Abstract
A somatic point mutation (V617F) in the JAK2 tyrosine kinase was found in a majority of patients with polycythemia vera (PV), essential thrombocythemia, and primary myelofibrosis. However, contribution of the JAK2V617F mutation in these 3 clinically distinct myeloproliferative neoplasms (MPNs) remained unclear. To investigate the role of JAK2V617F in the pathogenesis of these MPNs, we generated an inducible Jak2V617F knock-in mouse, in which the expression of Jak2V617F is under control of the endogenous Jak2 promoter. Expression of heterozygous mouse Jak2V617F evoked all major features of human polycythemia vera (PV), which included marked increase in hemoglobin and hematocrit, increased red blood cells, leukocytosis, thrombocytosis, splenomegaly, reduced serum erythropoietin (Epo) levels and Epo-independent erythroid colonies. Homozygous Jak2V617F expression also resulted in a PV-like disease associated with significantly greater reticulocytosis, leukocytosis, neutrophilia and thrombocytosis, marked expansion of erythroid progenitors and Epo-independent erythroid colonies, larger spleen size, and accelerated bone marrow fibrosis compared with heterozygous Jak2V617F expression. Biochemical analyses revealed Jak2V617F gene dosage-dependent activation of Stat5, Akt, and Erk signaling pathways. Our conditional Jak2V617F knock-in mice provide an excellent model that can be used to further understand the molecular pathogenesis of MPNs and to identify additional genetic events that cooperate with Jak2V617F in different MPNs.
Introduction
The myeloproliferative neoplasms (MPNs) polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are clonal stem cell–derived hematologic malignancies characterized by excessive production of one or more myeloerythroid lineage cells. A somatic point mutation (V617F) in JAK2 has been detected in most patients with PV and in 50% to 60% of patients with ET and PMF.1-5 The basis for the various pathologies associated with the JAK2V617F mutation, however, remains unclear.
Although most patients with MPN are heterozygous for JAK2V617F, a subset of patients, more commonly with PV than ET, are homozygous for the JAK2V617F allele.1-4 Homozygosity of JAK2V617F results from acquired uniparental disomy (UPD) at chromosomal locus 9p24, which includes JAK2.4,6 Scott et al observed that homozygous JAK2V617F mutant erythroid colonies are present in almost all patients with PV, but are rare in patients with ET.7 These observations led to the speculation that JAK2V617F gene dosage may play a role in MPN phenotype.
JAK2, a member of the Janus family of nonreceptor tyrosine kinases, plays an important role in signaling through type I cytokine receptors including erythropoietin (Epo) receptor.8 JAK2V617F is a constitutively active tyrosine kinase, which can transform factor-dependent hematopoietic cell lines to cytokine independence.1,2 Expression of JAK2V617F results in constitutive activation of downstream signaling pathways, including the signal transducer and activator of transcription 5 (Stat5), extracellular signal-regulated kinase (Erk), and phosphatidylinositol 3-kinase/Akt pathways.1,2 It has been shown that coexpression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation of hematopoietic cells.9
Murine bone marrow transplantation (BMT) models using retrovirally transduced bone marrow (BM) cells demonstrated that overexpression of Jak2V617F results in a PV-like disorder without thrombocytosis.10-13 Recently, 3 groups have generated transgenic mice expressing Jak2V617F allele.14-16 Depending on the promoter and the level of Jak2V617F expression, the phenotypes of these transgenic mice were different. Moreover, incomplete penetrance of the MPNs was observed in these mice models.
Although retroviral BMT and transgenic mice models provided some insights into the role of JAK2V617F in the pathogenesis of MPN, the contribution of the JAK2V617F gene dosage in signaling and phenotype remained unclear. Moreover, the existing models do not provide the appropriate genetic context to compare the effects of heterozygous and homozygous JAK2V617F expression on MPN phenotype. To get better insight into the effects of JAK2V617F at a physiologic gene dosage on hematopoietic cells, we generated an inducible Jak2V617F knock-in mouse, in which the expression of Jak2V617F is under control of the endogenous Jak2 promoter. Using this conditional Jak2V617F knock-in allele, we have characterized the effects of heterozygous and homozygous Jak2V617F expression in vivo.
Methods
Generation of conditional Jak2V617F knock-in mice
The V617F mutation and a unique DraI restriction site were introduced into the Jak2 locus by site-directed mutagenesis. A loxP-flanked cassette containing the 3′-245 base pairs of intron 12 including the splice acceptor, the mouse Jak2 cDNA containing exons 13 to 24, the mouse Jak2 polyadenylation sequences, and a PGK-Neo-Stop cassette was placed 5′ of exon 13. Two correctly targeted embryonic stem (ES) clones were injected into C57/BL6 (B6) blastocysts. Both clones gave rise to germline transmission and produced similar phenotypes. The targeted mutant (V617F/+) mice were crossed to MxCre mice to obtain MxCre;V617F/+ mice. Cre expression was induced by intraperitoneal injection of 3 doses of 300 μg of polyinosine-polycytosine (pI:pC; Amersham). Mice with C57BL/6 × 129Sv mixed background were used for all experiments except for transplantation into secondary recipients, in which MxCre;V617F/+ mice were backcrossed to C57BL/6 background for 4 generations. The details of targeting vector, Southern blotting, and genotyping protocol are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All animal studies were approved by the Committee for the Humane Use of Animals of SUNY Upstate Medical University.
Blood and tissue analysis
Peripheral blood cell counts were determined using Hemavet 950FS (Drew Scientific). Blood smears were stained with Wright-Giemsa, and reticulocytes were enumerated by New Methylene Blue staining. Mouse serum Epo levels were determined by enzyme-linked immunosorbent assay using the Quantikine Epo Immunoassay Kit (R&D Systems). For histopathologic analysis, mouse tissue specimens were fixed in 10% neutral-buffered formalin and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin and reticulin stain.
Flow cytometry
Flow cytometry was carried out as described in the supplemental Methods. Flow cytometry was performed with an LSRII (BD Biosciences) and analyzed by using FlowJo software (TreeStar).
Colony-forming assays
BM (2 × 104) or spleen (1 × 105) cells were plated in duplicate in complete methylcellulose medium (Methocult M3434; StemCell Technologies). Burst-forming units-erythroid (BFU-E), granulocyte-macrophage colony-forming unit (CFU-GM), and colony-forming unit-granulocyte, erythrocyte macrophage, megakaryocyte (CFU-GEMM) colonies were scored on day 7. To detect Epo-independent colony-forming unit-erythroid (CFU-E) colonies, spleen cells (1 × 105) were plated in duplicate in methylcellulose medium (Methocult M3234; StemCell Technologies) without any cytokine. CFU-E colonies were counted after 2 days by staining with benzidine solution (Sigma-Aldrich).
Quantitative PCR and allelic ratio
RNA was isolated from the BM, and reverse transcription was carried out using a Reverse Transcription Kit (QIAGEN). Quantitative polymerase chain reaction (PCR) was performed using the SYBR Green PCR Master mix and a set of primers that amplify a 182–base pair segment of Jak2 cDNA including Jak2V617F. The primers used for Jak2 were GCAGCAAGCATGATGAGTC and CAACTGCTTAGCCACTCCA. 18S was used for normalization of Jak2 expression level. The primers used for 18S were CGCCGCTAGAGGTGAAATTC and TTGGCAAATGCTTTCGCTC.
Quantitative real-time PCR was performed using a LightCycler 480 (Roche Applied Science) and analyzed with associated software. Relative expression values were calculated by the ΔΔCT method using BM sample from a wild-type (WT) mouse as the calibrator. The allelic ratio of mutant Jak2V617F to WT Jak2 in heterozygous Jak2V617F mice was determined by the T/G ratio as described previously.15 For this purpose, the real-time PCR products obtained from quadruplicate determination for each sample were combined, purified using the QIAGEN PCR purification kit, and directly sequenced using the forward primer used for amplification of Jak2 in real-time PCR. The T peak identifies the Jak2V617F mutant allele, whereas the G peak identifies the WT Jak2 allele. The height of the T and G peaks were determined directly using the 4 Peaks software (freely available online), and the percentage of T (mutant) and G (WT) peak fluorescence was calculated using the formula: % T = (height of T-peak)/(height of T-peak + G-Peak) × 100, whereas % G = (height of G-peak)/(height of T-peak + G-Peak) × 100. Standard curves were generated from known ratios of accurately measured pMSCV-IRES-GFP plasmids containing mouse Jak2WT and mouse Jak2V617F.
Erythroblast culture and immunoblotting
BM and spleen cells were cultured in a medium that enriches the erythroblasts.17 Cells were analyzed by flow cytometry after staining with CD71 and Ter-119 after culturing for 7 days. For signaling studies, erythroblasts were starved for 4 hours in Iscove modified Dulbecco's medium containing 0.5% BSA at 37°C and cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer. Immunoblotting was performed using anti-phosphotyrosine antibody (4G10; Upstate Biotechnology Associates) or phospho-specific antibodies against Stat5, Akt, or Erk1/2 (Cell Signaling Technologies), or antibodies against total Jak2 (Upstate Biotechnology Associates), Stat5, Akt, or Erk2 (Santa Cruz Biotechnology).
Statistical analysis
Results are expressed as mean plus or minus SEM, and data were analyzed by the 2-tailed Student t test. A value of P less than .05 was considered to be statistically significant.
Results
Generation of inducible Jak2V617F knock-in mice
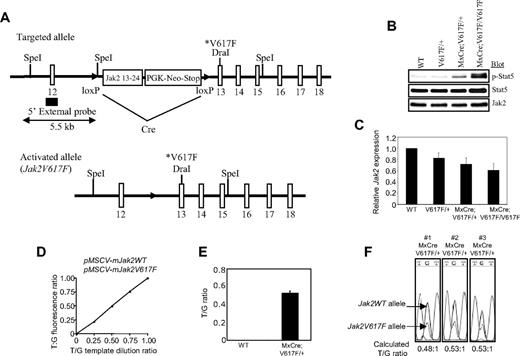
We used homologous recombination in ES cells to generate an inducible Cre-activated targeted allele of Jak2V617F (Figure 1A, supplemental Figure 1A). The targeted Jak2V617F allele was designed to express normal WT Jak2 before Cre-mediated recombination. Five properly targeted ES clones were obtained. We reconfirmed the positive ES clones by PCR as well as by Southern blotting (supplemental Figure 1B-C). Two independent ES clones were injected into blastocysts to obtain chimeras, both of which gave germline transmission (supplemental Figure 1D). Notably, breeding between heterozygous (V617F/+) male and female mice generated mice with all possible genotypes including WT (+/+), V617F/+, and V617F/V617F.
Generation of inducible Jak2V617F knock-in mice. (A) The targeted allele contains the floxed PGK-Neo-Stop cassette and the V617F mutation. This allele is transcriptionally silent, but can be induced in the presence of Cre to generate the activated Jak2V617F allele. (B) Constitutive phosphorylation of Stat5 in the BM of induced MxCre;V617F/+ and MxCre;V617F/V617F mice confirm expression of the mutant Jak2V617F protein. (C) Expression of total Jak2 mRNA was measured in the BM of WT, V617F/+, MxCre;V617F/+ (heterozygous), and MxCre;V617F/+V617F (homozygous) mice (n = 4) by real-time PCR. Total Jak2 mRNA expression was significantly reduced in the BM of both heterozygous and homozygous Jak2V617F mice compared with WT mice (P < .05 between WT and heterozygous Jak2V617F, P < .05 between WT and homozygous Jak2V617F; unpaired t test), whereas there were no significant differences between V617F/+ and heterozygous or V617F/+ and homozygous Jak2V617F mice. (D) A standard curve made from known ratios of accurately measured pMSCV-IRES-GFP plasmids containing mouse Jak2WT and mouse Jak2V617F showing the linearity and accuracy of the measurement of T/G fluorescence ratio (T-peak identifies the mutant, G-peak identifies the WT allele) for determination of allelic ratio. (E) Allelic ratio of the mutant Jak2V617F to WT Jak2 mRNA was determined by the T/G ratio after direct sequencing of the real-time PCR products from the BM of heterozygous Jak2V617F mice (n = 8). (F) The chromatograms of 3 sequenced real-time PCR products from the BM of heterozygous Jak2V617F mice are shown.
Generation of inducible Jak2V617F knock-in mice. (A) The targeted allele contains the floxed PGK-Neo-Stop cassette and the V617F mutation. This allele is transcriptionally silent, but can be induced in the presence of Cre to generate the activated Jak2V617F allele. (B) Constitutive phosphorylation of Stat5 in the BM of induced MxCre;V617F/+ and MxCre;V617F/V617F mice confirm expression of the mutant Jak2V617F protein. (C) Expression of total Jak2 mRNA was measured in the BM of WT, V617F/+, MxCre;V617F/+ (heterozygous), and MxCre;V617F/+V617F (homozygous) mice (n = 4) by real-time PCR. Total Jak2 mRNA expression was significantly reduced in the BM of both heterozygous and homozygous Jak2V617F mice compared with WT mice (P < .05 between WT and heterozygous Jak2V617F, P < .05 between WT and homozygous Jak2V617F; unpaired t test), whereas there were no significant differences between V617F/+ and heterozygous or V617F/+ and homozygous Jak2V617F mice. (D) A standard curve made from known ratios of accurately measured pMSCV-IRES-GFP plasmids containing mouse Jak2WT and mouse Jak2V617F showing the linearity and accuracy of the measurement of T/G fluorescence ratio (T-peak identifies the mutant, G-peak identifies the WT allele) for determination of allelic ratio. (E) Allelic ratio of the mutant Jak2V617F to WT Jak2 mRNA was determined by the T/G ratio after direct sequencing of the real-time PCR products from the BM of heterozygous Jak2V617F mice (n = 8). (F) The chromatograms of 3 sequenced real-time PCR products from the BM of heterozygous Jak2V617F mice are shown.
To induce expression of Jak2V617F in hematopoietic cells, V617F/+ mice were crossed to MxCre transgenic mice that express Cre recombinase in all hematopoietic tissues in response to pI:pC.18 Control (WT and V617F/+), MxCre;V617F/+ and MxCre;V617F/V617F mice were injected with pI:pC to induce Cre expression and subsequent excision of the Neo-stop cassette, which resulted in the expression of Jak2V617F in the hematopoietic system. Immunoblotting with a phospho-specific Stat5 antibody revealed constitutive phosphorylation of Stat5 in the BM of MxCre;V617F/+ (heterozygous Jak2V617F) and MxCre;V617F/V617F (homozygous Jak2V617F) mice (Figure 1B), confirming the expression/activation of the mutant Jak2V617F protein.
We measured the expression of total Jak2 mRNA (both WT Jak2 and Jak2V617F) in the BM of control (WT and V617F/+), heterozygous and homozygous Jak2V617F mice by quantitative real-time PCR. The expression of total Jak2 mRNA was significantly reduced in the BM of both heterozygous and homozygous Jak2V617F mice compared with WT mice (Figure 1C). However, no significant differences were observed between V617F/+ (control), heterozygous and homozygous Jak2V617F mice. To determine the allelic ratio of mutant Jak2V617F to WT Jak2 in heterozygous Jak2V617F mice, we directly sequenced the real-time PCR products of Jak2 cDNA from the BM and the ratio of T/G (T peak identifies the mutant, G peak identifies the WT allele) was estimated as described previously15 (also described in “Quantitative PCR and allelic ratio”). Standard curve was generated from known ratios of accurately measured pMSCV-mouse Jak2WT-IRES-GFP and pMSCV-mouse Jak2V617F-IRES-GFP plasmids (Figure 1D). The standard curve showed the linearity and accuracy of the measurement of the T/G ratio for determination of the mutant to WT Jak2 allelic ratio. The expression of Jak2V617F mutant mRNA was almost half of the WT Jak2 in the BM of heterozygous Jak2V617F mice (Figure 1E-F).
Expression of Jak2V617F in hematopoietic cells results in a PV-like disease
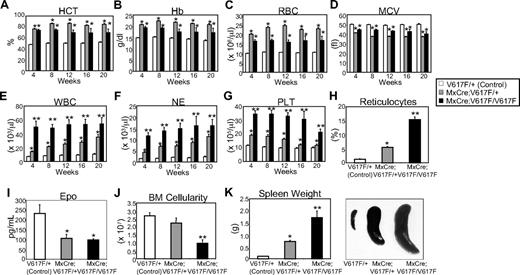
All mice expressing either heterozygous or homozygous Jak2V617F exhibited markedly increased blood hematocrit, hemoglobin, and red blood cell (RBC) mass that were evident within 4 weeks after pI:pC injection and sustained for more than 20 weeks (Figure 2A-C). Microcytosis was observed in heterozygous and homozygous Jak2V617F-expressing mice (Figure 2D). Leukocytosis, neutrophilia, and thrombocytosis were also observed in both heterozygous and homozygous Jak2V617F-expressing mice, although increases in the white blood cells (WBCs), neutrophils, and platelets were much more pronounced in peripheral blood of homozygous Jak2V617F mice compared with heterozygous mice (Figure 2E-G). Polycythemia was accompanied by significant increase in circulating reticulocytes (∼ 5% in heterozygous and ∼ 15% in homozygous Jak2V617F mice) (Figure 2H). Serum Epo level was significantly reduced in both heterozygous and homozygous Jak2V617F mice (Figure 2I), as commonly observed in PV patients.19,20 BM cellularity (total BM cell count) was markedly reduced particularly in homozygous Jak2V617F mice (Figure 2J), probably due to fibrosis in the BM (see “Discussion”). Both heterozygous and homozygous Jak2V617F mice showed splenomegaly, although homozygous Jak2V617F expression resulted in much larger spleen size compared with heterozygous Jak2V617F (Figure 2K). All these features were consistently observed in 100% of the animals expressing heterozygous or homozygous Jak2V617F.
Mice expressing Jak2V617F develop MPN. Peripheral blood hematocrit (A), hemoglobin (B), and RBC (C) counts were significantly increased in heterozygous and homozygous Jak2V617F mice compared with controls (V617F/+). Mean corpuscular volume (MCV; D) was significantly reduced in both heterozygous and homozygous Jak2V617F mice compared with controls (V617F/+). WBC (E), neutrophil (F), and platelet (G) counts were also significantly increased in both heterozygous and homozygous Jak2V617F mice compared with controls. However, the WBC, neutrophil, and platelet counts were much greater in peripheral blood of Jak2V617F homozygous mice compared with heterozygous Jak2V617F mice. Blood counts at 4, 8, 12, 16, and 20 weeks after induction with pI:pC are shown. (n = 30 at all time points for V617F/+ control; n = 30 at all time points for heterozygous Jak2V617F; n = 10 at 4, 8, 12 weeks, n = 6 at 16 and 20 weeks for homozygous Jak2V617F mice). (H) Reticulocyte counts were markedly increased in the peripheral blood of homozygous Jak2V617F mice. (I) Serum Epo level was significantly reduced in both heterozygous (n = 9) and homozygous Jak2V617F (n = 6) mice compared with controls (n = 9). (J) BM cellularity (total BM cell count; was significantly reduced (12 to 16 weeks after induction) in mice expressing homozygous Jak2V617F. (K) Spleen weight/size was significantly increased in Jak2V617F heterozygous (n = 20) and homozygous (n = 9) mice compared with controls (n = 20; 12 to 16 weeks after induction). *Significance between control and heterozygous or between control and homozygous; **significance between control and homozygous as well as between heterozygous and homozygous Jak2V617F mice; P < .05 determined by unpaired, 2-tailed Student t test.
Mice expressing Jak2V617F develop MPN. Peripheral blood hematocrit (A), hemoglobin (B), and RBC (C) counts were significantly increased in heterozygous and homozygous Jak2V617F mice compared with controls (V617F/+). Mean corpuscular volume (MCV; D) was significantly reduced in both heterozygous and homozygous Jak2V617F mice compared with controls (V617F/+). WBC (E), neutrophil (F), and platelet (G) counts were also significantly increased in both heterozygous and homozygous Jak2V617F mice compared with controls. However, the WBC, neutrophil, and platelet counts were much greater in peripheral blood of Jak2V617F homozygous mice compared with heterozygous Jak2V617F mice. Blood counts at 4, 8, 12, 16, and 20 weeks after induction with pI:pC are shown. (n = 30 at all time points for V617F/+ control; n = 30 at all time points for heterozygous Jak2V617F; n = 10 at 4, 8, 12 weeks, n = 6 at 16 and 20 weeks for homozygous Jak2V617F mice). (H) Reticulocyte counts were markedly increased in the peripheral blood of homozygous Jak2V617F mice. (I) Serum Epo level was significantly reduced in both heterozygous (n = 9) and homozygous Jak2V617F (n = 6) mice compared with controls (n = 9). (J) BM cellularity (total BM cell count; was significantly reduced (12 to 16 weeks after induction) in mice expressing homozygous Jak2V617F. (K) Spleen weight/size was significantly increased in Jak2V617F heterozygous (n = 20) and homozygous (n = 9) mice compared with controls (n = 20; 12 to 16 weeks after induction). *Significance between control and heterozygous or between control and homozygous; **significance between control and homozygous as well as between heterozygous and homozygous Jak2V617F mice; P < .05 determined by unpaired, 2-tailed Student t test.
To examine whether the phenotype observed in the Jak2V617F knock-in mice is cell autonomous, we transplanted BM or spleen cells (2 × 106 per recipient) from diseased MxCre;V617F/+ mice (which were backcrossed to C57BL/6 background for 4 generations) 20 weeks post-pI:pC induction into lethally irradiated C57BL/6 recipients. Elevated RBCs, hemoglobin, and hematocrit were observed in the recipients (n = 8) within 4 weeks after transplantation (supplemental Table 1). Thus, the phenotype observed in MxCre;V617F/+ mice is cell autonomous.
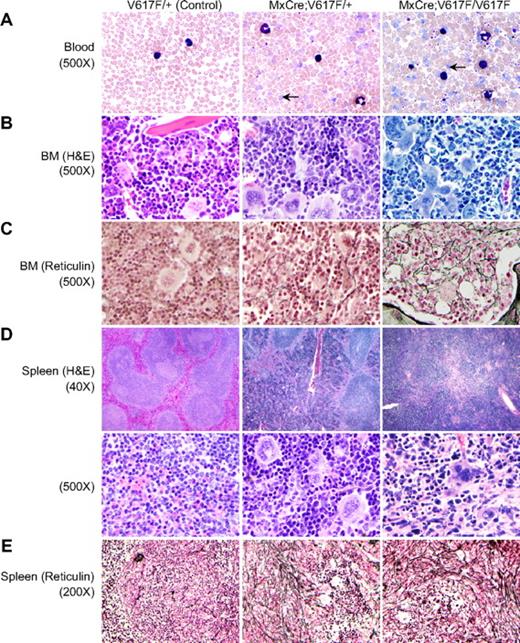
Histopathologic analyses also revealed polycythemia in mice expressing both heterozygous and homozygous Jak2V617F. Peripheral blood smears showed increased RBCs, reticulocytes, neutrophils, and platelets in heterozygous and homozygous Jak2V617F mice, with greater elevations in peripheral blood of homozygous compared with heterozygous mice (Figure 3A). BM from induced heterozygous and homozygous Jak2V617F mice showed hypercellularity with trilineage hyperplasia (Figure 3B). Reticulin staining indicated the presence of mild fibrosis in the BM of older heterozygous Jak2V617F mice (24 weeks after induction) that was noticeably increased in the BM of homozygous Jak2V617F mice (Figure 3C). Spleen sections from heterozygous and homozygous Jak2V617F mice exhibited effacement of normal splenic architecture with attenuated white pulp and markedly expanded red pulp, increased numbers of megakaryocytes and clusters of immature erythroid precursors (Figure 3D). Reticulin staining showed pronounced fibrosis of the white pulp in the spleens of both heterozygous and homozygous Jak2V617F mice (Figure 3E). Spleens of homozygous Jak2V617F mice showed somewhat increased reticulin fibrosis in the red pulp compared with that of heterozygous mice (Figure 3E).
Histopathologic characterization of the MPN induced by Jak2V617F. (A) Peripheral blood smears show increased RBCs, leukocytes, and platelets in induced heterozygous and homozygous Jak2V617F mice (12 weeks after induction). Leukocytosis and thrombocytosis were more pronounced in homozygous Jak2V617F mice compared with heterozygous Jak2V617F animals. Arrows point to reticulocytes. (B) BM sections from induced Jak2V617F heterozygous and homozygous mice show trilineage hyperplasia (hematoxylin and eosin staining, ×500). (C) Reticulin staining on the BM sections (×500) shows mild fibrosis (grade 1) in older Jak2V617F heterozygous mice (24 weeks after induction), whereas homozygous mice show more reticulin fibrosis (grade 2) than heterozygous mice. (D) Spleens from heterozygous and homozygous Jak2V617F mice display extensive destruction of normal splenic architecture (×40 and ×500) with attenuated white pulp and markedly expanded red pulp, increased numbers of megakaryocytes, and clusters of immature erythroid precursors. (E) Reticulin staining of the heterozygous spleen shows increased fibrosis of the white pulp and slight reticulin fibrosis of the red pulp. Spleens from homozygous mice show pronounced reticulin fibrosis in the white pulp and also increased fibrosis in the red pulp compared with heterozygous mice.
Histopathologic characterization of the MPN induced by Jak2V617F. (A) Peripheral blood smears show increased RBCs, leukocytes, and platelets in induced heterozygous and homozygous Jak2V617F mice (12 weeks after induction). Leukocytosis and thrombocytosis were more pronounced in homozygous Jak2V617F mice compared with heterozygous Jak2V617F animals. Arrows point to reticulocytes. (B) BM sections from induced Jak2V617F heterozygous and homozygous mice show trilineage hyperplasia (hematoxylin and eosin staining, ×500). (C) Reticulin staining on the BM sections (×500) shows mild fibrosis (grade 1) in older Jak2V617F heterozygous mice (24 weeks after induction), whereas homozygous mice show more reticulin fibrosis (grade 2) than heterozygous mice. (D) Spleens from heterozygous and homozygous Jak2V617F mice display extensive destruction of normal splenic architecture (×40 and ×500) with attenuated white pulp and markedly expanded red pulp, increased numbers of megakaryocytes, and clusters of immature erythroid precursors. (E) Reticulin staining of the heterozygous spleen shows increased fibrosis of the white pulp and slight reticulin fibrosis of the red pulp. Spleens from homozygous mice show pronounced reticulin fibrosis in the white pulp and also increased fibrosis in the red pulp compared with heterozygous mice.
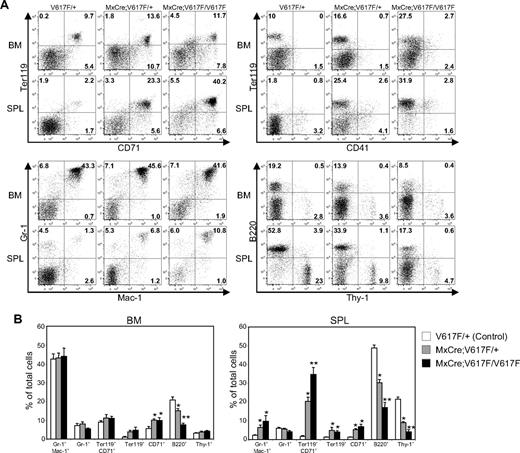
Flow cytometric analysis showed an approximately 10-fold increase in CD71/Ter-119–positive populations in the spleen of heterozygous Jak2V617F mice compared with control (V617F/+) mice (Figure 4A-B). Spleens of homozygous Jak2V617F mice showed an even greater increase (∼20-fold) in CD71/Ter-119–positive populations. In addition, there was an increase in myeloid cells (Gr-1/Mac-1–positive) in the spleens of both heterozygous and homozygous Jak2V617F mice (Figure 4A-B). However, B-cell populations (B220-positive) were significantly reduced in the BM and spleens of heterozygous and homozygous Jak2V617F mice compared with control animals (Figure 4A-B). Together, these results suggest that expression of either heterozygous or homozygous Jak2V617F gives rise to a phenotype closely resembling human PV.
Flow cytometric analysis of BM and spleen from mice expressing Jak2V617F. (A) Dot plots demonstrate a marked increase in the Ter-119/CD71–postive populations in the spleens of heterozygous and homozygous mice compared with control (V617F/+) mice. Modest increases in Gr-1/Mac-1–positive cells in the spleen of heterozygous and homozygous Jak2V617F mice were observed. However, B-cell populations (B220-positive) were proportionately decreased in the BM and spleens of heterozygous and homozygous Jak2V617F mice compared with control animals. (B) Percentages of myeloid, erythroid, B- and T-lymphoid populations are shown in histograms as mean ± SEM. Data are presented as percentage of total cells (control, n = 11; heterozygous, n = 11; homozygous, n = 5). *Significance between control and heterozygous or between control and homozygous; **significance between control and homozygous as well as between heterozygous and homozygous; significant difference at P < .05.
Flow cytometric analysis of BM and spleen from mice expressing Jak2V617F. (A) Dot plots demonstrate a marked increase in the Ter-119/CD71–postive populations in the spleens of heterozygous and homozygous mice compared with control (V617F/+) mice. Modest increases in Gr-1/Mac-1–positive cells in the spleen of heterozygous and homozygous Jak2V617F mice were observed. However, B-cell populations (B220-positive) were proportionately decreased in the BM and spleens of heterozygous and homozygous Jak2V617F mice compared with control animals. (B) Percentages of myeloid, erythroid, B- and T-lymphoid populations are shown in histograms as mean ± SEM. Data are presented as percentage of total cells (control, n = 11; heterozygous, n = 11; homozygous, n = 5). *Significance between control and heterozygous or between control and homozygous; **significance between control and homozygous as well as between heterozygous and homozygous; significant difference at P < .05.
Jak2V617F expression alters the hematopoietic progenitor compartments
To determine how expression of Jak2V617F affects the hematopoietic progenitors, we examined the hematopoietic progenitor compartments in the BM and spleens of heterozygous and homozygous Jak2V617F mice. The Lin−Sca1+c-kit+ (LSK) compartment (containing hematopoietic stem cell [HSC]) and myeloid progenitor populations were significantly increased in both BM and spleen of induced heterozygous and homozygous Jak2V617F mice compared with control V617F/+ littermates (Figure 5A-B). Subsequent analyses of myeloid progenitors revealed that megakaryocyte/erythroid progenitors (MEP) were extensively expanded in mice expressing Jak2V617F (Figure 5A-B). The expansion of MEP population is more striking in the spleens of homozygous Jak2V617F mice compared with that of heterozygous Jak2V617F mice. Modest elevation of common myeloid progenitors (CMP) and granulocyte/macrophage progenitors (GMP) were also observed in the BM and spleen of heterozygous and homozygous Jak2V617F mice (Figure 5A-B).
Effects of Jak2V617F on hematopoietic progenitors. (A) Flow cytometric analysis of the LSK compartment (Lin−Sca1+c-kit+) and subsets of myeloid progenitors including CMP (Lin−Sca1−c-kit+CD34+FcγRII/IIIlo), GMP (Lin−Sca1−c-kit+CD34+FcγRII/IIIhigh), and MEP (Lin−Sca1−c-kit+CD34−FcγRII/III−) in the BM and spleen from control (n = 8), heterozygous (n = 8), and homozygous Jak2V617F (n = 5) mice. (B) Representative contour plots are shown. The percentage of LSK, CMP, GMP, and MEP is shown in histograms as mean ± SEM. Data are presented as percentage of total cells. *Significance between control and heterozygous or between control and homozygous; **significance between control and homozygous as well as between heterozygous and homozygous Jak2V617F mice; significant difference of P < .05. (C) Hematopoietic progenitor colonies. BM (2 × 104) and spleen (1 × 105) cells from control (n = 6), heterozygous (n = 6), and homozygous Jak2V617F (n = 5) mice were plated in complete methylcellulose (Methocult M3434) medium. BFU-E, CFU-GM, and CFU-GEMM colonies were counted on day 7. (D) Epo-independent CFU-E colonies. Spleen cells (1 × 105) from control, heterozygous, and homozygous Jak2V617F mice were plated in methylcellulose medium without any cytokine. CFU-E colonies were counted after 2 days.
Effects of Jak2V617F on hematopoietic progenitors. (A) Flow cytometric analysis of the LSK compartment (Lin−Sca1+c-kit+) and subsets of myeloid progenitors including CMP (Lin−Sca1−c-kit+CD34+FcγRII/IIIlo), GMP (Lin−Sca1−c-kit+CD34+FcγRII/IIIhigh), and MEP (Lin−Sca1−c-kit+CD34−FcγRII/III−) in the BM and spleen from control (n = 8), heterozygous (n = 8), and homozygous Jak2V617F (n = 5) mice. (B) Representative contour plots are shown. The percentage of LSK, CMP, GMP, and MEP is shown in histograms as mean ± SEM. Data are presented as percentage of total cells. *Significance between control and heterozygous or between control and homozygous; **significance between control and homozygous as well as between heterozygous and homozygous Jak2V617F mice; significant difference of P < .05. (C) Hematopoietic progenitor colonies. BM (2 × 104) and spleen (1 × 105) cells from control (n = 6), heterozygous (n = 6), and homozygous Jak2V617F (n = 5) mice were plated in complete methylcellulose (Methocult M3434) medium. BFU-E, CFU-GM, and CFU-GEMM colonies were counted on day 7. (D) Epo-independent CFU-E colonies. Spleen cells (1 × 105) from control, heterozygous, and homozygous Jak2V617F mice were plated in methylcellulose medium without any cytokine. CFU-E colonies were counted after 2 days.
Hematopoietic progenitor colony assays showed significant increase in BFU-E, CFU-GM, and CFU-GEMM colonies in the BM and spleens of heterozygous and homozygous Jak2V617F mice compared with control animals (Figure 5C). Spleens of homozygous Jak2V617F mice showed greater increase in BFU-E colonies compared with heterozygous Jak2V617F mice (Figure 5C). We also observed a large number of Epo-independent CFU-E colonies in the spleens of heterozygous and homozygous Jak2V617F mice (Figure 5D) indicating the presence of endogenous erythroid colonies (EEC), a hallmark feature of PV.21 The number of Epo-independent CFU-E colonies in the spleens of homozygous Jak2V617F mice was much higher than that of heterozygous Jak2V617F mice, suggesting that homozygous Jak2V617F expression causes greater expansion of the erythroid progenitors.
Differential effects of Jak2V617F gene dosage on cell signaling
To determine the effects of Jak2V617F gene dosage on hematopoietic signaling, we used primary erythroblasts derived from the BM and spleen of control, heterozygous, and homozygous Jak2V617F mice. Because Jak2V617F has much greater effects on erythroid progenitors than other hematopoietic progenitors, primary erythroblasts are more likely to reveal the actual effects of Jak2V617F on signaling that are relevant to the development of MPN. We obtained approximately 94% pure erythroblasts (CD71-positive) after 7 days of culture in an erythroblast medium (supplemental Figure 2A). Deletion of the Neo-stop cassette was also confirmed in the erythroblasts obtained from heterozygous and homozygous Jak2V617F mice by PCR (supplemental Figure 2B).
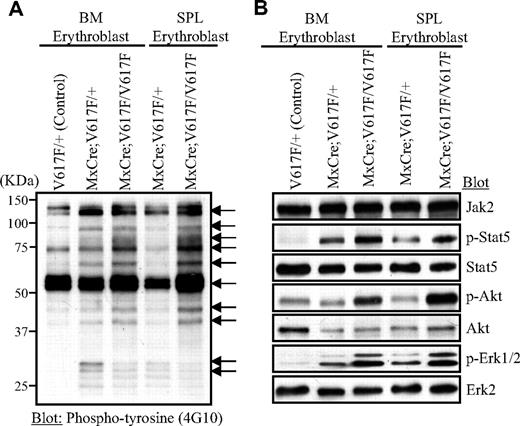
We analyzed the effect of Jak2V617F expression on total tyrosyl phosphorylation by immunoblotting of cell lysates from the growth factor starved erythroblasts using anti-phosphotyrosyl antibody. Several proteins (sizes 125, 95, 85, 73, 68, 56, 44, 42, 30, 28 kDa) exhibited increased tyrosyl phosphorylation compared with control erythroblasts, suggesting that they might be substrates of the activated Jak2V617F mutant (Figure 6A). Although the identities of these hyperphosphorylated proteins were not defined, they might play important role in signaling downstream of Jak2V617F. Marked differences were also observed in tyrosyl phosphoproteins between heterozygous and homozygous Jak2V617F-expressing erythroblasts (Figure 6A). Basal activation of Stat5, Akt, and Erk1/2, as monitored by immunoblotting with phospho-specific antibody, was increased in Jak2V617F-expressing erythroblasts compared with control erythroblasts (Figure 6B). However, homozygous Jak2V617F expression resulted in much higher activation of these signaling pathways than heterozygous Jak2V617F. Thus, Jak2V617F activates multiple downstream signaling pathways known to be important for transformation in a gene dosage-dependent manner.
Signaling effects of heterozygous and homozygous Jak2V617F in erythroid progenitors. Primary erythroblasts were derived from the BM and spleen of control (V617F/+), heterozygous, and homozygous Jak2V617F mice. For signaling studies, erythroblasts were starved in Iscove modified Dulbecco medium plus 0.5% BSA for 4 hours. Cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer and subjected to immunoblotting with anti-phosphotyrosine (4G10) antibody (A) or phospho-specific antibodies against Stat5, Akt, and Erk1/2 (B).
Signaling effects of heterozygous and homozygous Jak2V617F in erythroid progenitors. Primary erythroblasts were derived from the BM and spleen of control (V617F/+), heterozygous, and homozygous Jak2V617F mice. For signaling studies, erythroblasts were starved in Iscove modified Dulbecco medium plus 0.5% BSA for 4 hours. Cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer and subjected to immunoblotting with anti-phosphotyrosine (4G10) antibody (A) or phospho-specific antibodies against Stat5, Akt, and Erk1/2 (B).
Discussion
The identification of the JAK2V617F mutation in most patients with PV, ET, and PMF increased our understanding of the molecular basis of MPNs. Initial studies using BMT assays showed that overexpression of Jak2V617F results in polycythemia.10-13 However, in contrast to patients with PV, thrombocytosis was absent in the transplanted animals.10-13 Recently, 3 groups have generated transgenic mice expressing Jak2V617F.14-16 The phenotypes observed in these transgenic mice were variable. Moreover, incomplete penetrance of the disease was observed in these transgenic mice models. Although informative, the randomly integrated transgenic mice models have inherent variability in expression patterns and may not provide the best genetic context to study the effects of Jak2V617F gene dosage on signaling and MPN phenotype.
To assess the in vivo effects of Jak2V617F expression at appropriate physiologic gene dosages, we generated a conditional Jak2V617F knock-in mouse in which the expression of Jak2V617F is under control of the endogenous murine Jak2 promoter. Heterozygous Jak2V617F expression induces all the features of human PV in the knock-in mice, including polycythemia due to excessive production of erythrocytes, increased hematocrit and hemoglobin, neutrophilia, leukocytosis, thrombocytosis, and splenomegaly due to extramedullary hematopoiesis, and low serum Epo levels (Figure 2). All these features were consistently observed in 100% of the animals expressing heterozygous Jak2V617F. Thus, heterozygous Jak2V617F mutation is directly responsible and sufficient for the induction of PV.
Similar to the expression of heterozygous Jak2V617F, expression of homozygous Jak2V617F resulted in a PV-like phenotype. However, homozygous Jak2V617F expression was associated with a much greater increase in reticulocytes, leukocytes, neutrophils, and platelets. Splenomegaly was more pronounced in mice expressing homozygous Jak2V617F compared with heterozygous Jak2V617F. We also observed markedly increased myelofibrosis (grade 2) in the BM of Jak2V617F homozygous mice, while less reticulin fibrosis (grade 1) was detected in the BM of Jak2V617F heterozygous mice (24 weeks after induction) (Figure 3C). Myelofibrosis was evident in the BM of homozygous Jak2V617F mice as early as 10 weeks after induction. Total BM cell counts were significantly decreased in mice expressing homozygous Jak2V617F (Figure 2J), probably due to increased myelofibrosis in the BM, suggesting that homozygous Jak2V617F mutation may accelerate the progression of PV to post-PV myelofibrosis. Recent clinical studies also found an association of JAK2V617F homozygosity with increased leukocytosis, larger spleen size and secondary myelofibrosis in patients with PV.22,23
Patients homozygous for the JAK2V617F mutation exhibited higher leukocyte counts at diagnosis.23,24 Moreover, leukocytosis was identified as a major risk factor for thrombosis in patients with PV and ET.25,26 Several studies suggested an association of JAK2V617F homozygosity with thrombosis and major cardiovascular events in patients with PV and ET,24,27,28 although other studies could not find a good correlation between homozygous JAK2V617F expression and the risk of thrombosis in PV patients.23,29 We observed that several mice expressing homozygous Jak2V617F died within one week after induction of expression of the mutant Jak2V617F allele (data not shown). Postmortem analyses showed thrombosis of atria and ventricles in the hearts of these animals. Further studies are needed to determine whether homozygous Jak2V617F expression enhances the chances of cardiac thrombosis.
Quantification of the expression of Jak2V617F and WT Jak2 in the BM of heterozygous Jak2V617F knock-in mice showed 0.53:1 allelic ratio of mutant Jak2V617F to WT Jak2 (Figure 1E). Shide et al observed a low allelic ratio (0.25:1) of Jak2V617F to WT Jak2 in mice with ET, and slightly higher allelic ratio (∼ 0.45:1) in mice with PV, although one highest expresser in PV group had a ratio of 1:1.15 Overall, the correlation between expression of mutant allele and the phenotype reported in this study was not strong.15 Tiedt et al observed an allelic ratio of approximately 0.5:1 in mice with ET-like phenotype and an allelic ratio of approximately 1:1 in mice with PV.14 Although the allelic ratio in our heterozygous Jak2V617F mice (mutant to WT = 0.53:1) is lower than the allelic ratio in transgenic mice with PV (∼ 1:1), and slightly higher than the allelic ratio seen in transgenic mice with ET (∼ 0.5:1),14 we consistently observed a PV-like phenotype in all the heterozygous Jak2V617F mice (n = 40). One major difference between this and the study described by Tiedt et al is the use of human JAK2V617F transgene as opposed to mouse Jak2V617F used in our Jak2V617F knock-in mice. There is evidence that the mouse Jak2V617F is more active than the human JAK2V617F,14 therefore, we cannot directly compare our results with the study by Tiedt and colleagues.14
We also have analyzed the effects of Jak2V617F mutation on HSC and progenitor cells. Our results show that expression of Jak2V617F causes marked expansion of the MEP population in the BM and spleens of heterozygous or homozygous Jak2V617F mice, although a modest increase in HSC, CMP, and GMP populations was also observed in these animals (Figure 5A-B). The increase in MEP populations is more striking in the spleens of homozygous Jak2V617F mice than in heterozygous Jak2V617F mice. The presence of a huge number of cytokine-independent CFU-E colonies (Figure 5D) in the spleens of homozygous Jak2V617F mice suggests a robust expansion of the erythroid progenitors in these animals. Dupont et al also observed that most homozygous JAK2V617F erythroid progenitors in humans were Epo-independent and much more sensitive to Epo compared with heterozygous JAK2V617F erythroid progenitors.30 A previous study indicated that the differentiation potential of PV HSC was already skewed toward the erythroid lineage.31 Moreover, enforced expression of JAK2V617F in human cord blood progenitors resulted in enhanced erythroid colony formation.32 Thus, consistent with previous findings in humans, our current studies suggest a direct link between expression of Jak2V617F and expansion of erythroid progenitors. Future studies will determine whether transformation of erythroid progenitors by Jak2V617F would be sufficient to cause PV.
Previous studies have indicated an influence of genetic background on Jak2V617F-induced disease phenotype.10,13 In BMT models, expression of the Jak2V617F in Balb/c mice resulted in more pronounced leukocytosis, neutrophilia, and myelofibrosis than in C57BL/6 mice.10,13 These suggest that host genetic modifiers may act in concert with Jak2V617F in MPNs. Future studies using our Jak2V617F knock-in mice will determine whether genetic background alters the MPN phenotype, and identify the host genetic modifiers in MPNs. Interestingly, recent studies have identified single nucleotide polymorphisms in JAK2 that were associated with PV or ET.33,34
Our results also provide new insight into the effects of Jak2V617F gene dosage on hematopoietic signaling. Basal activation of Stat5, Akt, and Erk1/2 was significantly enhanced in erythroblast cells expressing homozygous Jak2V617F compared with heterozygous Jak2V617F (Figure 6B), consistent with the idea that WT Jak2 might compete with the mutant Jak2V617F protein when coexpressed in the same cells1,30 and that this competition might be lost in homozygous Jak2V617Fexpressing cells. Therefore, the degree of activation of downstream signaling pathways would be affected by the Jak2V617F gene dosage, which may explain the robust expansion of erythroid progenitors, marked leukocytosis and splenomegaly in mice expressing homozygous Jak2V617F. Future studies will determine the role and requirement of different signaling molecules/pathways in Jak2V617F-mediated MPN.
By using a novel inducible Jak2V617F knock-in allele, we have shown that Jak2V617F is primarily responsible for PV. Whereas heterozygous mouse Jak2V617F expression is sufficient to cause PV, homozygous Jak2V617F results in a PV associated with increased reticulocytosis, leukocytosis, thrombocytosis, splenomegaly, and a marked increase in erythroid progenitors. Further studies using this model should lead to a better understanding of the molecular pathogenesis of Jak2V617F-associated MPNs. Moreover, our inducible Jak2V617F knock-in mouse provides a unique and reproducible animal model to test novel therapies for Jak2V617F-associated diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Gordon Chan (Ontario Cancer Institute, Toronto, ON) and Dr David Dankort (University of California, San Francisco) for helpful discussion on the targeting construct, and Saeko Hamada for maintaining and genotyping mice. We acknowledge the assistance of the Dartmouth Transgenic Facility in generating the mice.
This work was supported in part by a grant from the National Institutes of Health (R01HL095685; M.G.M.).
National Institutes of Health
Authorship
Contribution: H.A. performed research and analyzed data; D.Y. and H.Z. performed research; S.F. provided expert help with the gene targeting in ES cells and revised the paper; R.E.H. performed histopathologic analysis and revised the paper; and M.G.M. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Golam Mohi, Department of Pharmacology, SUNY Upstate Medical University, 750 East Adams St, Syracuse, NY 13210; e-mail: mohim@upstate.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal