Abstract
The microenvironment provides essential growth and survival signals to chronic lymphocytic leukemia (CLL) cells and contributes to their resistance to cytotoxic agents. Pharmacologic inhibition of spleen tyrosine kinase (SYK), a key mediator of B-cell receptor (BCR) signaling, induces apoptosis in primary CLL cells and prevents stroma contact-mediated cell survival. This report demonstrates a role of SYK in molecularly defined pathways that mediate the CLL-microenvironmental crosstalk independent from the BCR. Chemokine and integrin stimulation induced SYK phosphorylation, SYK-dependent Akt phosphorylation, and F-actin formation in primary CLL cells. Inhibition of SYK by 2 pharmacologic inhibitors and siRNA-knockdown abrogated downstream SYK signaling and morphologic changes induced by these stimuli. CLL cell migration toward CXCL12, the major homing attractor, and CLL cell adhesion to VCAM-1, a major integrin ligand expressed on stromal cells, were markedly reduced by SYK inhibition. In combination with fludarabine, the SYK inhibitor R406 abrogated stroma-mediated drug resistance by preventing up-regulation of the antiapoptotic factor Mcl-1 in CLL cells. SYK blockade in CLL is a promising therapeutic principle not only for its inhibition of the BCR signaling pathway, but also by inhibiting protective stroma signals in a manner entirely independent of BCR signaling.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is the most prevalent B-cell malignancy in adults in Western countries and is defined as an expansion of monoclonal, mature B cells.1,2 Despite encouraging scientific and therapeutic advances, the disease remains incurable with standard therapy. Although CLL cells accumulate and are resistant to cell death in vivo, these cells rapidly become apoptotic on in vitro culture. Nonmalignant accessory cells in the tumor microenvironment, that is, stromal cells in the bone marrow (BM) and lymph nodes, are known to induce up-regulation of antiapoptotic molecules in CLL cells and to protect them thereby from spontaneous and chemotherapy-induced apoptosis.3 The latter phenomenon has been termed cell adhesion–mediated drug resistance (CAM-DR).4 Residual leukemic cells in protective microenvironmental niches potentially contribute to disease relapse after cytoreductive therapy.3
We and others have previously identified the B-cell receptor (BCR)–associated tyrosine kinase spleen tyrosine kinase (SYK) as a rational therapeutic target in CLL.5-10 Pharmacologic SYK inhibition exerts prominent proapoptotic effects on CLL cells in vitro. Because SYK is primarily known as a key proximal signal transducer of the BCR, the effects of SYK inhibition in CLL are commonly interpreted as the consequence of the disruption of BCR signaling. Of note, the proapoptotic effect of SYK inhibition is not affected by the presence of stroma.5,7 We therefore hypothesized that SYK inhibition does not only block BCR signaling but might also inhibit stroma cell–derived signals, especially because SYK has been implicated in chemokine receptor11 and integrin signaling12,13 in nonmalignant cell types of various origins. Therefore, we investigated in this study whether SYK plays a significant role in the intracellular stroma-CLL signaling and whether SYK inhibition can block stroma-induced survival signals and CAM-DR.
To dissect the relevant candidate stromal pathways, we analyzed chemokine and integrin signaling, which has been identified as important microenvironmental signals for CLL cell survival and drug resistance: The chemokine CXCL12 (SDF1α) is known to direct homing of CLL cells toward protective niches, and competitive CXCR4 antagonists reduce CAM-DR in CLL in vitro cultures.14,15 Likewise, inhibition of VCAM-1 binding to very late antigen-4 (VLA-4; α4β1 integrin; CD49d/CD29) partially prevents stroma-mediated protection of apoptosis in CLL.15-17 Furthermore, enhanced expression of the α chain of VLA4, CD49d, correlates with poor overall survival in CLL.18
Methods
CLL samples
This study was approved by the Institutional Review Board of University Medical Center Freiburg. Peripheral blood samples were obtained with informed consent in accordance with the Declaration of Helsinki from B-CLL patients who were either untreated or off therapy for at least 6 months (Table 1). All cases were characterized for IgVH mutational status,19,20 ZAP70 expression,21 disease stage according to Binet22 or Rai criteria,23 and history of treatment. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll gradient centrifugation. For CLL samples containing less than 90% tumor cells according to flow cytometric analysis with anti-CD19 (BioLegend), anti-CD5 (BD Biosciences), and anti-CD23 (Beckman Coulter) monoclonal antibodies (mAbs), CD19+ B cells were isolated by negative selection (B-cell isolation kit II; Miltenyi Biotec). Cells were either used fresh or cryopreserved in fetal calf serum (FCS)/10% dimethyl sulfoxide (DMSO) until use.
Patient and sample characteristics
| ID . | Sex . | Age, y . | IgVH . | ZAP-70 . | Genetic aberration . | Rai stage . | Previous therapy . |
|---|---|---|---|---|---|---|---|
| 3 | Male | 57 | UM | Positive | Del 13q14 | 2 | Clb, ClbP |
| 9 | Male | 61 | UM | Positive | None | 2 | Clb, ClbP, R |
| 14 | Male | 71 | M | Negative | Del 13q14 homozygotic | 0 | None |
| 23 | Male | 67 | UM | ND | Del 13q14, Del 17p13 | 2 | None |
| 28 | Female | 66 | UM | Positive | None | 1 | Clb |
| 37 | Male | 65 | M | Negative | Del 13q14, trisomy 12 | 3 | Clb, ClbP, B |
| 39 | Male | 64 | M | ND | Del 13q14 | 2 | None |
| 43 | Male | 57 | M/UM | Negative | Trisomy12 | 2 | None |
| 45 | Male | 59 | M | Negative | Del 13q14 | 2 | None |
| 52 | Male | 74 | M | Negative | Del 13q14 | 2 | None |
| 54 | Male | 46 | UM | Positive | Del 18p11, trisomy 2p11 | 2 | F |
| 58 | Male | 72 | M | ND | Del 13q14, Del 5q15 | 2 | None |
| 59 | Male | 56 | UM | Positive | Del 17p13 | 4 | None |
| 61 | Male | 70 | M | ND | None | 2 | Clb |
| 85 | Male | 73 | M | Negative | Del 13q14 | 2 | None |
| 90 | Male | 74 | UM | ND | Del 13q14 | 3 | Clb |
| 95 | Male | 69 | M | Positive | Del 13q14 | 2 | Clb |
| 100 | Female | 76 | M | Negative | Del 13q14 | 2 | Clb, F |
| 101 | Male | 67 | M | Negative | Del 13q14 | 4 | Clb, ClbP |
| 107 | Female | 74 | M | Negative | Del 13q14 | 2 | Clb |
| 140 | Male | 55 | M | ND | Del 13q14 | 2 | None |
| 146 | Male | 69 | M | Negative | Del 13q14 | 0 | None |
| 159 | Male | 68 | M | Negative | Del 11q22 | 2 | Clb, R |
| 161 | Male | 71 | M | ND | Del 13q14 homozygotic | 0 | None |
| 163 | Male | 76 | M | ND | None | 0 | None |
| 186 | Male | 50 | M | ND | None | 1 | Clb |
| 202 | Male | 69 | M | Negative | Del 13q14 | 4 | F |
| 209 | Male | 68 | UM | ND | Del 2q13, trisomy 12 | 1 | Clb |
| 210 | Female | 68 | M | Negative | ND | 1 | None |
| 225 | Female | 58 | M | ND | Del13q14 | 3 | Clb, F |
| 300 | Male | 79 | ND | Negative | ND | 2 | None |
| 301 | Male | 75 | UM | ND | None | 4 | Clb, F |
| ID . | Sex . | Age, y . | IgVH . | ZAP-70 . | Genetic aberration . | Rai stage . | Previous therapy . |
|---|---|---|---|---|---|---|---|
| 3 | Male | 57 | UM | Positive | Del 13q14 | 2 | Clb, ClbP |
| 9 | Male | 61 | UM | Positive | None | 2 | Clb, ClbP, R |
| 14 | Male | 71 | M | Negative | Del 13q14 homozygotic | 0 | None |
| 23 | Male | 67 | UM | ND | Del 13q14, Del 17p13 | 2 | None |
| 28 | Female | 66 | UM | Positive | None | 1 | Clb |
| 37 | Male | 65 | M | Negative | Del 13q14, trisomy 12 | 3 | Clb, ClbP, B |
| 39 | Male | 64 | M | ND | Del 13q14 | 2 | None |
| 43 | Male | 57 | M/UM | Negative | Trisomy12 | 2 | None |
| 45 | Male | 59 | M | Negative | Del 13q14 | 2 | None |
| 52 | Male | 74 | M | Negative | Del 13q14 | 2 | None |
| 54 | Male | 46 | UM | Positive | Del 18p11, trisomy 2p11 | 2 | F |
| 58 | Male | 72 | M | ND | Del 13q14, Del 5q15 | 2 | None |
| 59 | Male | 56 | UM | Positive | Del 17p13 | 4 | None |
| 61 | Male | 70 | M | ND | None | 2 | Clb |
| 85 | Male | 73 | M | Negative | Del 13q14 | 2 | None |
| 90 | Male | 74 | UM | ND | Del 13q14 | 3 | Clb |
| 95 | Male | 69 | M | Positive | Del 13q14 | 2 | Clb |
| 100 | Female | 76 | M | Negative | Del 13q14 | 2 | Clb, F |
| 101 | Male | 67 | M | Negative | Del 13q14 | 4 | Clb, ClbP |
| 107 | Female | 74 | M | Negative | Del 13q14 | 2 | Clb |
| 140 | Male | 55 | M | ND | Del 13q14 | 2 | None |
| 146 | Male | 69 | M | Negative | Del 13q14 | 0 | None |
| 159 | Male | 68 | M | Negative | Del 11q22 | 2 | Clb, R |
| 161 | Male | 71 | M | ND | Del 13q14 homozygotic | 0 | None |
| 163 | Male | 76 | M | ND | None | 0 | None |
| 186 | Male | 50 | M | ND | None | 1 | Clb |
| 202 | Male | 69 | M | Negative | Del 13q14 | 4 | F |
| 209 | Male | 68 | UM | ND | Del 2q13, trisomy 12 | 1 | Clb |
| 210 | Female | 68 | M | Negative | ND | 1 | None |
| 225 | Female | 58 | M | ND | Del13q14 | 3 | Clb, F |
| 300 | Male | 79 | ND | Negative | ND | 2 | None |
| 301 | Male | 75 | UM | ND | None | 4 | Clb, F |
UM indicates unmutated; M, mutated, ND, not done; Clb, chlorambucil; ClbP, chlorambucil + prednisone; F, fludarabine; B, bendamustin; and R, rituximab.
CLL cell/stroma coculture
CLL cells were cultured for the indicated time periods on confluent layers of either the murine M2-10B4 or the human HS-5 stromal cell line (both ATCC) in RPMI/10% FCS. For primary BM-derived stromal cells, BM aspirate of healthy donors was cultured in Dulbecco modified Eagle medium/10% FCS for 1 week, and adherent cells were subsequently replated twice every 3 weeks. For CLL coculture, BM stroma cells were transferred to 24-well plates until a confluent layer was formed.
CLL cell stimulation
Complete crosslinking of BCR on primary CLL cells was performed with 10 μg/mL goat anti–human IgM F(ab′)2 fragment and 10 μg/mL anti–goat F(ab′)2 antibody fragment (both Jackson ImmunoResearch Laboratories).
For integrin stimulation, plates were coated with 10 μg/mL VCAM-1 (R&D Biosystems) at 4°C overnight and washed twice with phosphate-buffered saline (PBS) before addition of primary CLL cells. Human recombinant CXCL12 (Chemicon International) was added directly to the medium at a concentration of 200 ng/mL.
Analysis of the SYK phosphorylation site Y352
The phosphorylation status of SYK Y352 was measured for different stimulation conditions by flow cytometry. For chemokine stimulation, CLL cells were incubated in suspension for 15 seconds with 200 ng/mL CXCL12. For integrin and stroma cell contact studies, CLL cells were centrifuged at 100g for 10 seconds onto wells precoated with 10 μg/mL VCAM-1 or a confluent layer of stromal cells and incubated for 15 seconds. Then, cells were fixed, washed, permeabilized, and incubated with a primary phospho-SYK (pSYK)Y352 antibody (Cell Signaling Technology). Cells were then stained with a fluorescein isothiocyanate-labeled anti–rabbit F(ab′)2 fragment (Jackson ImmunoResearch Laboratories).
Immunoblotting
CLL cells were cultured alone or in contact to fibronectin, VCAM-1, or stromal cells for the indicated time frames. Total cell protein was extracted as described,7 and total protein concentration was determined (BCA Protein Assay kit). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Membranes were incubated at 4°C overnight or 1 hour at room temperature with primary antibodies to SYK, pSYKY525/526, pSrc-family Y416, pAktS473 (all Cell Signaling Technology), Mcl-1 (Epitomics), or β actin (Sigma-Aldrich). Binding of secondary horseradish peroxidase-conjugated antibodies (Cell Signaling Technology; Sigma-Aldrich) was detected using enhanced chemiluminescence and autoradiography with hyperfilm ECL (GE Healthcare). Densitometric analysis of immunoblots was performed using ImageJ software (National Institutes of Health).
Immunoprecipitation
Total phosphorylation of SYK was determined by immunoprecipitation for SYK and subsequent immunoblotting for phosphotyrosine residues. A total of 10 μg of protein lysate was incubated with anti-SYK antibody (Cell Signaling Technology) overnight; then protein G Sepharose beads (Sigma-Aldrich) were added for 90 minutes. After centrifugation and at least 3 washing steps, the precipitated protein was resuspended in loading buffer and stored at −20°C. Samples were heated to 70°C for 10 minutes, centrifuged, and the supernatant was analyzed by phosphotyrosine immunoblotting using the antibody pTyr-100 (Cell Signaling Technology).
SYK inhibition and siRNA-mediated SYK knockdown
The SYK inhibitor R406 (Rigel Pharmaceuticals) was dissolved in DMSO and added to CLL cells 30 minutes before stimulation at a final concentration of 4μM. This R406 concentration is achievable in vivo.24 For siRNA transfection, 7 × 106 CLL cells were resuspended in Nucleofector solution (Nucleofector kit V; Amaxa), containing 1μM nontarget control siRNA or SYK siRNA (Dharmacon RNA Technologies), and transfected using the Amaxa Nucleofector program U-013. After 24 hours, cells were plated in a round-bottom 96-well plate in 400 μL of RPMI/10% FCS. Experiments were performed 48 hours after transfection.
Expression and phosphorylation of SYK downstream targets
Phosphorylation of Akt was determined after CXCL12 or VCAM-1 stimulation by flow cytometry (30-second stimulation) or immunoblotting (15-minute stimulation; for procedure see “Immunoblotting”) with an anti-pAktS473 antibody (Cell Signaling Technology).
F-actin formation was determined after 30 seconds of stimulation with CXCL12 or VCAM-1, or stromal contact by staining with phalloidin-Alexa488 (Invitrogen) and analyzed by confocal microscopy and/or flow cytometry.
Expression levels of Mcl-1 in CLL cells were determined after 48 hours with or without stromal cell coculture in the presence or absence of R406 either by flow cytometry with intracellular staining or by immunoblotting as described in “Immunoblotting” using the anti–Mcl-1 antibody (Epitomics).
Confocal microscopy
For VCAM-1 stimulation, adhesion slides (Marienfeld) were precoated overnight with 10 μg/mL human recombinant VCAM-1. CLL cells were preincubated with 4μM R406 or vehicle control for 30 minutes and then placed on the slides and allowed to settle for 30 minutes at 37°C. Cells were fixed with 2% formamide, permeabilized, and stained with Phalloidin-Alexa594 (Invitrogen) using 2.5% nonfat milk powder (AppliChem) in 0.05% Tween PBS to block unspecific binding. Slides were mounted using 4,6-diamidino-2-phenylindole containing Vectrashield mounting medium (Vector Laboratories). Slides were examined using a Leica TCS SP2 AOBS spectral confocal microscope (Leica Microsystems) and analyzed using the LSM Image Browser software (Carl Zeiss).
Chemotaxis assay
Chemotaxis assay was performed as previously described.14 Briefly, 5 × 105 CLL cells were cultured in RPMI 1640 containing 0.5% bovine serum albumin and were preincubated for 30 minutes with R406 or DMSO, before they were added to the inserts of transwell chambers (5 μm pore size; Corning). Inserts were then transferred into wells containing 200 ng/mL CXCL12. After 2 hours, cell count was determined for the upper and lower well in duplicates using Guava Viacount (Guava Technologies). Chemotaxis indices were calculated as: [number of cells in the lower chamber × 100]/[number of cells in the lower and upper chambers]. The chemotaxis index with R406 was then calculated as percentage of the DMSO index.
Flow chamber experiments
Flow chamber slides μ-Slide VI (Ibidi BioDiagnostics) were coated with 10 μg/mL VCAM-1 overnight at 4°C and then washed with 1 mL dH2O and dried. Cells were pretreated with R406 or the equivalent DMSO concentration for 30 minutes at a concentration of 3 × 106/mL and then loaded on the slides in triplicates. After adhesion overnight, flow chambers were perfused with medium at a constant flow of 50 mL/h for 10 minutes. The remaining cells on the VCAM-1–coated surface were photographed, and the cell number was determined using the particle count function of the ImageJ software.
Expression of the active conformation of CD29
For analysis of the expression of the active conformation of CD29, cells were preincubated with R406 or vehicle control for 3 hours and then stained using the anti-CD29 antibody HUTS-21 (BD Biosciences) for 20 minutes at 4°C in PBS containing 1mM mangan-chloride-tetrahydret (Merck).25
Analysis of CAM-DR and synergistic drug effects
CLL cells were incubated for 48 hours with 4μM R406 (Rigel Pharmaceuticals) and/or 20μM F-ara-A (Sigma-Aldrich) in the presence or absence of M2-10B4 cells.
For formal calculations of potential synergistic drug actions, concentrations of F-ara-A and R406 were titrated from 20.0μM and 8.0μM, respectively. Combined treatment of F-ara-A + R406 was titrated at a constant F-ara-A to R406 concentration ratio of 2.5:1.
Cell viability was determined by annexin V/propidium iodide staining after 48 hours of culture (Annexin V Apoptosis Detection Kit I; BD Biosciences) by flow cytometry (FlowJo, Version 3.3 software; TreeStar). To normalize viability measurements with respect to individual CLL variations in spontaneous apoptosis rates, the absolute viability in the respective control cultured with or without stroma was defined as 100%, and relative viabilities were calculated for statistical comparisons.
Drug-induced cytotoxicity was calculated as follows: 1 − [viability(treated)/viability(control)]. For analysis of drug combinations, the median-effect principle and the multiple drug effect equation were applied as described by Chou and Talalay using the CalcuSyn software (Version 2.1; Biosoft).26,27 Combination indices were calculated by the mean drug effects on 8 CLL samples.
Results
Enhanced SYK phosphorylation in CLL cells after coculture with stromal cells
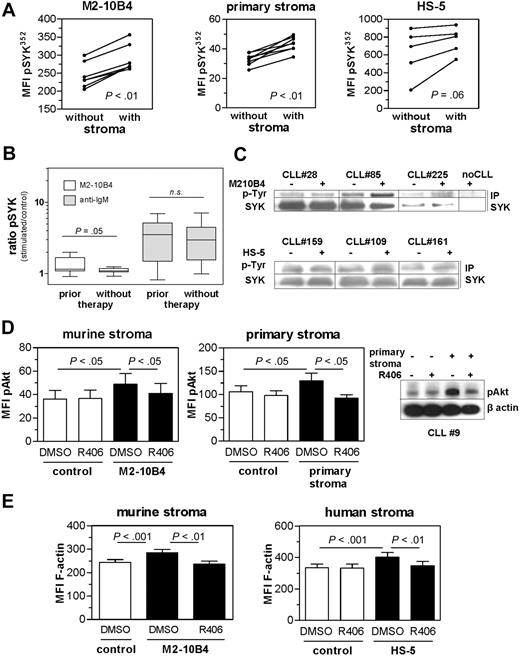
In vivo cell contact of CLL cells to marrow stromal cells is mimicked in vitro by coculture with a stromal cell line.28 A potential role of SYK in the crosstalk between CLL cells and stroma was indicated by increased phosphorylation of SYK at tyrosine 352 on contact to the murine stroma cell line M2-10B4, to the human cell line HS-5, and to primary stromal cells (Figure 1A). Phosphorylation of Y352 is an early event of SYK activation and results in the release of the SYK kinase domain from an autoinhibitory configuration.29
Stromal contact induced SYK phosphorylation and SYK-dependent Akt phosphorylation and F-actin expression. (A) Coculture of CLL samples with the stromal cell line M2-10B4 (n = 6; P < .01), with primary bone marrow–derived stromal cells (n = 8; P < .01) or with the human stromal cell line HS-5 (n = 5; P = .06) resulted in an increase of pY352 SYK, determined by the mean fluorescent intensity (MFI). (B) Parallel stimulation of 9 CLL samples from pretreated patients and 10 samples from patients who have not been treated revealed differential amounts of SYK phosphorylation induced by stroma but not IgM stimulation in these subgroups with higher induction in the pretreated subset. n.s. indicates not significant. (C) Immunoprecipitation of SYK with subsequent immunoblotting for pY revealed SYK phosphorylation induced by 15 minutes of murine (top) or human (bottom) stromal cell contact shown for 3 representative CLL samples. For sample 225, only 5 μg protein lysate was used for the IP. (D) Bar diagram represents mean values ± SEM of MFI for phospho-Akt-stained primary CLL samples (n = 8) with and without M2-10B4 (left panel) or primary (right panel) stromal contact, in the presence and absence of SYK inhibitor R406. Displayed is a representative immunoblot of pAkt after 15 minutes of stromal cell coculture in the presence and absence of R406. (E) Murine and human stromal coculture induced F-actin formation in control-treated cells (P < .01); this was markedly reduced in R406 pretreated cells (P < .01) as determined by phalloidin-Alexa488 staining and flow cytometric analysis (n = 8).
Stromal contact induced SYK phosphorylation and SYK-dependent Akt phosphorylation and F-actin expression. (A) Coculture of CLL samples with the stromal cell line M2-10B4 (n = 6; P < .01), with primary bone marrow–derived stromal cells (n = 8; P < .01) or with the human stromal cell line HS-5 (n = 5; P = .06) resulted in an increase of pY352 SYK, determined by the mean fluorescent intensity (MFI). (B) Parallel stimulation of 9 CLL samples from pretreated patients and 10 samples from patients who have not been treated revealed differential amounts of SYK phosphorylation induced by stroma but not IgM stimulation in these subgroups with higher induction in the pretreated subset. n.s. indicates not significant. (C) Immunoprecipitation of SYK with subsequent immunoblotting for pY revealed SYK phosphorylation induced by 15 minutes of murine (top) or human (bottom) stromal cell contact shown for 3 representative CLL samples. For sample 225, only 5 μg protein lysate was used for the IP. (D) Bar diagram represents mean values ± SEM of MFI for phospho-Akt-stained primary CLL samples (n = 8) with and without M2-10B4 (left panel) or primary (right panel) stromal contact, in the presence and absence of SYK inhibitor R406. Displayed is a representative immunoblot of pAkt after 15 minutes of stromal cell coculture in the presence and absence of R406. (E) Murine and human stromal coculture induced F-actin formation in control-treated cells (P < .01); this was markedly reduced in R406 pretreated cells (P < .01) as determined by phalloidin-Alexa488 staining and flow cytometric analysis (n = 8).
Analysis of 19 CLL samples (Table 2) in parallel revealed no difference in the degree of stroma-induced SYK phosphorylation according to prognostic factors (IgVH mutational status and/or 17p deletion; Table 2; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Strong BCR stimulation by IgM crosslinking was included as a positive control. In accordance with published data,30 differential signaling capacity toward IgM stimulation was observed in the prognostic subgroups (supplemental Figure 1A). However, CLL samples that were obtained from patients who had previously received chemotherapy had a trend for higher SYK phosphorylation on stromal contact but not on IgM stimulation compared with samples from untreated patients (Figure 1B).
Patient and sample characteristics (Figure 1B; supplemental Figure 1A)
| ID . | Sex . | Age, y . | IgVH . | ZAP-70 . | Genetic aberration . | Binet stage . | Previous therapy . |
|---|---|---|---|---|---|---|---|
| UM1 | Male | 75 | UM | Negative | Del11q, Del13q, Del14q | A | None |
| UM2 | Male | 61 | UM | Negative | Del11q, Del13q | NA | F, FCA, hu anti-CD20 |
| UM3 | Male | 73 | UM | Negative | Del13q, Gain12q | B | Clb |
| UM4 | Male | 66 | UM | Positive | Del11q, Gain12q, t(14q32) | A | None |
| UM5 | Female | 62 | UM | ND | Del11q | B | Clb, F, CHOP, A |
| UM6 | Male | 62 | UM | ND | Del11q, Del13q | A | F |
| UM7 | Female | 79 | UM | ND | Gain12q | A | FC |
| 17p− 1 | Female | 52 | UM | Negative | Del17p | C | None |
| 17p− 2 | Female | 66 | UM | Positive | Del17p, Del14q32 | C | B, FCR |
| 17p− 3 | Female | 43 | UM | ND | Del17p | A | RB, RC |
| 17p− 4 | Male | 71 | UM | Negative | Del17p, Del11q, Del13q | B | None |
| 17p− 5 | Male | 77 | UM | Negative | Del17p, Del13q | C | None |
| M1 | Male | 53 | M | Negative | Del13q | B | None |
| M2 | Female | 80 | M | Negative | Del13q | C | None |
| M3 | Female | 75 | M | Negative | Del13q | A | None |
| M4 | Female | 65 | M | Negative | None | A | None |
| M5 | Male | 72 | M | Negative | Del13q | A | None |
| M6 | Male | 81 | M | ND | Del13q | A | F |
| M7 | Male | 60 | M | Negative | Del13q | A | F |
| ID . | Sex . | Age, y . | IgVH . | ZAP-70 . | Genetic aberration . | Binet stage . | Previous therapy . |
|---|---|---|---|---|---|---|---|
| UM1 | Male | 75 | UM | Negative | Del11q, Del13q, Del14q | A | None |
| UM2 | Male | 61 | UM | Negative | Del11q, Del13q | NA | F, FCA, hu anti-CD20 |
| UM3 | Male | 73 | UM | Negative | Del13q, Gain12q | B | Clb |
| UM4 | Male | 66 | UM | Positive | Del11q, Gain12q, t(14q32) | A | None |
| UM5 | Female | 62 | UM | ND | Del11q | B | Clb, F, CHOP, A |
| UM6 | Male | 62 | UM | ND | Del11q, Del13q | A | F |
| UM7 | Female | 79 | UM | ND | Gain12q | A | FC |
| 17p− 1 | Female | 52 | UM | Negative | Del17p | C | None |
| 17p− 2 | Female | 66 | UM | Positive | Del17p, Del14q32 | C | B, FCR |
| 17p− 3 | Female | 43 | UM | ND | Del17p | A | RB, RC |
| 17p− 4 | Male | 71 | UM | Negative | Del17p, Del11q, Del13q | B | None |
| 17p− 5 | Male | 77 | UM | Negative | Del17p, Del13q | C | None |
| M1 | Male | 53 | M | Negative | Del13q | B | None |
| M2 | Female | 80 | M | Negative | Del13q | C | None |
| M3 | Female | 75 | M | Negative | Del13q | A | None |
| M4 | Female | 65 | M | Negative | None | A | None |
| M5 | Male | 72 | M | Negative | Del13q | A | None |
| M6 | Male | 81 | M | ND | Del13q | A | F |
| M7 | Male | 60 | M | Negative | Del13q | A | F |
UM indicates unmutated; NA, not applicable; M, mutated, ND, not done; F, fludarabine; C, cyclophosphamide; A, alemtuzumab; FCA, fludarabine, cyclophosphamide, alemtuzumab; hu, human; Clb, chlorambucil; CHOP, cyclophosphamide, doxorubicin, vincristin, prednisone; B, bendamustin; and R, rituximab.
Enhanced SYK phosphorylation on contact with M2-10B4 and HS-5 stromal cells was also determined by immunoprecipitation of total SYK with subsequent phosphotyrosine immunoblotting (Figure 1C). Analysis of Src phosphorylation did not indicate activation induced by stromal contact (data not shown).
R406 inhibits stromal cell–induced Akt phosphorylation and actin polymerization in CLL cells
Because the signaling molecule Akt is activated by stromal contact in CLL cells,31 we analyzed the phosphorylation status of Akt in the presence and absence of stroma, and with and without treatment with the SYK inhibitor R406. Akt phosphorylation was significantly increased by coculture with M2-10B4 and primary stromal cells. Concomitant SYK inhibition prevented stroma-induced Akt phosphorylation but had no effect in the absence of stroma (Figure 1D).
Actin reassembly is a common response to the engagement of various cell surface receptors.14 SYK activity is known to be required for immunoreceptor tyrosine-based activation motif-mediated actin assembly.32 Human and murine stroma-dependent rearrangements of the CLL cytoskeleton were observed by F-actin staining and flow cytometric quantification (Figure 1E). Concomitant SYK inhibition significantly reversed this stromal cell–induced actin polymerization. SYK inhibition alone did not influence the F-actin expression.
CXCL12 as an important survival axis: SYK inhibition antagonizes CXCL12-induced Akt activation, actin polymerization, and chemotaxis
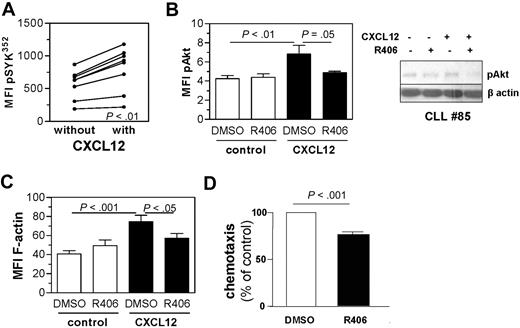
After demonstrating SYK phosphorylation in CLL cells as a response to stroma cell contact in vitro, we sought to define the molecular pathways that involve SYK activation. The chemokine CXCL12 is described as the major homing factor for CLL cells and is constitutively secreted in high amounts by the stromal cell line M2-10B4.14 Stimulation with recombinant CXCL12 induced activation of SYK and phosphorylation of its downstream target Akt (Figure 2A-B). Inhibition of SYK resulted in almost complete abrogation of CXCL12-induced Akt phosphorylation (Figure 2B). A representative example of flow cytometric analysis is displayed in supplemental Figure 1B. Furthermore, inhibition of SYK prevented CXCL12-induced F-actin formation in CLL cells (Figure 2C). Because actin reorganization is an early event of migratory response to chemokines, we also evaluated the effects of SYK inhibition on the migratory capacity of CLL cells toward CXCL12. SYK inhibition with R406 or SYK inhibitor II resulted in a significant reduction of the migratory capacity toward CXCL12 of primary CLL cells (Figure 2D; supplemental Figure 1C). Based on these observations, we conclude that SYK is a key mediator of CXCL12-induced responses in primary CLL cells.
SYK is involved in the CXCL12 pathway in CLL cells. (A) Stimulation of primary CLL samples (n = 8) for 15 seconds with human CXCL12 resulted in an increase of pY352 SYK, the activation site of SYK determined by the MFI (P < .001). (B) Concomitant SYK inhibition abrogated the phosphorylation of Akt induced by CXCL12 stimulation. Displayed is the MFI of CLL cells stained for pAkt after 30 seconds of stimulation with CXCL12 (n = 8; P < .01). A representative immunoblot is shown. (C) F-Actin formation after CXCL12 stimulation was determined by intracellular staining using Alexa488-labeled phalloidin and flow cytometric analysis of 7 different primary CLL samples. SYK inhibition alone did not significantly change F-actin formation, CXCL12 induced F-actin (P < .001), and R406 prevented up-regulation induced by CXCL12 (P < .05). (D) Transwell chemotaxis assays toward CXCL12 were performed without or with R406 for 7 CLL samples. The percentage of migrated cells in vehicle control was defined as 100% to determine the relative chemotaxis. R406 inhibited chemotaxis toward CXCL12 with a P value less than .001.
SYK is involved in the CXCL12 pathway in CLL cells. (A) Stimulation of primary CLL samples (n = 8) for 15 seconds with human CXCL12 resulted in an increase of pY352 SYK, the activation site of SYK determined by the MFI (P < .001). (B) Concomitant SYK inhibition abrogated the phosphorylation of Akt induced by CXCL12 stimulation. Displayed is the MFI of CLL cells stained for pAkt after 30 seconds of stimulation with CXCL12 (n = 8; P < .01). A representative immunoblot is shown. (C) F-Actin formation after CXCL12 stimulation was determined by intracellular staining using Alexa488-labeled phalloidin and flow cytometric analysis of 7 different primary CLL samples. SYK inhibition alone did not significantly change F-actin formation, CXCL12 induced F-actin (P < .001), and R406 prevented up-regulation induced by CXCL12 (P < .05). (D) Transwell chemotaxis assays toward CXCL12 were performed without or with R406 for 7 CLL samples. The percentage of migrated cells in vehicle control was defined as 100% to determine the relative chemotaxis. R406 inhibited chemotaxis toward CXCL12 with a P value less than .001.
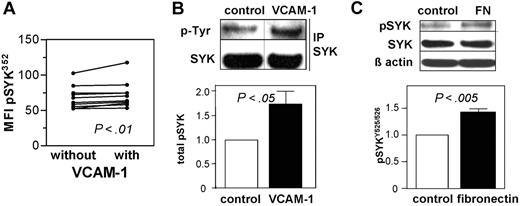
SYK protein phosphorylation by VCAM-1 and fibronectin
Integrin ligation constitutes another important microenvironmental prosurvival pathway for CLL.15 We analyzed SYK phosphorylation after culturing CLL cells on VCAM-1–coated plates. SYK was phosphorylated at Y352 during VCAM-1 contact (Figure 3A). To validate this finding, we performed immunoblotting of CLL cells with and without contact to VCAM-1 or fibronectin, another ligand for VLA-4, with phospho-SYK specific antibodies recognizing the autophosphorylation site YY525/526. Furthermore, we performed immunoprecipitation for SYK with subsequent immunoblot for phosphotyrosine to obtain the total amount of phosphorylated SYK. Both types of experiments showed similar results for VCAM-1 and fibronectin stimulations (data not shown). Densitometric analyses demonstrated significant phosphorylation of SYK by VCAM-1 (Figure 3B) and fibronectin contact after normalization to β actin (Figure 3C).
Integrin stimulation mediates activation of SYK in CLL cells. (A) Stimulation of CLL samples (n = 10) for 15 seconds with human recombinant VCAM-1 increased phosphorylation of SYK at Y352 determined by the MFI (P < .01). (B) Immunoprecipitation of SYK with subsequent immunoblotting for phosphotyrosine (pY) revealed SYK phosphorylation on VCAM-1 contact as shown in the representative immunoblot and the densitometric evaluation of 9 different CLL samples displayed as relative expression after 15 minutes with or without VCAM-1 contact (mean ± SEM; P < .05; bottom). (C) Immunoblotting for the autophosphorylation site 525/526 for SYK revealed an increase induced by fibronectin contact as shown by a representative immunoblot and densitometric evaluation after normalization to β actin for 6 CLL samples (bottom). Displayed is the relative expression as mean ± SEM (P < .005).
Integrin stimulation mediates activation of SYK in CLL cells. (A) Stimulation of CLL samples (n = 10) for 15 seconds with human recombinant VCAM-1 increased phosphorylation of SYK at Y352 determined by the MFI (P < .01). (B) Immunoprecipitation of SYK with subsequent immunoblotting for phosphotyrosine (pY) revealed SYK phosphorylation on VCAM-1 contact as shown in the representative immunoblot and the densitometric evaluation of 9 different CLL samples displayed as relative expression after 15 minutes with or without VCAM-1 contact (mean ± SEM; P < .05; bottom). (C) Immunoblotting for the autophosphorylation site 525/526 for SYK revealed an increase induced by fibronectin contact as shown by a representative immunoblot and densitometric evaluation after normalization to β actin for 6 CLL samples (bottom). Displayed is the relative expression as mean ± SEM (P < .005).
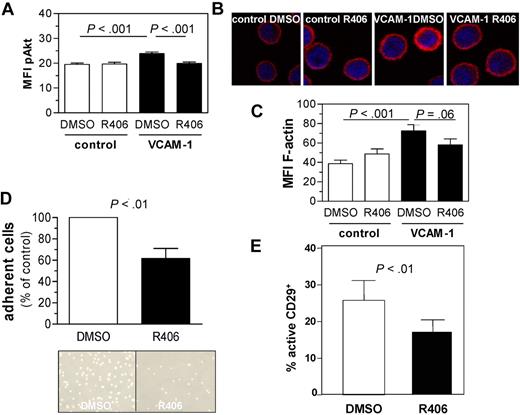
R406 inhibits VCAM-1–induced Akt activation, actin polymerization, and adhesion
Next, we tested the effects of SYK inhibition on phosphorylation of SYK downstream targets after integrin stimulation. SYK inhibition almost completely abrogated Akt phosphorylation after integrin stimulation (Figure 4A). Integrin stimulation may result in cytoskeleton remodeling.33 To analyze the effects of SYK inhibition on F-actin formation, we stimulated 8 different primary CLL samples with VCAM-1 in the absence and presence of R406 and analyzed F-actin expression by confocal microscopy and by flow cytometry. We observed that VCAM-1 contact induced F-actin formation, which was reversed by concomitant SYK inhibition (Figure 4B-C).
Responses on integrin ligation are attenuated by concomitant SYK inhibition. (A) Mean of MFI of pAkt expression in primary CLL cells cultured with or without VCAM-1–coated plates in the presence or absence of R406. Displayed are the mean values as assessed by flow cytometry (n = 9). (B) F-actin formation increased on VCAM-1 contact in DMSO control-treated cells but not in R406 pretreated cells, determined by confocal microscopy of CLL cells. Cells attached to VCAM-1 or poly-L-lysine (control)–coated slides and were stained with phalloidin-Alexa594 (representative example). (C) Quantification of F-actin formation by flow cytometry using phalloidin-Alexa488 for 7 primary CLL samples. The up-regulation induced by VCAM-1 contact in control cells (P < .001) was in part abrogated by concomitant SYK inhibition (P = .06). (D) CLL cells adhered less effective toward a VCAM-1–coated surface during SYK inhibition as determined by flow chamber analysis. The mean of triplicates for control and R406-treated samples (n = 10) were calculated, and the cell count of control-treated cells was determined as 100% (P < .01). Photographs of a representative example are displayed. (E) CLL cell treatment with R406 resulted in a reduced expression of the active conformation of CD29 compared with vehicle control (n = 20, P < .01).
Responses on integrin ligation are attenuated by concomitant SYK inhibition. (A) Mean of MFI of pAkt expression in primary CLL cells cultured with or without VCAM-1–coated plates in the presence or absence of R406. Displayed are the mean values as assessed by flow cytometry (n = 9). (B) F-actin formation increased on VCAM-1 contact in DMSO control-treated cells but not in R406 pretreated cells, determined by confocal microscopy of CLL cells. Cells attached to VCAM-1 or poly-L-lysine (control)–coated slides and were stained with phalloidin-Alexa594 (representative example). (C) Quantification of F-actin formation by flow cytometry using phalloidin-Alexa488 for 7 primary CLL samples. The up-regulation induced by VCAM-1 contact in control cells (P < .001) was in part abrogated by concomitant SYK inhibition (P = .06). (D) CLL cells adhered less effective toward a VCAM-1–coated surface during SYK inhibition as determined by flow chamber analysis. The mean of triplicates for control and R406-treated samples (n = 10) were calculated, and the cell count of control-treated cells was determined as 100% (P < .01). Photographs of a representative example are displayed. (E) CLL cell treatment with R406 resulted in a reduced expression of the active conformation of CD29 compared with vehicle control (n = 20, P < .01).
To analyze the adhesion capacity of CLL cells toward VCAM-1, we performed adhesion assays using VCAM-1–coated flow chambers. CLL cells treated with the SYK inhibitor R406 revealed a reduced adhesion capacity toward the VCAM-1–coated chambers compared with DMSO-treated cells (Figure 4D). Representative photographs of the adhered cells after flow application with and without R406 treatment are shown. The viability was tested simultaneously to the adhesion analysis and revealed no significant change after overnight incubation with R406 or DMSO (data not shown).
R406 inhibits integrin inside-out signaling
In response to a number of physiologic stimuli, including BCR ligation,34 integrins are activated by a conformational change that rendered them competent to mediate high-affinity adhesion. We analyzed the expression of a high-affinity epitope of CD29, the β1 chain of VLA-4, on CLL cells by flow cytometry with and without SYK inhibition and found a significant decrease of the expression induced by SYK inhibition (Figure 4E), whereas the expression of total CD29 decreased only approximately 10% (data not shown).
siRNA-mediated SYK knockdown confirms SYK involvement in the VCAM-1 and CXCL12 signaling pathway
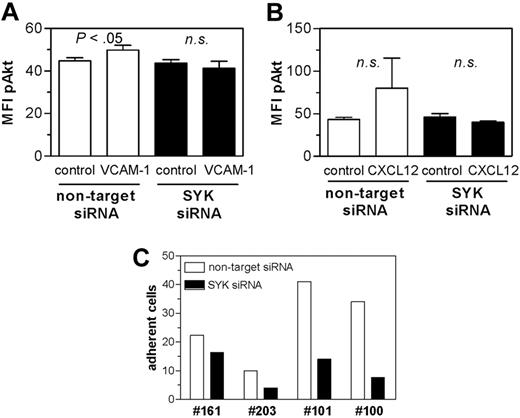
To verify the specificity of the effects of pharmacologic SYK inhibitors, we performed siRNA-mediated silencing of SYK in CLL cells. Transfection of the CLL derived cell line EHEB revealed a dose-dependent reduction of SYK expression after 48 hours as determined by immunoblot (supplemental Figure 2A). For primary CLL cells, we observed a down-regulation of SYK protein expression by flow cytometry (supplemental Figure 2B). Nevertheless, the viability of SYK down-regulated cells was not significantly affected compared with control-transfected cells until 72 hours after transfection7 (supplemental Figure 2C). We then stimulated control- and SYK siRNA-treated cells with VCAM-1 or CXCL12 and analyzed Akt phosphorylation. VCAM-1 significantly increased Akt phosphorylation in control but not in SYK siRNA-transfected cells (Figure 5A). For CXCL12, a similar, albeit more variable effect on Akt phosphorylation was observed (Figure 5B). The consequences of SYK knockdown were explored in functional adherence assays. In all cases, transduction with the SYK siRNA resulted in a substantially decreased fraction of adhering CLL cells compared with control (Figure 5C).
siRNA mediated SYK knockdown validated the results of the pharmacologic SYK inhibitor. Primary CLL cells (n = 4) were transfected with control siRNA or SYK-specific siRNA. After 48-hour cultures, cells were either stimulated with VCAM-1 (A) or CXCL12 (B) for 30 seconds or used for functional adhesion (C). (A) VCAM-1 stimulation up-regulated Akt phosphorylation in control transfected (P < .05), but not in SYK silenced CLL cells. n.s. indicates not significant. (B) Stimulation with CXCL12 resulted in up-regulation of pAkt by trend in nontarget siRNA but not in SYK siRNA-transfected CLL cells. (C) Flow chamber analysis revealed a clear trend to reduced adhesion capacity after SYK knockdown.
siRNA mediated SYK knockdown validated the results of the pharmacologic SYK inhibitor. Primary CLL cells (n = 4) were transfected with control siRNA or SYK-specific siRNA. After 48-hour cultures, cells were either stimulated with VCAM-1 (A) or CXCL12 (B) for 30 seconds or used for functional adhesion (C). (A) VCAM-1 stimulation up-regulated Akt phosphorylation in control transfected (P < .05), but not in SYK silenced CLL cells. n.s. indicates not significant. (B) Stimulation with CXCL12 resulted in up-regulation of pAkt by trend in nontarget siRNA but not in SYK siRNA-transfected CLL cells. (C) Flow chamber analysis revealed a clear trend to reduced adhesion capacity after SYK knockdown.
The SYK inhibitor R406 abrogates cell adhesion–mediated drug resistance in CLL
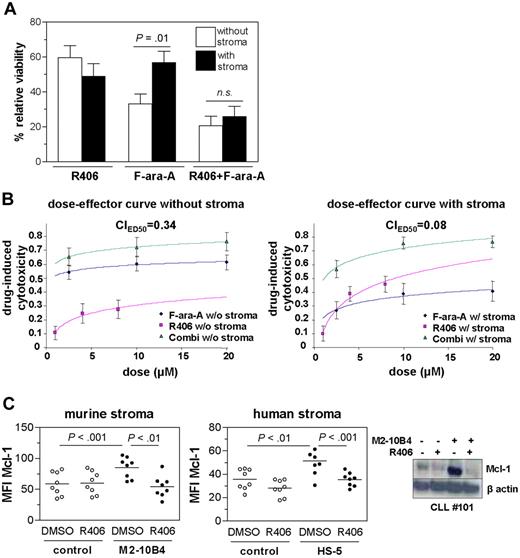
Coculture with the murine and the human stroma cell line significantly protected CLL cells from spontaneous apoptosis (supplemental Figure 3A). In similarity to our previous observations for the commercially available SYK inhibitor II,7 R406 effectively inhibited antiapoptotic effects of both cell lines on primary CLL cells (supplemental Figure 3B). In line with previous reports,14 M2-10B4 coculture effectively protected CLL cells against F-ara-A, the in vitro active derivate of fludarabine (Figure 6A). This CAM-DR effect toward F-ara-A by stromal cells was completely abrogated in the presence of R406.
SYK inhibition abrogates the protective effect of stroma and counteracts CAM-DR in context with F-ara-A. Relative viability of CLL cells was calculated by defining the viability of the respective DMSO control (with or without stroma) as 100%. (A) CLL samples were treated with R406, F-ara-A, or the combination of R406 and F-ara-A in the presence and absence of stroma. The effect of F-ara-A (20μM) was limited in stromal coculture as indicated by the significant higher relative viability in stromal coculture (P < .01; n = 14, 8 of which were from patients who had obtained previous therapy). The combination of R406 and F-ara-A prevented the protective effect of the stromal cells toward apoptosis, resulting in no change in relative viability of the combined treatment. n.s. indicates not significant. (B) Dose-effect curves of 8 CLL samples for R406, F-ara-A, and the combination of both at a ratio of 1:2.5 without (left) and with stromal coculture. Displayed are the mean effects ± SEM with logarithmic regression. (C) Mcl-1 stained primary CLL samples (n = 8) cultured with or without stroma in the presence or absence of R406 as assessed by flow cytometry (left). Stromal contact resulted in higher Mcl-1 levels compared with CLL cells cultured alone (murine stroma, P < .001; human stroma, P < .01), whereas concomitant SYK inhibition prevented this up-regulation (murine stroma, P < .01; human stroma, P < .001). A representative immunoblot analysis is shown.
SYK inhibition abrogates the protective effect of stroma and counteracts CAM-DR in context with F-ara-A. Relative viability of CLL cells was calculated by defining the viability of the respective DMSO control (with or without stroma) as 100%. (A) CLL samples were treated with R406, F-ara-A, or the combination of R406 and F-ara-A in the presence and absence of stroma. The effect of F-ara-A (20μM) was limited in stromal coculture as indicated by the significant higher relative viability in stromal coculture (P < .01; n = 14, 8 of which were from patients who had obtained previous therapy). The combination of R406 and F-ara-A prevented the protective effect of the stromal cells toward apoptosis, resulting in no change in relative viability of the combined treatment. n.s. indicates not significant. (B) Dose-effect curves of 8 CLL samples for R406, F-ara-A, and the combination of both at a ratio of 1:2.5 without (left) and with stromal coculture. Displayed are the mean effects ± SEM with logarithmic regression. (C) Mcl-1 stained primary CLL samples (n = 8) cultured with or without stroma in the presence or absence of R406 as assessed by flow cytometry (left). Stromal contact resulted in higher Mcl-1 levels compared with CLL cells cultured alone (murine stroma, P < .001; human stroma, P < .01), whereas concomitant SYK inhibition prevented this up-regulation (murine stroma, P < .01; human stroma, P < .001). A representative immunoblot analysis is shown.
The drug potency and the shape of the dose effect curve are relevant for analysis of drug combinations. Thus, we analyzed the effect of F-ara-A, R406, and the combination at 3 different concentrations with and without M2-10B4 stromal cells (Figure 6B). The combination index was calculated using the CalcuSyn software. In both culturing systems, the combination of F-ara-A and R406 revealed synergistic effects (indicated by a combination index < 1). For the setting of stromal coculture, a lower combination index than for the setting without stroma indicated a stronger synergy. Therefore, the synergism of both drugs is enhanced during stromal coculture.
Maximal apoptotic effects (Emax = 0.37) were determined after 48 hours at a dose of 20μM (data not shown).The half-maximal effect dose (IC50) revealed IC50R406 (without stroma) = 2.25μM in the absence of stroma and IC50R406(stroma) = 1.24μM in the presence of stroma.
Although we cannot completely exclude direct effects of R406 on stromal cells, we observed no noticeable morphologic changes or detached stromal cells after SYK inhibition (supplemental Figure 3C).
Inhibition of SYK by R406 reduces stromal cell–induced Mcl-1 expression in CLL cells
Mcl-1 is a critical factor for stromal cell–mediated chemoresistance in CLL.35 To further investigate the role of SYK in mediating CAM-DR, we tested the effect of pharmacologic SYK inhibition on Mcl-1 expression in the presence and absence of the stromal cell lines M2-10B4 and HS-5. After 48 hours, Mcl-1 was up-regulated in CLL cells cultured with stroma compared with CLL cells cultured alone, whereas SYK inhibition in stroma-cocultured CLL cells almost completely abolished the up-regulation of Mcl-1 (Figure 6C). A representative flow cytometric analysis is displayed in supplemental Figure 4A. In contrast to a published study,6 we observed only minor to no Mcl-1 down-regulation by SYK inhibition in the absence of stroma. The flow cytometry data were validated by immunoblot as shown in a representative example. Similar results were obtained for treatment with 4μM SYK inhibitor II (supplemental Figure 4B). As additional survival pathway may contribute to stroma-mediated CLL cell survival, we analyzed STAT3 phosphorylation with and without stromal coculture. In line with our previous findings,7 we observed down-regulation of STAT3 phosphorylation by R406 treatment. Nevertheless, primary stroma contact did not significantly induce STAT3 phosphorylation (supplemental Figure 4C). The transcription factor nuclear factor-κB (NF-κB) is implicated in the regulation of various cellular functions. Although we cannot exclude involvement of other NF-κB subunits, analysis of the most common NF-κB subset p50 revealed no increased activity during M2-10B4 coculture. R406 treatment, however, resulted in a moderate reduction of NF-κB activity (supplemental Figure 4D).
Discussion
The tyrosine kinase SYK was originally identified as a crucial mediator of immunoreceptor signaling in lymphocytes. Analysis of its molecular structure provided evidence for involvement of SYK in various signal transduction pathways.29
Several studies have already indicated modulatory effects of SYK inhibition on chemokine-mediated interactions between CLL and stroma cells: R406 was shown to inhibit the secretion of the chemokines CCL3 and CCL4 induced by nurse-like cell coculture in CLL cells.36 Homing of CLL cells toward CXCL12 is enhanced by BCR stimulation, and simultaneous treatment with R406 reduces this effect.5 However, all observed effects in both of these studies were interpreted as the consequence of the disruption of BCR signaling by SYK inhibition.
In this study, however, we demonstrate direct inhibition of molecularly defined pathways in the CLL-stroma crosstalk that are distinct from BCR signaling by pharmacologic inhibition of SYK: 2 recombinant proteins, CXCL12 and VCAM-1, were sufficient to induce SYK phosphorylation and SYK-dependent Akt phosphorylation and cytoskeleton reorganization in the absence of stroma cell contact. It has been shown that stromal contact-induced Akt phosphorylation31 is sufficient to increase the half-life of the antiapoptotic factor Mcl-1.37 The SYK-dependent Akt/Mcl-1 pathway is known to contribute to cell survival in CLL cells.8-10 Because fludarabine resistance was shown to be mediated by Mcl-1 expression,38 these combined observations are sufficient to explain the abrogation of CAM-DR through R406 in CLL-stroma coculture. This effect of SYK inhibition establishes its importance in the treatment of CLL beyond blockade of BCR signaling. SYK inhibition might be especially relevant for treatment strategies aimed to minimize CLL relapse through the reduction of resistant residual leukemic cells in protective niches.
The participation of SYK in integrin signaling in B-CLL cells is demonstrated for 2 distinct molecular processes. On resting lymphocytes, integrins are found as monomers in a bent, inactive conformation.39 Binding of specific ligands to the extracellular integrin domain results in “outside-in” activation. On the other hand, stimulation of distinct chemokine or immunoreceptors can result in integrin activation in an “inside-out” fashion.39 Our findings show a role for SYK in both integrin activation mechanisms in CLL cells: SYK inhibition prevented phosphorylation of the integrin downstream target Akt after stimulation, consistent with reduced “outside-in” signaling. In addition, as SYK inhibition reduced expression of the active conformation of CD29 in the absence of a ligand, “inside-out” activation seems to be reduced. This “inside-out” signaling might be mediated by the constitutive phosphorylated state of SYK in CLL cells,7 and activation of the BCR signaling cascade by IgM ligation induces further integrin activation39 (data not shown).
Besides these 2 major survival pathways, that is, CXCL12/CXCR4 and VCAM-1/VLA-4, other CLL survival factors might also be affected by SYK inhibition: SYK has been described to mediate NF-κB activity after engagement of CD74 by macrophage migration inhibiting factor. The macrophage migration inhibiting factor/CD74/CD44 axis represents another microenvironmental pathway with importance for survival of CLL cells.40,41
Pharmacologic inhibition of tyrosine kinase activity generally raises the issue of target specificity. Although developed as a specific SYK inhibitor, it has been suggested that R406 may interfere with other molecules. Recent studies, however, have shown that expression of the constitutive active TEL-SYK in BaF3 cells induces Akt and ERK phosphorylation. This effect can be abrogated by R406, confirming that the effects of R406 on downstream phosphorylation events are a consequence of SYK inhibition.6 We ourselves have assayed a second, commercially available and structurally different SYK inhibitor (SYK inhibitor II, Calbiochem) and observed similar effects compared with R406 (supplemental Figures 1C, 4B; and data not shown). Most importantly, we verified our observations by siRNA-mediated SYK knockdown experiments. Both VCAM-1– and CXCL12-induced phosphorylation of Akt was reduced in SYK-down-regulated cells. These effects were accompanied with marked reduction of the adhesion capacity of CLL cells to VCAM-1. Of note, although these results were obtained in primary cells that permit only limited knockdown efficacies (27% reduction, supplemental Figure 2B), they are in full accordance with studies using genetically engineered cells deficient for SYK.42-44 Taken together, these data indicate that SYK inhibition is indeed the specific mechanism underlying the effects of R406 on CLL cells described in this study.
The recognition of the effects of SYK inhibition on stroma interactions that are independent of BCR signaling have important implications for the clinical development of SYK inhibitors in CLL. In the first clinical trial of the oral prodrug of R406, fostamatinib disodium, the overall response rate of 55% in patients with relapsed/refractory CLL/small lymphocytic lymphoma suggested remarkable clinical activity.45 Unexpectedly, all of these CLL patients experienced an hitherto unexplained, transient but dramatic leukocyte flare during lymph node regression under SYK inhibition therapy.45 Our findings provide a plausible explanation for this phenomenon and permit to postulate that direct interruption of pathways that mediate the CLL-stroma interaction, rather than inhibition of BCR signaling, may represent the main effector mechanism in SYK inhibition therapy of CLL.
The observations that CLL samples from pretreated patients exert enhanced SYK phosphorylation by stromal contact (Figure 1B) suggest a selective survival advantage for CLL subclones that have been protected by particularly high dependence on microenvironmental factors against the previous cytotoxic chemotherapy. This resistance mechanism, on the other hand, would render such CLLs cells particularly vulnerable to the disruption of the stroma interaction and would be in accordance with the good response rate of fostamatinib even in heavily pretreated and refractory CLL patients.45
Alternative approaches to interfere with microenvironmental signals for treatment of CLL have been proposed. Extracellular CXCR4 antagonists can partially resensitize CLL cells to cytotoxic drugs in stromal coculture and are currently being evaluated in clinical trials in leukemia patients.46,47 However, intracellular blockade of signaling molecules involved in multiple survival pathways offers conceptual advantages over targeting a single survival mechanism. Recently, phosphatidylinositol 3-kinase (PI3K) inhibitors were shown to effectively antagonize migratory, survival, and drug-resistance signals derived from CXCL12 and CLL-marrow stromal cell interactions in vitro.48 In leukemic cells, PI3K activity depends on activation of upstream signaling components, such as SYK.49 The phosphorylation status of Akt reflects PI3K activity as a direct target. Our analyses revealed that SYK inhibition by R406 is sufficient to almost completely abrogate PI3K activity induced by various microenvironmental stimuli. In contrast to PI3K inhibitors, the oral SYK inhibitor fostamatinib disodium, the prodrug of R406, has already been safely tested in nonmalignant diseases and animal studies and revealed minimal functional immunotoxicity.50
In conclusion, our work establishes an important role of SYK in different prosurvival stimuli provided to CLL cells by the microenvironment. Our data contribute to the establishment of SYK inhibition as a rational therapeutic principle in CLL. In addition to reduction of BCR signaling in CLL, our study adds the important prospect of preventing survival of drug-resistant CLL cells in protective niches by BCR-independent, direct blockade of stroma signals by inhibition of SYK.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yasumichi Hitoshi from Rigel Pharmaceuticals Inc for valuable suggestions and for providing R406, Miguel Waterhouse for providing the primary stromal cells, Marie Follo for assistance with confocal microscopy, and Roland Nitschke for helpful advice for the flow chamber experiments.
This work was supported by the Deutsche Krebshilfe (grant 107275; M. Burger, H.V., K.Z.).
Authorship
Contribution: M. Buchner designed and performed research, analyzed data, and wrote the paper; C.B. and G.P. performed research and analyzed data; M. Burger, C.D., and H.J. designed research; T.Z. and S.S. provided patient samples; and H.V. and K.Z. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hendrik Veelken, Department of Hematology/Oncology, University Medical Center Freiburg, Hugstetter Strasse 55, 79106 Freiburg, Germany; e-mail: hendrik.veelken@uniklinik-freiburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal