Abstract
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is a treatment option for patients with hematopoietic malignancies that is hampered by treatment-related morbidity and mortality, in part the result of opportunistic infections, a direct consequence of delayed T-cell recovery. Thymic output can be improved by facilitation of thymic immigration, known to require precommitment of CD34+ cells. We demonstrate that Delta-like ligand-mediated predifferentiation of mobilized CD34+ cells in vitro results in a population of thymocyte-like cells arrested at a T/natural killer (NK)–cell progenitor stage. On intrahepatic transfer to Rag2−/−γc−/− mice, these cells selectively home to the thymus and differentiate toward surface T-cell receptor–αβ+ mature T cells considerably faster than animals transplanted with noncultured CD34+ cells. This finding creates the opportunity to develop an early T-cell reconstitution therapy to combine with HSCT.
Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has shown impressive results in the treatment of leukemia.1,2 HSCT is hampered by treatment-related mortality resulting from opportunistic infections.2-4 One obvious causative factor for enhanced susceptibility to pathogens is the slow recovery of T cells in the periphery.3,5 In mice, it is established that T-lineage–directed progenitors generated in vitro have the capacity to migrate to the thymus and complete their development into host-tolerant T cells.6,7 Because repopulation of the thymus after transplantation is considered a rate-limiting process, supplementation of in vitro pre–T-lineage–directed cells may be one approach with potential to improve T-cell levels after HSCT. However, not much is known about the in vitro T-lineage commitment potential of granulocyte colony-stimulating factor mobilized CD34+ (mCD34+) cells commonly used for haplo-HSCT. To stimulate development of T-lineage–directed cells, we use the TSt-4 murine thymic stromal cell line8 transduced with human Delta-like ligand (hDLL), demonstrated to have the capacity to generate T-lineage–directed cells from cord blood (CB) CD34+ cells.9 Importantly, the T-lineage–directed cells were arrested at the CD5+CD7+ stage, considered to be the murine equivalent of double-negative 2 or 3 thymocytes, the designated stage of T-lineage development suitable for direct thymic reconstitution.
The goal of this study is to examine the potential of mCD34+ cells to generate T-lineage–directed progenitors, determine whether this potential is influenced by a specific Notch ligand, and investigate whether mCD34+-cell–derived progenitors have the capacity to home to and mature in the thymus.
Methods
Cells
Purity of mobilized and CB cells, obtained after informed consent, was more than or equal to 95% CD34+ cells and less than or equal to 0.1% contaminating CD3/CD56+ cells. Natural killer (NK) cells were enriched from peripheral blood of healthy donors using the NK isolation kit according to the manufacturer's instructions (Miltenyi Biotec). Informed consent was obtained from all CD34+ cell donors in accordance with the Declaration of Helsinki. TSt-4 cell lines8-10 and K562 were grown in standard media.
Flow cytometry
All antibodies, materials, and equipment were obtained from BD Biosciences, unless stated otherwise in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Coculture
mCD34+ cells were seeded on monolayers of TSt-4 cells expressing Notch ligands DLL1 or DLL4 and maintained as described.9 IL-15 (20 ng/mL; R&D Systems) was added for differentiation toward NK-lineage. For transfer to mice, differentiated cells were sorted with a FACSAria. Purity of cells was 80% to 90%.
Polymerase chain reaction analysis
Primers and positive controls used for detection of T-cell receptor (TCR) rearrangements have been described.11
Cytotoxicity assay
A total of 2 × 104 fluorophor-labeled target cells (K562) were incubated with effectors at various effector/target ratios for 4 hours. Percentages of killed target cells were determined by flow cytometry.
Rag2−/−γc−/− mice
Mice were bred and maintained under specific pathogen–free conditions in accordance with the guidelines of the animal facility at the Institute for Research in Biomedicine. The Institutional Animal Care and Use Committee of the Institute for Research approved the transplantation procedure and maintenance of the mice. Newborn injection was done as described previously.12
Results and discussion
mCD34+ cells develop into T/NK-lineage progenitors on hDLL1/4 interaction
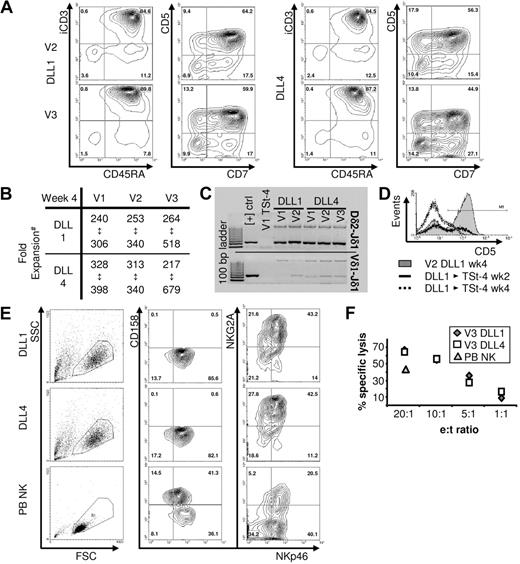
Sorted mCD34+ cells were cultured on monolayers of TSt-4 cells expressing either hDLL1 or hDLL4, and on TSt-4 control monolayers (supplemental Figure 1). First, we analyzed the time-dependent development of the phenotypic and genotypic markers associated with T-lineage development in human thymus.11,13 Intracellular CD3+ (iCD3) cells became detectable after 1 week of coculture, expanded strongly during the following weeks, and costained with T-lineage–associated surface markers (Figure 1A; supplemental Figure 2). After 4 weeks, the overall phenotype and percentage distribution of the cells had stabilized, similar to the differentiation kinetics described for CB-CD34+ cells on TSt-4-hDLL1.9 Within this time period, cell numbers had increased 200 to 600 times (Figure 1B) with on average 50% of the cells coexpressing iCD3, CD45RA, CD7, and CD5 (Figure 1A). Differences in overall phenotype of the cell populations were minimal between different mCD34+ cell donors, or whether TSt-4-hDLL1 or 4 was used to induce differentiation (Figure 1A).
mCD34+ cells develop into T/NK progenitors on DLL1/4 interaction. mCD34+ cells were obtained from 3 donors, V1 to V3. (A) Expression of indicated T-lineage–associated markers has stabilized after 4 weeks of culture on TSt-4-hDLL1/4 monolayers. For V2 and V3, the population percentage distribution of each marker combination falls within the following ranges: 88% to 92% for CD45RA+iCD3+ and 45% to 55% CD7+CD5+. For V1, these percentages are 63% to 68% and 37% to 58%, respectively. (B) #Numbers/donor represent minimum and maximum of fold increases observed in 2 or 3 independent experiments/donor. (C) DNA was isolated from cells cultured on TSt-4 and TSt-4-hDLL1/4 for polymerase chain reaction analysis of gene rearrangements at the TCR locus. At the δ-locus, all donors show clear Dδ-Jδ rearrangement products, whereas Vδ-Jδ rearrangement products are faint and therefore must have occurred less frequently. In contrast to positive controls, 2 products are amplified from DNA of mCD34+ cell–derived progenitors. Sequence analysis demonstrated that the large products (> 1000 bp) are partial rearrangements of the δ-locus. (D) When differentiated cells were cultured for another 4 weeks on TSt-4 without DLL, the population lost surface expression of the T-lineage–associated marker CD5. (E) Instead, cells gained expression of the NK-lineage–associated markers NKp46 and NKG2A but did not express the killer cell immunoglobulin-like receptor proteins CD158a-c. Again, there are no differences between hDLL1- or hDLL4-derived cells. Similar results were obtained with another donor. (F) TSt-4–derived cells have killing capacity when coincubated at indicated effector (e) target (t) ratios with the HLA class I–negative leukemia cell line K562. Each point represents the average of a triplicate analysis. Similar results were obtained with another donor. Purified peripheral blood (PB) NK cells from healthy controls always served as positive control.
mCD34+ cells develop into T/NK progenitors on DLL1/4 interaction. mCD34+ cells were obtained from 3 donors, V1 to V3. (A) Expression of indicated T-lineage–associated markers has stabilized after 4 weeks of culture on TSt-4-hDLL1/4 monolayers. For V2 and V3, the population percentage distribution of each marker combination falls within the following ranges: 88% to 92% for CD45RA+iCD3+ and 45% to 55% CD7+CD5+. For V1, these percentages are 63% to 68% and 37% to 58%, respectively. (B) #Numbers/donor represent minimum and maximum of fold increases observed in 2 or 3 independent experiments/donor. (C) DNA was isolated from cells cultured on TSt-4 and TSt-4-hDLL1/4 for polymerase chain reaction analysis of gene rearrangements at the TCR locus. At the δ-locus, all donors show clear Dδ-Jδ rearrangement products, whereas Vδ-Jδ rearrangement products are faint and therefore must have occurred less frequently. In contrast to positive controls, 2 products are amplified from DNA of mCD34+ cell–derived progenitors. Sequence analysis demonstrated that the large products (> 1000 bp) are partial rearrangements of the δ-locus. (D) When differentiated cells were cultured for another 4 weeks on TSt-4 without DLL, the population lost surface expression of the T-lineage–associated marker CD5. (E) Instead, cells gained expression of the NK-lineage–associated markers NKp46 and NKG2A but did not express the killer cell immunoglobulin-like receptor proteins CD158a-c. Again, there are no differences between hDLL1- or hDLL4-derived cells. Similar results were obtained with another donor. (F) TSt-4–derived cells have killing capacity when coincubated at indicated effector (e) target (t) ratios with the HLA class I–negative leukemia cell line K562. Each point represents the average of a triplicate analysis. Similar results were obtained with another donor. Purified peripheral blood (PB) NK cells from healthy controls always served as positive control.
To confirm T-lineage commitment, we analyzed early and late recombination events of TCR genes, Dδ-Jδ and Vδ-Jδ/Dβ-Jβ, respectively.11 After 4 weeks, T-lineage progenitors from all donors analyzed had either partially or completely rearranged the Dδ2-Jδ1 genes, whereas a smaller proportion had continued with rearrangement of the Vδ-Jδ genes (Figure 1C). There were no rearrangements of Dβ-Jβ genes detected (not shown).
Both phenotype and genotype of the T-lineage directed cells indicated that they were arrested at the T/NK pre-T-cell stages of T-lineage development.11,13-15 Indeed, on transfer to TSt-4 control monolayers, cells gradually lost the T-lineage–associated marker CD5 (Figure 1D) and gained expression of the NK-lineage–associated markers NKp46 and NKg2A (Figure 1E). Furthermore, the fact that the cells were able to kill the leukemia cell line K562 (Figure 1F) and did not express killer cell immunoglobulin-like receptor ligands (Figure 1E) established that they had developed into immature NK cells.16
T/NK progenitors fully mature on transfer to Rag2−/−γc−/− mice
To determine whether these T/NK progenitors were able to complete their maturation in vivo, adoptive transfer experiments were performed using Rag2−/−γc−/− mice. Figure 2A shows that human T/NK progenitors home to the thymus of these mice and become CD4+CD8+ double-positive (DP) thymocytes within 5 weeks after transfer. Furthermore, a clear population of surface CD3 and TCR-αβ–expressing thymocytes was observed within 7 weeks (Figure 2A). Along with the appearance of TCR-αβ+ cells, there was a concomitant decrease in total number of huCD45+ cells in these thymuses (Figure 2A). This demonstrates that the T/NK progenitors are able to complete their TCR gene rearrangements and implicates that a single shot of T/NK progenitors provides one spatiotemporal wave of developing thymocytes. No CD56 expression was detected in the thymus (supplemental Figure 3), nor could huCD45+ cells be found outside of the thymus in mice given T/NK progenitors (Figure 2C), indicating that the lineage development was restricted to T cells in Rag2−/−γc−/− mice.
T/NK progenitors fully mature on transfer to Rag2−/−γc−/− mice. mCD34+ were differentiated on monolayers of TSt-4 expressing hDLL for 4 weeks, after which they were purified by cell sorting and injected intrahepatically into newborn Rag2−/−γc−/− mice. (A) Both hDLL1- and hDLL4-derived T/NK progenitors are found exclusively in the thymus at both time points analyzed and have progressed toward the CD4+CD8+ DP stage at 4.5 weeks. A limited number of DP and few CD8 single-positive (SP) cells already have CD3 surface expression (supplemental Figures 3-4). The CD4+ cells at this time point are immature single-positive cells (ISP); sCD3− and CD5+CD7+. At 6.5 weeks, the percentages of huCD45+ have dropped sharply, but there is clear surface expression of sCD3 and TCR-αβ on T/NK progenitor-derived cells, on both DP and CD4/CD8 SP cells (supplemental Figure 4). (B-C) Next to T/NK progenitors, mice were given mCD34+ or CB-CD34+ cells. (B) Thymus compartment was analyzed at indicated time points. Thymocyte subsetting is based on coexpression of CD5 and CD7 only (double-negative), CD4+sCD3− (ISP), CD4+CD8+ (DP), and CD4+sCD3+ or CD8+sCD3+ (SP). The left 2 bars of each graph represent the data from mice displayed in panel A. Compared with mCD34+ and CB-CD34+ cells, predifferentiation of mCD34+ cells results in a higher percentage of cells at the DP stage at 4.5 weeks, but few cells are left at 6.5 weeks. (C) The total number of huCD45+ cells/tissue shows that T/NK progenitors are present temporarily and exclusively in the thymus.
T/NK progenitors fully mature on transfer to Rag2−/−γc−/− mice. mCD34+ were differentiated on monolayers of TSt-4 expressing hDLL for 4 weeks, after which they were purified by cell sorting and injected intrahepatically into newborn Rag2−/−γc−/− mice. (A) Both hDLL1- and hDLL4-derived T/NK progenitors are found exclusively in the thymus at both time points analyzed and have progressed toward the CD4+CD8+ DP stage at 4.5 weeks. A limited number of DP and few CD8 single-positive (SP) cells already have CD3 surface expression (supplemental Figures 3-4). The CD4+ cells at this time point are immature single-positive cells (ISP); sCD3− and CD5+CD7+. At 6.5 weeks, the percentages of huCD45+ have dropped sharply, but there is clear surface expression of sCD3 and TCR-αβ on T/NK progenitor-derived cells, on both DP and CD4/CD8 SP cells (supplemental Figure 4). (B-C) Next to T/NK progenitors, mice were given mCD34+ or CB-CD34+ cells. (B) Thymus compartment was analyzed at indicated time points. Thymocyte subsetting is based on coexpression of CD5 and CD7 only (double-negative), CD4+sCD3− (ISP), CD4+CD8+ (DP), and CD4+sCD3+ or CD8+sCD3+ (SP). The left 2 bars of each graph represent the data from mice displayed in panel A. Compared with mCD34+ and CB-CD34+ cells, predifferentiation of mCD34+ cells results in a higher percentage of cells at the DP stage at 4.5 weeks, but few cells are left at 6.5 weeks. (C) The total number of huCD45+ cells/tissue shows that T/NK progenitors are present temporarily and exclusively in the thymus.
As mentioned, in T/NK progenitor–injected mice, all huCD45+ cells were CD4+CD8+ DP by week 5, in contrast to animals that had received either CB- or mCD34+ cells. In these latter groups, T-lineage development was delayed by approximately 2 weeks, as percentage DP along with total number of huCD45+ cells at week 7 corresponded with week 5 data of mice supplied with T/NK progenitors (Figure 2B; supplemental Figure 4), confirming the advantage provided by in vitro DLL-mediated predifferentiation.
When compared, there were not many huCD45+ cells left in bone marrow at week 7 in mCD34+ cell–transplanted mice (Figure 2C). Apparently, the long-term engraftment potential of CB is favorable over mCD34+ cells in Rag2−/−γc−/− mice.
At 7 weeks, one mouse given hDLL4 T/NK progenitors had very few huCD45+ cells left in the thymus (Figure 2B-C), and none had mature T cells in the periphery. This is not unexpected because more than 95% of the thymocytes do not survive the positive/negative selection process, and the underdeveloped thymuses of Rag2−/−γc−/− mice have considerably reduced capacity. Even in CB-CD34+ cell–transplanted mice it requires continuous immigration of pro-thymocytes for 8 to 10 weeks before mature T cells can be found outside the thymus.12
The most important finding of this study is that mCD34+ cell–derived, in vitro–generated T/NK progenitors are able to migrate to the thymus and continue their development toward mature T cells. Conceptual similar findings were recently described for CB-derived progenitors.17 Prospectively, cotransplantation or sequential transplantation of CD34+ cells and T/NK progenitors will narrow the immunocompromised window of HSCT patients considerably. Furthermore, the donor-derived thymus-matured T cells will also contain a patient-tolerant regulatory T-cell repertoire that may reduce graft-versus-host disease–related complications.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Henk van Rie and Jozien Jasper-Spits for cell sorting.
This work was supported by Senternovum (W.T.V.G., G.M.J.B.) and the Bill and Melinda Gates Foundation Grand Challenges in Global Health Program (M.G.M.).
Authorship
Contribution: B.M. designed and performed experiments and designed and wrote the paper; S.C. designed and performed experiments and wrote the paper; C.B. and C.H.M.J.V.E. designed and performed experiments; J.V., M.C.A.S., M.W.M.v.d.P., and B.L. performed experiments; R.H. organized research at PharmaCell; M.G.M., Y.K., and H.K. organized research, designed experiments at Institute for Research in Biomedicine and RIKEN, respectively, and helped write the paper; W.T.V.G. designed the project, designed experiments, and wrote the paper; and G.M.J.B. designed and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wilfred T. V. Germeraad, Department of Internal Medicine, Maastricht University Medical Center, P O Box 616, 6200 MD Maastricht, The Netherlands; e-mail: w.germeraad@immuno.unimaas.nl.
References
Author notes
*B.M. and S.C. contributed equally to this study.
†C.B. and C.H.M.J.V.E. share second authorship.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal