis–induced serum IgE levels were significantly attenuated (Figure 8B), and in vivo production of both IL-4 and IL-13 after N brasiliensis infection was reduced in recipients of WAS−/− CD4+ T cells (Figure 8C-D). These changes were not due to significant differences in the number of WT and WAS−/− CD4 T cells after reconstitution (Figure 8E). Anti–N brasiliensis IL-4 production, on restimulated in vitro with soluble N brasiliensis antigens, was also impaired in WAS−/−-reconstituted mice (Figure 8F). Similarly, ex vivo MHC class II–dependent IL-13 production was absent in mice reconstituted with WAS−/− CD4+ T cells (Figure 8G). Thus, WAS−/− CD4+ T cells appear unable to support a protective Th2 response in vivo. Consistent with a posttranscriptional role for WASp in cytokine production (Figure 4D), we found robust induction of Th2 cytokine mRNAs by WAS−/− CD4+ T cells in LNs after infection (Figure 8H) despite significantly reduced IL-4 protein (Figure 8C).
WASp-deficient CD4+ T cells fail to support Th2 immune responses to N brasiliensis infection in vivo. WT or WAS−/− CD4+ T cells (BALB/c) were adoptively transferred into TCR Ca−/− mice. After 24 hours, mice were infected with 500 infective N brasiliensis third-stage larvae (Nb). Ten days after infection, small intestines were excised for direct counting of adult worms. Symbols represent individual mice, 6 mice per group. *P ≤ .01 for WT versus WAS−/− CD4-reconstituted; Student t test. (B) Serum IgE levels measured by ELISA 10 days after infection. (C) In vivo cytokine capture for IL-4 in TCR Ca−/− mice reconstituted with WT or WAS−/− CD4+ T cells, 10 days after Nb infection. (D) Serum IL-13 in TCR Ca−/− mice reconstituted with WT or WAS−/− CD4+ T cells, 10 days after Nb infection. (E) CD4 T-cell numbers in mesenteric LNs 10 days after Nb infection; symbols represent individual mice. (F) ELISPOT for cytokine-secreting cells from the lungs and mesenteric LNs of infected mice restimulated ex vivo for 18 hours with Nb antigen. Statistical analysis by Student t test: lung tissue, *P < .008; mesenteric LN, *P < .004 for difference between IL-4 production by WT and WAS−/− cells. Data are from 1 experiment representative of at least 3 independent experiments. (G) Ex vivo ELISPOT for IL-13 with or without addition of blocking anti–MHC class II Ab. (H) mRNA analysis of Th2 cytokine gene expression in WT and WAS−/− CD4+ T cells from the mesenteric LNs of Nb-infected mice at day 10. Transcripts were normalized to HPRT and expressed relative to uninfected CD4+ T cells from the mesenteric LN. (I) Innate cell number in the lung, 10 days after Nb infection. Noninfected lungs contained 306 ± 60 basophils, 1130 ± 248 eosinophils, and 507 ± 140 mast cells. Results are the mean of individual mice from 3 independent experiments. Differences between WT and WAS−/− recipients were not statistically significant. Error bars indicate mean and SEM.
WASp-deficient CD4+ T cells fail to support Th2 immune responses to N brasiliensis infection in vivo. WT or WAS−/− CD4+ T cells (BALB/c) were adoptively transferred into TCR Ca−/− mice. After 24 hours, mice were infected with 500 infective N brasiliensis third-stage larvae (Nb). Ten days after infection, small intestines were excised for direct counting of adult worms. Symbols represent individual mice, 6 mice per group. *P ≤ .01 for WT versus WAS−/− CD4-reconstituted; Student t test. (B) Serum IgE levels measured by ELISA 10 days after infection. (C) In vivo cytokine capture for IL-4 in TCR Ca−/− mice reconstituted with WT or WAS−/− CD4+ T cells, 10 days after Nb infection. (D) Serum IL-13 in TCR Ca−/− mice reconstituted with WT or WAS−/− CD4+ T cells, 10 days after Nb infection. (E) CD4 T-cell numbers in mesenteric LNs 10 days after Nb infection; symbols represent individual mice. (F) ELISPOT for cytokine-secreting cells from the lungs and mesenteric LNs of infected mice restimulated ex vivo for 18 hours with Nb antigen. Statistical analysis by Student t test: lung tissue, *P < .008; mesenteric LN, *P < .004 for difference between IL-4 production by WT and WAS−/− cells. Data are from 1 experiment representative of at least 3 independent experiments. (G) Ex vivo ELISPOT for IL-13 with or without addition of blocking anti–MHC class II Ab. (H) mRNA analysis of Th2 cytokine gene expression in WT and WAS−/− CD4+ T cells from the mesenteric LNs of Nb-infected mice at day 10. Transcripts were normalized to HPRT and expressed relative to uninfected CD4+ T cells from the mesenteric LN. (I) Innate cell number in the lung, 10 days after Nb infection. Noninfected lungs contained 306 ± 60 basophils, 1130 ± 248 eosinophils, and 507 ± 140 mast cells. Results are the mean of individual mice from 3 independent experiments. Differences between WT and WAS−/− recipients were not statistically significant. Error bars indicate mean and SEM.
Although IL-4 and IL-13 are essential for N brasiliensis worm expulsion, a T-cell source of these cytokines is not mandatory.27 Therefore, in our transfers, where the innate compartment is wild-type, the failure of WAS−/− CD4+ T cells to make IL-4/IL-13 cannot alone account for the defects in parasite clearance. Consistent with the notion that Th2 cells aid recruitment of innate effectors to tissue sites of infection,27 we found that the numbers of eosinophils, basophils, and mast cells recruited to the lung were consistently reduced in recipients of WAS−/− CD4+ T cells (Figure 8I), although this difference was not statistically significant. Thus, in vivo production of IL-4/IL-13 (Figure 8C-D) and recruitment of innate effectors to infected tissues are both compromised when CD4 T cells lack WASp and likely conspire to limit parasite clearance. A defect in IL-4 production was also seen in WAS−/− CD4+ T cell–reconstituted TCR Cα−/− BALB/c mice infected with the protozoa parasite L major24 (supplemental Figure 2). Thus, WAS−/− CD4+ T cells show defects in Th2-dependent responses to 2 pathogenically distinct infectious agents independent of the genetic background of the WAS−/− mouse.
Discussion
We demonstrate a distinct requirement for WASp in the function of Th2 immune responses in vitro and in vivo. In vitro, WASp was not required for initial Th2 differentiation but was required for subsequent Th2 effector function. WAS−/− CD4+ T cells failed to mount a protective Th2 response against the parasitic nematode N brasiliensis or Th2 responses to L major. WASp requirements were specific to Th2 function as WAS−/− CD4+ T cells were capable of making both IFNγ and IL-17 in vitro and orchestrating a protective Th1 response to L major. Thus, WASp plays a nonredundant role in CD4+ Th2 effector function. Susceptibility to the loss of WASp was both cytokine (IL-4 not IFNγ)– and cell type (αβ not γδ or basophils)–specific.
WAS−/− CD4+ T cells exhibited intact signaling for the induction of the Th2 differentiation program, as shown by early transcriptional up-regulation of IL-4 and GATA3 and IL-4 protein synthesis. However, we found a profound defect in the synthesis of IL-4 and other Th2 cytokines in Th2-primed WAS−/− effectors. Steady-state transcripts for IL-4 were comparable with that of WT Th2 effectors, but protein production was severely impaired. Several signaling molecules have been described as important in Th2 responses, including PKCθ, VAV-1, and Itk. In each case, provision of a calcium ionophore restored IL-4 function, suggesting these molecules aid in IL-4 expression via a common downstream mediator such as NFAT. We were surprised therefore to find that neither anti-TCR/ionomycin or PMA/ionomycin rescued the IL-4 defect in Th2-primed WAS−/− effectors. Rather, our results suggest WASp plays a distinct posttranscriptional role in IL-4 expression. In the study of WAS patient T-cell lines, the IL-4 response was only minimally reduced compared with healthy controls.11 However, the authors did make note of the fact that they observed higher IL-4 mRNA, but not protein, in patients with WAS and raised the possibility of a posttranscriptional defect in IL-4 production in human WAS CD4+ T cells, similar to the uncoupling of IL-4 transcription and protein production in mouse WAS−/− CD4+ T cells.
At this stage, it is not clear how WASp may influence protein production. WASp could regulate a common control point for which IL-4 and IFNγ are differentially sensitive (relative requirement), or WASp could regulate a posttranscriptional control point specific to the cytokine itself (selective requirement), such as translation regulation via cytokine-specific AU-rich elements in the 3′ untranslated region of cytokine mRNAs.28 WAS−/− Th2 cells were markedly impaired in ERK activation after restimulation (Figure 4). Our results show that IL-4 and IFNγ are differentially sensitive to the loss of ERK activity, supporting a relative rather than absolute role for ERK in IL-4 production. ERK could be functioning at multiple levels, but relevant to our current observations, ERK has been shown to be required for general translation via phosphorylation of eIF4E,29,30 production of ribosomal components for increased protein production in Th2 effectors,31 and to regulate protein stability.32 Analysis of translational control points such as the intregrated stress response33 and transcriptional regulation of rRNA processing factors31 failed to reveal a key role for WASp at these control points (data not shown). Further work is needed to determine the precise role for WASp in bridging cytokine transcription with protein production. It is striking that WASp regulates cytokine production in CD4+ T cells at multiple levels: IL-2 at the transcriptional level, IL-4 posttranscriptionally for protein production, and IFNγ at protein secretion. How WASp regulates these seemingly disparate processes will require further analysis of the signal pathways emanating from WASp activation.
Our results show that the signals for Th2 differentiation and the signals for Th2 effector function are differentially sensitive to the loss of WASp. Differential requirements for production of the same cytokine in differentiating and effector cells could reflect differences in transcriptional requirements for transcriptional enhancer sites in effectors34 or signals needed for accelerated protein production in effectors.31 Several recent studies have also highlighted differential requirements for naive and effector/memory induction of cytokine production.35 IL-2 production was heavily dependent on NFAT in memory but not naive cells.36 Itk was found to be required for the transcriptional up-regulation of IL-4 in Th2 effector cells but not in naive T cells during Th2 priming.37 These differences may be linked to the distinct synapse structure in naive and effector/memory T cells and/or between Th1 and Th2 to which WASp may contribute. In the absence of WASp, a lack of synapse stabilization and subsequent signaling may affect amplification of the signals for the transcription of cytokines and for protein translation, stabilization, and/or secretion.
The functional phenotype when WASp deficiency is limited to the CD4+ T-cell compartment is distinct from the immune deficiencies observed or predicted from WASp deficiency in all hematopoietic cells. Patients with WAS have recurrent bacterial and fungal infections that suggest type 1 immunity is compromised in the absence of WASp.38 It is likely that the outcome of a given infection is influenced at multiple levels and in different cell types. WASp-deficient dendritic cells show defects in homing to the draining LNs and poor T-cell priming.39 The absence of WASp in macrophages leads to defects in phagocytic cup and podosome formation that both limit macrophage activity and migration.40 Defects in multiple innate effectors in the absence of WASp have been documented including neutrophils, NK cells, and mast cells.38 A recent study of CD4+ T-cell lines derived from patients with WAS identified defects in IFNγ mRNA and protein production with anti-CD3/CD28 Ab stimulation.11 It remains to be determined if patients with WAS have defects in CD4 T-cell IFNγ production under physiologic conditions. In contrast, our studies demonstrated a posttranscriptional requirement for WASp in IFNγ secretion12 that can be rescued by non-TCR/CD28 signals provided by APCs in vitro (Figure 1) and in vivo (Figure 7). Thus, the sensitivity to WASp requirements for IFNγ function will likely be context-dependent. Interestingly, we did observe a decrease in the accumulation of WAS−/− Th1 cells at the infected tissue. Although changes in Th1 numbers did not alter L major clearance in this study, clearance of other pathogens may be more reliant on Th1 numbers. Interestingly, studies in WAS−/− mice point to a role for WASp not in initial pathogen clearance but rather in the ability to develop protective memory.41 Additional modulating effects on T-cell function in the absence of WASp in vivo may come from the disruption of regulatory T-cell homeostasis.42-44
Defects in IL-4 production in the absence of WASp in this mouse model were unexpected given the elevated serum IgE and eczema symptoms in patients with WAS.23 Interestingly, we show that IL-4 production remained intact in WAS-deficient basophils and γδ T cells, unlike CD4+ αβ T cells. Moreover, serum IgE was significantly elevated in T cell–deficient mice also lacking WASp. Non-αβ T-cell production of IL-4 could account for the recent observation of a Th2-like inflammatory bowel disease in mice in the absence of WASp.45 The apparent disconnect between high baseline serum IgE levels and defective Th2 effector function observed in the WASp-deficient mice is shared by mice with a deficiency in Itk,46 and is thought to be mediated in part by IL-4 from γδ T cells.47,48 We speculate that this might reveal homeostatic or developmental cross regulation between innate and adaptive Type 2 compartments. Elevated IgE could come from “natural” IgE in the absence of MHC class II cognate help49 or from antigen-specific cells such as γδ T cells. In patients with WAS, the non-αβ T cells may be able to compensate for the loss of Th2 function and account for the absence of reports on WAS deficiency and problems with helminth infection. However, our studies do raise the possibility that a type 2 response in humans with WAS may be mechanistically distinct (innate rather than T cell–driven) and could require different approaches for treatment.
This is the first study to directly address the role of WASp in CD4+ T cells in an otherwise WASp-sufficient immune environment. Collectively, our results suggest a nonredundant posttranscriptional role for WASp in the regulation of Th2 effector function. These results are surprising given the symptoms of WAS in humans include eczema, which appears much like atopic dermatitis, and elevated serum IgE. It appears from our studies in the mouse that IL-4 production from non–CD4-immune cell types is WASp-independent and that in the absence of a CD4+ T-cell source of IL-4, the innate compartment may be dysregulated. The challenge for the future is to understand the relative role of WASp deficiency in individual immune cell types and their relationship to the overall ability to control infection in patients with WAS.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Kathy Siminovitch (University of Toronto) for the OTII WAS−/− mice and the WASpΔVCA construct. The authors thank Angie Hughson and Adam Kasper for technical help and the Fowell laboratory for helpful suggestions. Special thanks are due to Jim Miller for insightful comments on the manuscript.
This work was supported by National Institutes of Health grants R01-AI50201 (to D.J.F.), T32-AI007362 (to V.M.-T.), T32-A107285 (to S.D.K. and D.K.S.), and R01 AI052099 (to F.D.F.).
National Institutes of Health
Authorship
Contribution: V.M.-T., D.K.S., S.D.K., C.A.L., and D.J.F. designed and performed research and analyzed data; F.D.J. performed and analyzed data; J.F.U. provided N brasiliensis and edited the paper; and V.M.-T., D.K.S., and D.J.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deborah J. Fowell, David H. Smith Center for Vaccine Biology and Immunology, University of Rochester, 601 Elmwood Ave, Box 609, Rochester, NY 14642; e-mail: deborah_fowell@urmc.rochester.edu.
References
Author notes
V.M.-T. and D.K.S. contributed equally to this article.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal