The Kindlin family of intracellular proteins has recently emerged as key regulators of cellular functions and cell-matrix interactions. The 3 members of this family, Kindlin-1, -2, and -3, perform an essential role in activation of integrin adhesion receptors, and expression of at least 1 Kindlin paralog is required to enable integrin activation in physiologically relevant settings. In humans, deficiencies in Kindlin-3 lead to a number of abnormalities affecting hemostasis, the immune system, and bone function, whereas the lack of Kindlin-1 causes profound skin defects. The importance of Kindlins is underscored by the results of animal knockout studies, which clearly show the indispensable and nonredundant functions of all 3 Kindlins in development and normal physiology. This review discusses recent progress in the studies of Kindlin protein family, emphasizing newly identified functions and potential mechanisms underlying differential activities of the family members.
Introduction
Cell adhesion to extracellular matrix (ECM) is crucial for tissue integrity in all metazoans. In solid tissues, cells incorporate into their ECM environment, and their survival and proliferation depend on their adherence to the substratum. One of the notable exceptions to this paradigm of tissue structure is blood. Unlike adherent cells, most of the blood cells do not bind their ECM ligands constitutively and do not depend on adhesion for their survival. Cell-ECM interactions are mediated by the integrin family of noncovalent heterodimeric adhesion receptors, which provide a major mechanical link between cellular signaling machinery and the surrounding ECM. On blood cells, integrins mediate cell-cell and cell-ECM interactions that are vital for a number of physiologic processes, including but not limited to leukocyte adhesion and extravasation into inflamed tissues, and platelet aggregation during thrombus formation.
Most blood cells are constantly surrounded by an excess of integrin ligands in plasma, such as fibrinogen or fibronectin; however, under quiescent conditions, integrins remain inactive and cells are not adherent. The activity of integrins is tightly regulated to permit rapid adhesion and aggregation of blood cells in response to injury but yet prevent excessive reactions. Two interrelated but conceptually distinct mechanisms, affinity and avidity modulation, provide such tight control of integrin function. Affinity modulation entails a rapid and specific change in integrin conformation to increase affinity for soluble ligands, whereas avidity modulation alters the number and density of ligand-receptor bonds on the cell surface.1 Collectively, these changes are commonly referred to as integrin activation and are the consequence of inside-out signaling triggered by stimulation of the integrin-bearing cell by agonists. Integrin activation is set in motion by a cascade of intracellular signaling events that creates a unique pathway that ultimately converges at the short cytoplasmic tails (CTs) of the integrins (Figure 1). Tight regulation of integrin affinity is particularly crucial for blood cells, where signaling-independent integrin activation would have undesirable consequences, such as thrombosis. Thus, the understanding of integrin activation will provide insights into a mechanism that is of fundamental importance in blood cell biology. At the same time, an understanding of the details of integrin activation in anchorage-independent cells of hematopoietic origin may allow design of novel strategies for treatment of diseases, which are among the leading causes of mortality: myocardial infarction, stroke, and cancer.
Schematic representation of the signaling pathway from an agonist stimulation to integrin activation. CalDAG GEFI, a GDP/GTP exchange factor specific for Rap small G proteins, and protein kinase C (PKC) activated by a receptor-dependent stimulation of phospholipase C. As a result, Rap1*GTP interaction with RIAM protein (not shown) stimulates talin-1 function in the inside-out pathway. Protein kinase C has been implicated in the signaling as well, although the substrates important for the signaling are currently unknown. Talin-1 and Kindlin FERM domain are shown as spheres with 3 subdomains; the intersecting PH domain in Kindlin is shown as the dark-colored segment.
Schematic representation of the signaling pathway from an agonist stimulation to integrin activation. CalDAG GEFI, a GDP/GTP exchange factor specific for Rap small G proteins, and protein kinase C (PKC) activated by a receptor-dependent stimulation of phospholipase C. As a result, Rap1*GTP interaction with RIAM protein (not shown) stimulates talin-1 function in the inside-out pathway. Protein kinase C has been implicated in the signaling as well, although the substrates important for the signaling are currently unknown. Talin-1 and Kindlin FERM domain are shown as spheres with 3 subdomains; the intersecting PH domain in Kindlin is shown as the dark-colored segment.
In the current review, we discuss recent advances regarding the family of Kindlin proteins, also known by several other names (GenBank designation FERMT1, FERMT2, and FERMT3). For consistency, we refer to this family as Kindlins throughout this review. The Kindlin family members are now known to be indispensable for integrin inside-out signaling (Figure 1), and expression of a Kindlin protein is necessary to enable integrin activation. This function of Kindlins requires cooperation with talin-1, a large cytoskeletal protein.2,,,,,–8 Engagement of both Kindlin and talin represents the final events of integrin inside-out signaling. The presence of only one of these components is not sufficient to achieve productive integrin activation under physiologic conditions. In this review, we place a particular emphasis on Kindlin-3 because of its importance in the responses of hematopoietic cells. An underlying premise of this review is that, in addition to their common roles in integrin activation, the 3 Kindlins might have distinct functions, with Kindlin-3 being particularly suited for rapid integrin activation of integrins in blood cells.
Kindlin expression patterns
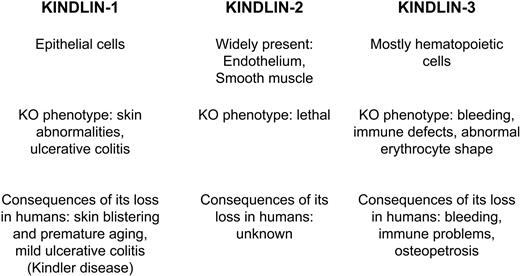
Unlike talin-1, which is expressed ubiquitously, Kindlins are expressed in a tissue-restricted manner (Figure 2).9 Thus, cell type–specific nuances of integrin regulation might be defined by expression of the individual Kindlin protein. Kindlin-1 is expressed in epithelial cells; Kindlin-2 (referred to as Mig-2 or PLEKHC1 in some studies) has broad expression in all solid tissues of mesenchymal origin; and Kindlin-3 (also referred to as URP2, Mig-2B) is expressed primarily in hematopoietic cells. These expression patterns might determine differences in the function of integrins in these tissues. Epithelial cells that form a defensive barrier against the external environment, such as skin keratinocytes and colonic epithelium, use Kindlin-1 to establish a continuous layer of adherent cells. Likewise, cells composing major organs use Kindlin-2 to embed in their 3-dimensional matrix scaffold, which, in turn, controls their organization. In blood cells, where Kindlin-3 is a main player, integrins and cell adhesion need to transit quickly from an inactive to a fully active state. Within mammalian cells, Kindlin-2 localizes to integrin containing focal adhesion complexes (FA),9 whereas Kindlin-3 is not targeted to FA at least when overexpressed in murine fibroblasts. This difference in FA association probably reflects differences in specific binding partners of the 2 Kindlins, which mediate their association with FA. Endogenous Kindlin-3 is present in podosomes,9 integrin adhesion structures formed by migratory cells.10 Podosomes are more transient in nature than FA, and they have different protein composition. Kindlin-1 association with FA requires its N-terminal F0 domain,11 which diverges significantly from that of Kindlin-3 (see next section). FA and other adhesion structures change in composition and location as cells attach, spread, and migrate, and the associations of Kindlins will undoubtedly be found to change dynamically during cellular responses. Nuclear localization of Kindlin-212 and Kindlin-113 has been observed as well, although the functional significance of this localization remains unknown.
Schematic representation of the Kindlin protein family in mammals. Tissue distribution and consequences of Kindlins' loss in animal models and human genetic disorders are shown side by side for comparison.
Schematic representation of the Kindlin protein family in mammals. Tissue distribution and consequences of Kindlins' loss in animal models and human genetic disorders are shown side by side for comparison.
Structural insights
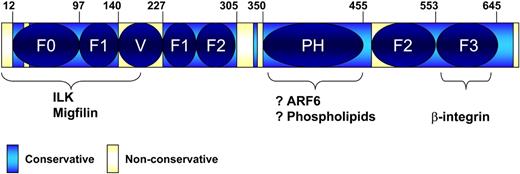
All 3 paralogs, Kindlin-1, -2, and -3, are highly conserved with 53% identity between the least similar members, Kindlin-2 and -3.9 Sequence comparison reveals invariable regions interspaced with variable sequences (Figure 3). A characteristic feature of the invariable region of Kindlins is a FERM domain, which constitutes a major portion of the entire proteins. FERM domains (so named for their presence in 4.1, ezrin, radixin, and moesin) are interaction domains and are commonly found in many cytoskeletal proteins. FERM domains in Kindlins and other proteins are composed of 3 subdomains (F1, F2, and F3), and the core FERM sequence is often preceded by a less well-defined F0 subdomain. The domain architecture of Kindlins is shown in Figure 3. The F3 subdomain of Kindlins resembles a phosphotyrosine-binding domain (PTB), which is also found in the FERM domain of talin-1.14 Mutational analyses suggest that it is the F3 subdomain that is involved in binding of both talin and Kindlins to integrin-β CT.14 A general property of PTB domains is their ability to bind NXXY motifs. Integrin-β subunits typically contain 2 NXXY. As discussed in the binding partners section, one of these NXXY motifs is involved in talin binding and the other in Kindlin binding.
Schematic domain architecture of Kindlins. Overlap of the defined subdomains with the conserved and nonconserved regions. F0, F1, F2, and F3 indicate subdomains of the FERM domain; and PH, pleckstrin homology domain. Variable region (V) intersects the F1 subdomain, splitting it into 2 parts. PH domain intersects the F2 subdomain. Numbers of Kindlin-3 amino acid residues are shown on the top.
Schematic domain architecture of Kindlins. Overlap of the defined subdomains with the conserved and nonconserved regions. F0, F1, F2, and F3 indicate subdomains of the FERM domain; and PH, pleckstrin homology domain. Variable region (V) intersects the F1 subdomain, splitting it into 2 parts. PH domain intersects the F2 subdomain. Numbers of Kindlin-3 amino acid residues are shown on the top.
Originally, Kindlin-3 was cloned as a gene highly homologous to Kindlin-1,15 which highlights the extent of sequence identity among the Kindlins. Similar to Kindlin-1 and -2, the F3 subdomain of Kindlin-3 is also able to interact with the CT of at least 3 integrin subclasses: β-1, -2, and -3.5,6 Considering the high degree of amino acid identity in the F3 subdomains of the 3 Kindlins, any difference in the functions of Kindlin-2 and -3 in integrin activation is probably not defined by this region per se but by the influence of other domains on the F3 interactions.
The F2 subdomain of all 3 Kindlins is interrupted by a pleckstrin homology (PH) domain, which are broadly distributed and exhibit phospholipid-binding properties.16 In general, the PH domain is another invariant region of the Kindlins but is less well conserved than the F3. PH domains bind phospholipids and a variety of other ligands.16 Phosphoinositides do bind to the Kindlin-2 PH domain (Y.-Q. Ma, E.F.P., unpublished data, 2008), although the functional importance of such interactions remains to be demonstrated. Notably, deletion of the PH domain from Kindlin-2 slightly diminishes but does not eliminate its coactivator function.3 In contrast, deletions of either N-terminal region encompassing F0 and partial F1 subdomains, or the F3 subdomain, as well as QW614/615AA point mutations in F3 all incapacitate the ability of Kindlin-2 to coactivate integrins.3 Moderate sequence variations among the PH domains might impart binding properties to Kindlin-3 that are different from those of Kindlin-2. For instance, the PH domain in Kindlin-3, like the majority of PH domains, may be able to bind ligands other than phospholipids.16 Interactions between PH domains and small G-proteins, including ARF1 and ARF6, might allow targeting of PH-containing molecules to specific subcellular compartments.17,18 ARF-6's GTP/GDP exchange factor (GEF), Cytohesin-1, has been implicated in activation of integrins through its GEF activity.19 Thus, ARF6 might contribute to selective localization of one of the Kindlins by targeting it to the inner surface of cell membrane, providing a mechanism for differential localization and function of the 3 Kindlin paralogs. Another basis for the differential regulation of cellular responses by the different family members also resides in the PH domain of Kindlin-3. There are 2 known splice variants of Kindlin-3: the longer isoform has a 4-amino acid insert resulting from usage of an alternative splicing site. The variable sequence resides in the N-terminal region of PH domain and has the characteristics of a potential SH3-binding site (PXXP). The shorter isoform of Kindlin-3 lacks this motif.
Comparative studies of the N-terminal domain of Kindlin-1 with that of talin-1 led to the proposal that this region of Kindlins coincides with a previously unrecognized F0 subdomain.11 Thus, the structural organizations of Kindlins and talin-1 might be even more similar than originally anticipated.11 In agreement with structure-function studies of Kindlin-1 and -2, Kindlin-3's N-terminal region is also required for the protein function as Kindlin-3Δ1-180 (corresponding to a truncated form derived by use of an alternative initiation codon) is not able to rescue inside-out signaling in Kindlin-3–deficient lymphocytes.4 By analogy with Kindlin-2, the N-terminal region of Kindlin-3 might be necessary for the interaction with integrin-β CT because the interaction of Kindlin-2 with its N-terminal region deleted is reduced3,20,21 compared with the full-length protein.
The F1 regions in the FERM domains of Kindlin proteins are interrupted by nonconservative inserts of variable length, and such an insert is also present in talin-1.11 Side-by-side alignment of the Kindlin-1, -2, and -3 reveals that the inserts are strikingly less conserved than the surrounding sequences. Moreover, the lengths of the inserts and extent of their similarities among the 3 paralogs set Kindlin-3 apart from the other 2 family members.11 The most N-terminal region in Kindlin-3, corresponding to amino acid residues 1 to 12, is another highly variable region among the Kindlins. As discussed in “Kindlin binding partners,” this part of Caenorhabditis elegans Kindlin (UNC-112) is required for interaction with ILK,22 thus identifying this small region of sequence variability as a potential site for paralog-specific functions.
In addition to these overall organization differences, variations in single amino acid also may provide a basis for paralog specific functions. As a specific example, threonine T91 in Kindlin-1 (T93 in Kindlin-2) is a potential site of phosphorylation by proline-dependent serine/threonine kinases, whereas Kindlin-3 has a glycine in the corresponding position of the otherwise conservative region of the F0 subdomain. Indeed, Kindlin-1 can be phosphorylated,23 pointing to modifications by protein kinases as another potential mechanism for influencing the function of Kindlins and doing so in a paralog-specific manner.
To summarize these structural considerations, although a body of evidence suggests a basis for common mechanisms of action for all 3 Kindlins, a mechanism dependent on their binding to integrin-β CT via their conserved PTB-like domain, paralog-specific regulation, can be envisioned based on differences in their sequences, which reside primarily in inserts in the F0 regions as well as on single amino acid variations.
Kindlin binding partners
At present, most functional studies were performed with Kindlin-2 and are now just being extended to the other 2 family members. Based on their high sequence homologies, it is predicted that many findings on Kindlin-2 will extrapolate to Kindlin-1 and -3; but based on the differences in tissue distribution and structure noted in “Structural insights,” some exceptions can be anticipated. In addition to F3-mediated interaction with integrin-β CT, there are 2 other known binding partners for Kindlin-2. The N-terminal domain (residues 1-217 of Kindlin-2) contains binding sites for migfilin and ILK.20,22,24 This region includes the F0 subdomain, the N-terminal part of F1, and a significant portion of the F1 insert. Deficiency of ILK has a profound effect on activation of integrins in platelets both in vitro and in vivo,25,26 raising the possibility that the function of the Kindlins in integrin activation involves ILK binding (Figure 1). The C elegans homolog of ILK, PAT4, interacts directly with UNC-112, the Kindlin homolog, in yeast 2-hybrid assays.22 The first 32 amino acid residues of UNC-112 are required for this interaction and are not well conserved among the 3 mammalian Kindlins. The N-terminal part of human Kindlin-2 is most similar to UNC-112, whereas Kindlin-3 is the least similar isoform. It remains to be established whether the coimmunoprecipitation of Kindlin-2 and ILK20 signifies a direct interaction and whether Kindlin-3 is able to form a similar complex.
The second Kindlin-2-binding partner is migfilin (Figure 1), a member of the zyxin family of LIM-containing proteins. Migfilin is a filamin-binding protein24,27 and filamin is an actin-binding protein.28 Therefore, migfilin provides a link between Kindlins -1, -2, and the actin cytoskeleton through its interaction with filamin,24 and it may influence integrin activation through this network of interactions. Kindlin-3 also appears to coimmunoprecipitate with migfilin (N.L.M., unpublished observation, 2009). Of interest, Kindlin-1 and -2 both coimmunoprecipitated with migfilin in the same cells,13 which may indicate a direct interaction with Kindlin-1 and -2, or migfilin may bridge the 2 Kindlins into a complex. The interaction of the Kindlins with other zyxin family members has yet to be reported. Knockdown of migfilin or introduction of a peptide that blocks its binding to filamin enhances integrin activation in several cell systems, including platelets.29 However, at this juncture, it is unclear whether the role of Kindlins in integrin activation depends on interaction with migfilin.
Of the binding partners most clearly linked to the functions of the Kindlin proteins are their interactions with integrin-β subunits. Compared in distant phyla, Kindlins are evolutionarily conserved proteins; Kindlin-2 and C elegans homolog UNC-112 have more than 40% identity, which implies conservation of function among these proteins. The absence of unc-112 gives rise to a phenotype similar to that observed when the homologs of the integrin-α and -β subunits are missing. Indeed, UNC-112 colocalizes with PAT-3, the integrin-β subunit, and a relationship between Kindlin and integrin expression patterns has been observed in all cells and organisms examined.22
There is a general agreement that each Kindlin family member interacts with at least 3 classes of integrins (β-1,-2, and -3). Unlike talin, which binds to the membrane proximal NXXY motif, NPLY 747 turns within the β3 CT, Kindlin-2 interacts with the membrane distal NXXY motif, NITY 759 in β3. The S752P mutation is shown to abrogate Kindlin-2 binding in vitro, suggesting an extended and complex interface for Kindlin/β3 integrin interaction.3 Kindlin-3 binding to the β3 CT was also shown to be abrogated by the S752P mutation within the β3 integrin,6 thus providing a potential explanation for the inability of the mutant integrin found in a patient with Glanzmann thrombasthenia to become activated.30 Although β1 CT interacts with both Kindlins, the functional consequence of Kindlin expression on talin-mediated activation of integrin α5β1 was inhibitory rather than stimulatory.21 This effect seemed to be independent of the Kindlins' ability to interact with integrin cytoplasmic domain because a point mutation in the F3 domain known to disrupt the interaction with integrins did not abolish this inhibitory effect. In case of integrin αIIβ3, both Kindlin-1 and -2 have stimulatory effects on talin-induced integrin activation, and both proteins were incapable of enhancing integrin activation without talin. A point mutation in the integrin binding site for Kindlins reversed their stimulatory effect on talin-induced activation, but their inhibitory effect on integrin function was still retained.21 In β2 integrins, the talin/Kindlin-binding NXXY motifs are, indeed, NXXF. This phenylalanine is still sufficient for Kindlin-3 binding,5 but a phenylalanine mutation to alanin F767A no longer supports this interaction.5 F755A mutation disrupting the membrane-proximal talin-binding site does not affect Kindlin-3 binding but precludes interaction with talin.5
Kindlin function: animal models and human diseases
Historically, the first described human genetic disorder caused by the deficiency of a Kindlin was Kindler syndrome, caused by a mutation in Kindlin-1 and characterized by skin blistering, severe periodontitis, and poikilodermia.31,32 Clinical manifestations of this disease were noted to resemble abnormalities in humans known to be associated with defects in ECM indicating potential defects in cell-matrix adhesion.33 Later, malfunctions in keratinocyte adhesion were shown to be associated with mutations in Kindlin-1,34,35 and in general the phenotype of animals deficient in Kindlin-1 concurs with these observations.36 Kindlin-1−/− mice die postnatally and exhibit symptoms of skin atrophy and reduced keratinocyte proliferation, consistent with defects in cell adhesion and spreading observed in vitro36 and with the symptoms of Kindlin-1 deficiency in humans.34,35 The cause of mortality in the mice was traced to ulcerative colitis resulting from shearing of intestinal epithelial cells.36 The phenotype of the mice illustrates the nonredundant functions of Kindlin-1 and -2 because the latter is also expressed in intestinal epithelial cells. Intracellular distribution of the 2 paralogs is different, with Kindlin-2 confined to cell-cell contacts and Kindlin-1 found throughout the cytoplasm.36 Intestinal symptoms have also been reported in some patients with Kindlin-1 deficiency; but unlike the animal model, Kindlin-1 mutations in humans do not result in fatal ulcerative colitis, highlighting interspecies variations. A high rate of squamous cell carcinoma has been reported in Kindler syndrome patients,37 but a seemingly opposite correlation was found in patients with lung and colon carcinomas with nonmutated Kindlin-1, where these cells displayed a 60% to 70% increase in Kindlin-1 mRNA level.15
Mouse Kindlin-2 knockout is embryonic lethal. The phenotype of Kindlin-2 knockdown in zebrafish embryos is consistent with defective integrin function, exerting a prominent effect on cardiac development.38 In vitro studies with cells derived from the Kindlin-2−/− mouse embryos or cells in which Kindlin-2 was knocked down by siRNA point to its significant role in integrin function.3,20 Human deficiencies of Kindlin-2 have not been reported and are predicted to be embryonic lethal similar to Kindlin-2 ablation in mice and zebrafish.20,38 The essential role of Kindlin-2 in development is also supported by its very broad expression compared with other members of the Kindlin family, with particularly high levels in smooth and skeletal muscle cells. Malignant transformation can alter the expression patterns of Kindlin-2 as has been reported in breast cancer cells and leiomyomas.12,39
Results of animal studies in which the Kindlin-3 gene was ablated provided critical insights into the function of the protein in cell adhesion. Kindlin-3 knockout mice have several pronounced phenotypic characteristics that do resemble the symptoms of patients with Kindlin-3 deficiency, including defects in hemostasis and inflammation. However, although humans seem to survive through their first years with dysfunctional Kindlin-3, Kindlin-3 deficiency in mice results in postnatal mortality within a week after birth.5,6,40 Mice deficient in Kindlin-3 have a decreased hematocrit,40 hemorrhage in multiple organs, and severe osteopetrosis.6 The cause of bleeding was traced to impaired activation of platelet integrin αIIbβ3. Platelet shape change in response to agonists was preserved, emphasizing a selective effect of Kindlin-3 deficiency on inside-out integrin signaling.6 Bypassing inside-out signaling by treatment of the cells with MnCl2 to activate integrin αIIbβ3 resulted in platelet adhesion on fibrinogen, but the platelets still failed to form lamellipodia, indicating that, in addition to its function in integrin activation, Kindlin-3 might regulate integrin-independent aspects of cytoskeletal rearrangements.6 A follow-up study of these mice revealed that impaired integrin activation is not restricted to platelets but also manifests a generalized defect in integrin activation affecting other hematopoietic cells, including leukocytes.5 Similar to patients with Kindlin-3 mutations (Table 1), mice transplanted with Kindlin-3 null bone marrow have 10-fold higher levels of circulating polymorphonuclear cells (PMNs) than wild-type animals.5 Administration of tumor necrosis factor-α reduced total leukocyte counts in wild-type but much less so in Kindlin-3 null mice, indicating that PMNs in the Kindlin-3−/− mice are unable to marginate or extravasate, which may account for the high blood levels of these cells. In vitro, Kindlin-3−/− PMNs did not adhere and spread in response to phorbol myristate acetate (PMA) when plated on ICAM-1, a ligand for αLβ2 and αMβ2, and did not bind soluble fibrinogen, a ligand for β2 and β3 integrins. This lack of response to PMA resembles the behavior of cells from patients with mutations in Kindlin-3 gene. Abnormal erythrocyte shape was observed in Kindlin-3 null animals40 but was not noted in humans.
Blood cell counts in male subject with bleeding and immune problems caused by mutation in Kindlin-3
| . | Blood cell count, ×109/L . | Normal range, ×109/L . |
|---|---|---|
| Neutrophils | 20-40 | 3-4 |
| Pan CD3+ T cells | 6.35 | 0.7-4.2 |
| CD4+ T cells | 4.65 | 0.3-2.7 |
| CD19+ B cells | 6.35 | 0.1-0.9 |
| CD16+ NK cells | 1.4 | 0-0.8 |
| . | Blood cell count, ×109/L . | Normal range, ×109/L . |
|---|---|---|
| Neutrophils | 20-40 | 3-4 |
| Pan CD3+ T cells | 6.35 | 0.7-4.2 |
| CD4+ T cells | 4.65 | 0.3-2.7 |
| CD19+ B cells | 6.35 | 0.1-0.9 |
| CD16+ NK cells | 1.4 | 0-0.8 |
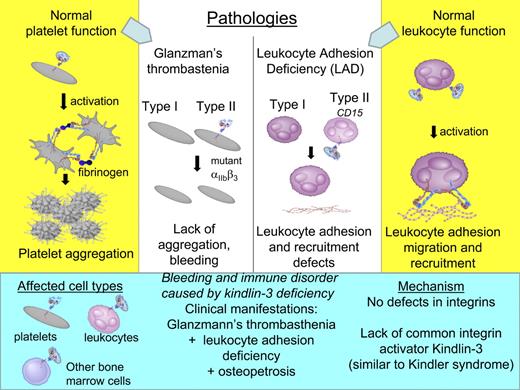
Although the role of Kindlins in integrin inside-out signaling has been considered for some time,14 identification of Kindlin-3 mutations as a cause of a severe bleeding and immune disorder in humans finally solidified the role of this family of proteins as key regulators of cell adhesion.4,7 As first described in 1996 by Shurin et al41 for a male patient, this disorder is characterized by a combination of life-threatening bleeding, immune deficiency, and bone problems. Blood cell counts, especially neutrophils, were significantly higher than in normal subjects (Table 1). Clinically, this disorder combined the symptoms of type II Glanzmann thrombasthenia, leukocyte adhesion deficiency-I (LAD-I), and severe osteopetrosis (Figure 4), and these symptoms were recapitulated in a younger sister. Importantly, although both patients had symptoms of LAD, it was bleeding that was their most troublesome clinical problem. The initial studies showed that, despite normal levels of αIIbβ3 integrin expression, platelets were not able to bind fibrinogen and aggregate in response to any agonist. Likewise, leukocytes, although expressing normal levels of β2 integrins, were not able to bind their ligands, resulting in abnormal neutrophil adhesion and a depressed respiratory burst. It was concluded that this disease is characterized by a defect in the activation of several integrins.
Molecular mechanisms of Glanzmann thrombasthenia and leukocyte adhesion deficiency syndrome in contrast to bleeding and immune disorder caused by Kindlin-3 deficiency (shown on blue background on the bottom). Mechanisms of normal platelet and leukocyte functions are shown on yellow background in the far left and right columns, respectively.
Molecular mechanisms of Glanzmann thrombasthenia and leukocyte adhesion deficiency syndrome in contrast to bleeding and immune disorder caused by Kindlin-3 deficiency (shown on blue background on the bottom). Mechanisms of normal platelet and leukocyte functions are shown on yellow background in the far left and right columns, respectively.
Studies from other groups aimed to understand the molecular mechanisms of this novel disorder were focused mainly on characteristics of primary and immortalized leukocytes from patients with similar sets of symptoms and demonstrated the lack of firm leukocyte adhesion and transmigration under flow.42,–44 To emphasize the defects in leukocytes, it was suggested to name this disorder LAD-III,45 although “LAD-I variant” was also proposed as an alternative designation.42 As shown in Figure 4, LAD type I is characterized by defects in leukocyte adhesion caused by deficiencies of integrin β2 subunit46 and LAD type II by mutation inactivating a fucose transporter resulting in abnormal expression and function of selectin family members.47 By 2005, there were several reports describing LAD-III–like patients from different families,41,,–44 and it became evident that, in contrast to LAD-I and -II, none of those patients has mutations in integrins or selectins themselves, but rather an essential common activator of integrins was missing.
In search of such an activator, it was reported that patients' cells express low levels of CalDAG GEF1 protein, resulting in decreased RAP1*GTP levels.48 It was quite plausible that this defect might be responsible for the defective integrin activation on leukocytes. The phenotype of CalDAG GEF1-deficient mice was largely in agreement with this conclusion.48,49 The only overlooked discrepancy was that platelets from CalDAG GEF1 null mice aggregated in response to PMA49 because PMA bypasses the requirement for CalDAG GEF1, whereas platelets and other cells from patients were nonresponsive to PMA.4
The report of the bleeding phenotype of Kindlin-3 knockout mice6 prompted an array of papers describing point mutations in Kindlin-3 human genomic DNA from LAD-III–like patients.50,51 The comprehensive study by Kuijpers et al demonstrated that many, but not all, patients with what they termed the LAD-I variant symptoms have mutations in CalDAG GEF1, whereas all of the patients have mutations in Kindlin-3.50 These results were most suggestive that CalDAG GEF1 is not a cause of LAD-III symptoms. Independently, 2 different groups using genetic7 and proteomic4 approaches identified Kindlin-3 mutations in these patients. Together, these reports provided the most complete documentation of the defects in platelets,4 primary4 and immortalized leukocytes4,7 and mesenchymal cells,4 which underlie bleeding, immune, and bone defects, respectively. Importantly, clinical manifestations of osteopetrosis (high bone density), which, in addition to bleeding, makes this disease distinct from LAD, were documented for these patients.4 Normal integrin expression but impaired activation has been documented for 3 subfamilies of integrins on blood cells: β1,4,7 β2,3 and β3.4,7
A crucial piece of information finally establishing that the cause of LAD-III–like symptoms is a lack of Kindlin-34,7 was the demonstration that expression of Kindlin-3, but not CalDAG GEF1, completely rescued defective integrin function in immortalized cells from the patients.52 The key role of Kindlin-3 in immune functions was further solidified by the report that Kindlin-3 null mice exhibit impaired leukocyte adhesion and transmigration, resulting in immune deficiencies, which clearly resembled the symptoms of LAD-III–like patients.5 Thus, all functional responses of hematopoietic cells (including platelets and leukocytes) dependent on integrin inside-out signaling require normal expression and function of Kindlin-3. Direct interaction of Kindlin-3 and integrin cytoplasmic tail might be crucial for these processes.3,5,6 In the absence of Kindlin-3, the only possible way to achieve activation is to artificially force integrins to acquire an active conformation by the treatment with Mn2+ or dithiothreitol.3 Thus, in live cell context, unlike reconstituted systems where the presence of talin-1 was proposed as sufficient for integrin activation, Kindlin expression is indispensable for the functionally significant integrin activation. In addition, it is important to emphasize that the lack of Kindlin-3 does not abolish functions of hematopoietic cells, which do not depend on integrin activation, and several processes, such as leukocyte rolling, are still preserved in the absence of Kindlin-3.
Once the lack of Kindlin-3 was identified as a cause for bleeding and immune problems in several cases, it became apparent that this disease is even less similar to LADs because it is not limited to immune deficiencies and is not caused by integrin mutations or dysfunctional integrin ligands (Figure 4). Mechanistically, this disease appears to be a variant of Kindler syndrome in hematopoietic cells or Kindler-3 syndrome (to distinguish from Kindler-1 syndrome caused by mutations in Kindlin-1). Recent advances in understanding of the underlying cause need to be considered to avoid further confusion in nomenclature for this complex disorder.
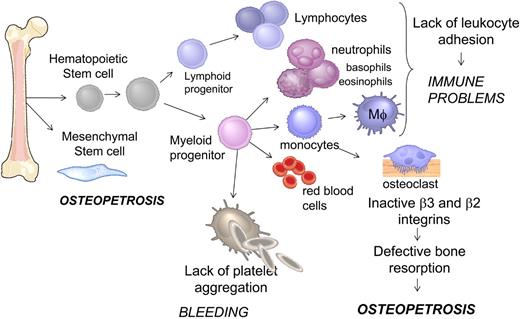
Although bone marrow transplantation largely resolved most of the clinical manifestations of bleeding and immune disorder,4 there is a possibility that the role of Kindlin-3 is not restricted to hematopoietic cells (Figure 5). Indeed, osteopetrosis, observed both in patients and Kindlin-3 null animals,4,6 was traced to abnormalities of bone marrow–derived mesenchymal, not hematopoietic, cells.4 The details of Kindlin-3 function in integrin-dependent and -independent functions of cells of hematopoietic as well as nonhematopoietic origin remain to be established.
Schematic representation of hematopoietic and mesenchymal cell differentiation in bone marrow. Cell types affected by the loss of Kindlin-3 and the functional consequences in human subjects leading to a number of clinical manifestations, including immune defects, osteopetrosis, and bleeding.
Schematic representation of hematopoietic and mesenchymal cell differentiation in bone marrow. Cell types affected by the loss of Kindlin-3 and the functional consequences in human subjects leading to a number of clinical manifestations, including immune defects, osteopetrosis, and bleeding.
In conclusion, identification of Kindlin-3 mutations resulting in ablation of integrin activation in leukocytes and platelets, along with complementary data on Kindlin-2 and Kindlin-1, showing their ability to regulate integrins, place the Kindlin family as integral components in the inside-out signaling pathway leading to integrin activation (Figure 1). Although all 3 Kindlins are structurally similar and share certain overlapping functions, they also appear to have distinct functions based on cell type and the adhesive status of the cells. Kindlin-1 and -2 function in adherent cells constantly embedded in the ECM, and forming cell-cell contacts, whereas Kindlin-3 promotes rapid acquisition of an adhesive phenotype in circulating blood cells. The differences in the regulation of Kindlin-1 and -2 versus Kindlin-3 are based on differences in their sequences, most probably in the variable insert region in their N-termini, and on the availability of unique tissue-restricted binding partners. In the near future, we can anticipate rapid progress in our understanding of the functions of the Kindlin proteins and in their mechanisms of action, including those that uniquely regulate Kindlin-3 and set it apart from the other 2 Kindlin paralogs.
Authorship
Contribution: N.L.M., E.F.P., and T.V.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tatiana V. Byzova, Joseph J. Jacobs Center for Thrombosis and Vascular Biology, Department of Molecular Cardiology, Lerner Research Institute, Cleveland Clinic, NB50, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: byzovat@ccf.org.
References
National Institutes of Health


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal