The polycistronic miRNA cluster miR-17-92 promotes tumor angiogenesis in a paracrine fashion. However, in this issue of Blood, Doebele and colleagues report cell-intrinsic, antiangiogenic properties of individual members of this miRNA cluster in endothelial cells. These context-dependent activities may explain the lack of effect for the systemic inhibition of miR-17-92 on tumor angiogenesis, highlighting the need for cell type–specific targeting strategies for antagomir-based therapeutic approaches.
MicroRNA (miRNAs) have emerged as an important new class of posttranscriptional regulators of gene expression in development and disease. Recent studies have revealed important functions for miRNAs in regulating angiogenesis. The endothelial cell–restricted miRNA miR-126 is an essential regulator of developmental angiogenesis,1-3 whereas the polycistronic miRNA cluster miR-17-92, also known as oncomir-1, promotes tumor angiogenesis in a non-cell, autonomous manner.4 The miR-17-92 cluster, one of the best-characterized oncogenic miRNAs, is up-regulated in multiple human tumor types, most notably undergoing amplification in diffuse large B-cell lymphomas, and promotes tumor progression in various mouse models of cancer.5,6 The proangiogenic functions of miR-17-92 have been ascribed to direct repression of the secreted, antiangiogenic molecules thrombospondin-1 (TSP-1) and connective tissue growth factor (CTGF) within tumor cells, thereby promoting angiogenesis in the adjacent tumor endothelium by a paracrine, cell-nonautonomous mechanism.7
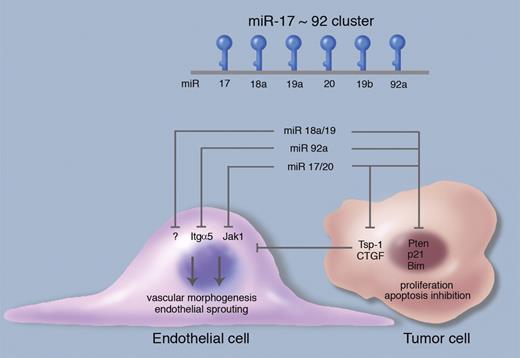
Angiogenesis regulation by the miR-17-92 cluster. The polycistronic miR-17-92 cluster encodes 6 mature miRNAs: miR17, miR18a, miR-19a, miR-20a, miR-19b, and miR-92a, all processed from a single primary transcript. miR-18a/19 directly repress the expression of the antiangiogenic, secreted molecules thrombospondin-1 (Tsp-1) and connective tissue growth factor in tumor cells, thereby promoting angiogenesis in the adjacent tumor endothelium by a paracrine, cell-nonautonomous mechanism. Conversely, miR-17/20 and miR-92a cell-autonomously target Janus kinase 1 (Jak1) and integrin α5 (Itgα5) in endothelial cells, thus negatively regulating endothelial cell sprouting and vascular morphogenesis. The miR-17-92 cluster miRNAs also repress targets in tumor cells that promote proliferation and survival (Pten, Bim, p21); these relationships are simplified for purposes of this schematic. Professional illustration by Marie Dauenheimer.
Angiogenesis regulation by the miR-17-92 cluster. The polycistronic miR-17-92 cluster encodes 6 mature miRNAs: miR17, miR18a, miR-19a, miR-20a, miR-19b, and miR-92a, all processed from a single primary transcript. miR-18a/19 directly repress the expression of the antiangiogenic, secreted molecules thrombospondin-1 (Tsp-1) and connective tissue growth factor in tumor cells, thereby promoting angiogenesis in the adjacent tumor endothelium by a paracrine, cell-nonautonomous mechanism. Conversely, miR-17/20 and miR-92a cell-autonomously target Janus kinase 1 (Jak1) and integrin α5 (Itgα5) in endothelial cells, thus negatively regulating endothelial cell sprouting and vascular morphogenesis. The miR-17-92 cluster miRNAs also repress targets in tumor cells that promote proliferation and survival (Pten, Bim, p21); these relationships are simplified for purposes of this schematic. Professional illustration by Marie Dauenheimer.
The miR-17-92 cluster represents a prototypical polycistronic miRNA gene, encoding 6 mature miRNAs: miR-17, miR-18a, miR-19a, miR-20a, miR-19b, and miR-92a, all processed from a single primary transcript (see figure).8 Based on seed sequence composition, these 6 miRNAs can be grouped into 4 families: miR-17/20, miR-18, miR-1, and miR-92. The presence of 2 miR-17-92 paralog clusters, miR-106b-25 and miR-106a-363, generated by gene duplication and containing homologous miRNAs to a subset of the miR-17-92 components, adds additional complexity to this system in mammalian cells.9 The authors previously demonstrated that miR-92a inhibits angiogenesis in endothelial cells, in part by repression of the target gene integrin α5, whereas antagomir-based inhibition of miR-92a in mice augmented neovascularization and functional recovery after ischemia.4 In this issue of Blood, Doebele et al perform an impressive systematic investigation of the functional roles of the remaining members of the miR-17-92 cluster in endothelial cells.10 Similar to the earlier miR-92a study, overexpression of miR-17, miR-18a, miR-19a, and miR-20a inhibited endothelial cell sprouting in vitro, individual knockdown enhanced endothelial sprouting, and miR-17 produced the strongest loss- and gain-of-function effects. The protein kinase Jak1 was identified as an endothelial miR-17 target and was functionally validated as a proangiogenic molecule downstream of miR-17.
The Doebele et al study clearly indicates that the individual miRNAs of the miR-17-92 cluster can exhibit cell-autonomous, antiangiogenic activity within endothelial cells. Notably, these results add substantial complexity to current models of miR-17-92 angiogenesis regulation. The proposed endothelial cell–autonomous, antiangiogenic activity contrasts with previous data indicating that miR-17-92 promotes angiogenesis within tumor cells via a paracrine mechanism11 and in endothelial cells lacking Dicer.12 Second, the authors propose that the miR-17-92 miRNAs exert distinct functions in physiologic versus pathologic angiogenesis, because the simultaneous inhibition of miR-17 and miR-20 via antagomir increased vascularization in Matrigel plugs, consistent with the antiangiogenic function of these miRNAs in endothelium, but did not affect tumor angiogenesis.
Several experimental possibilities can reconcile the apparent discrepancies between these models, including potential differential antagomir dosage requirements for these 2 angiogenesis models or the presence of tumor type–specific effects. Alternatively, such differences could be explained by context-dependent activities of the miR-17-92 cluster in endothelium versus tumor cells. The numerous elegant previous studies overexpressing or deleting miR-17-92 in the tumor compartment8 have unfortunately not been able to address questions of endothelial cell–intrinsic functionality. Such cell type–specific differences could also underlie the observed differences between miR-17-92 miRNA in physiologic (Matrigel plug) versus pathologic (tumor) angiogenesis in the study of Doebele et al. Where the endothelial cell predominancein the Matrigel plug assay could allow proangiogenic effects of miR-17/miR-20 inhibition to prevail, in vivo tumor models such as Lewis lung carcinoma (LLC) possess both endothelial and tumor cell compartments. Within such a mixed milieu, systemic administration of an antagomir that would ablate miR-17/miR-20 in tumor cells and tumor endothelium alike could elicit opposing proangiogenic and antiangiogenic signals emanating from endothelial and tumor cells, respectively, resulting in a zero sum net effect on tumor angiogenesis.
Given the potential for opposing effects of the miR-17-92 miRNAs depending on cellular compartment, the precise genetic dissection of this cluster's angiogenic functions in vivo may well require cell type–specific deletion, for instance in endothelium versus tumor parenchyma. Such strategies could also cleanly reveal endothelial functions in mice lacking either miR-17-92 or both miR-7-92 and miR-16b-25, which die postnatally and at midgestation, respectively,8 but in which developmental vascular patterning has not been formally examined, or in physiologically relevant angiogenesis models such as corpus luteum formation or wound healing. From a therapeutic standpoint, cell type–specific antagomir targeting strategies may be required to fully exploit miR-17-92 miRNA inhibition for applications such as tumor angiogenesis, although this requirement notably does not seem to be present for proangiogenic uses as in postischemic revascularization.4 The future development of such cell type–specific targeting strategies could greatly enlarge the therapeutic repertoire of antagomirs, considering that miRNAs may exhibit cell type–specific functions as a general property, as exemplified by the paradigm of miR-17-92.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■