In this issue Blood, Bleakley and colleagues describe improved techniques to identify mHAgs in donor-recipient pairs prior to transplantation, which have implications for the immunotherapy of leukemia.1
The success or failure of a human leukocyte antigen (HLA)–matched allogeneic stem cell transplantation (SCT) for a patient with leukemia is controlled to a large extent by the powerful alloresponse of the donor T cells against the recipient's minor histocompatibility antigens (mHAgs). These antigens are peptides that are “self” to the recipient but foreign to the donor, nestling in the peptide binding groove of the major histocompatibility (MHC) molecules. The antigenic difference is typically the result of a single nucleotide polymorphisms, giving rise to the inheritance of proteins differing in donor and recipient by a single amino acid—not enough to affect protein function, but occasionally enough to generate powerful T-cell responses to the alternate allele resulting in favorable graft-versus-leukemia (GVL) and deleterious graft-versus-host (GVH) reactions.
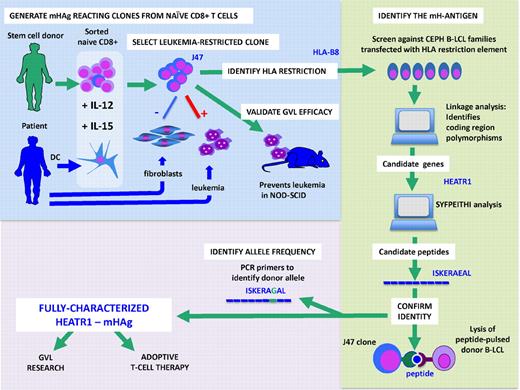
Discovery and characterization of mHAg HEATR1: Pretransplantation cells from donor and recipient are used to generate an HLA-B8–restricted hematopoiesis-specific CD8 T-cell clone (J47). This clone was used to identify the parent gene of the mHAg as HEATR1. One peptide candidate from this gene, ISKERAEAL, was recognized by J47. The allele in the donor was found to differ by one amino acid.
Discovery and characterization of mHAg HEATR1: Pretransplantation cells from donor and recipient are used to generate an HLA-B8–restricted hematopoiesis-specific CD8 T-cell clone (J47). This clone was used to identify the parent gene of the mHAg as HEATR1. One peptide candidate from this gene, ISKERAEAL, was recognized by J47. The allele in the donor was found to differ by one amino acid.
Although the human MHC system has been characterized over the years in ever greater detail, the repertoire and identity of mHAgs have remained largely unexplored. If we assume that humans harbor at least as many mHAgs as mice do, estimated to range in the many hundreds,2 we still have a long way to go. Since Goulmy and colleagues in Leiden first characterized HA-1, HA-2, and SMCY,3 more than 30 human mHAgs have been identified, but progress has been slow—about 2 to 3 mHAgs a year.4 While alloreacting T cells are readily isolated from alloimmunized persons after transfusion, pregnancy, or SCT, it has proven difficult to obtain the stable T-cell clones necessary for the identification of leukemia-restricted mHAg or for use in adoptive therapy of leukemia. The reliable generation of mHAg-specific donor T cells before the transplantation by Bleakley et al therefore represents a breakthrough. Their strategy facilitates the manufacture of GVL reacting T cells for adoptive therapy after SCT and advances the technology for discovering new mHAg. Studying HLA-identical donor-recipient pairs they first confirmed, as has been hypothesized, that in the unsensitized donor, mHAg-specific T cells reside in the naive T-cell subset. Selected CD8+ naive T cells were stimulated with recipient dendritic cells and a cocktail of cytokines,which critically included IL-15 to drive T-cell expansion and IL-12 to maintain cytotoxicity. They went on to show that G-CSF–mobilized PBSC were a suitable T-cell source for generating alloreacting T-cell lines in 9 selected donor-recipient pairs where fibroblasts were available from the recipient. Forty-two T-cell lines were screened against fibroblasts to eliminate T cells that displayed GVH reactivity. Ten lines that recognized a hematopoiesis-restricted mHAg in the context of common HLA alleles were studied further (see figure). One robust T-cell clone was studied in detail. They showed its specificity for the patient's leukemia and its ability to eliminate leukemia-initiating cells in a NOD-SCID mouse.5 To identify the mHAgs, they transfected the HLA-B8 restriction element into a panel of genotypically well-characterized immortalized B-cell lines (B-LCL) from informative families from the Center d'Etude de Polymorphism Humaine (CEPH) to narrow the chromosomal region encoding the mHAg via linkage analysis,6,7 and molecular databases to identify candidate genes that might function as mHAgs. Of 6 candidate genes thus identified, further analysis of known coding region polymorphisms that would alter the amino acid sequence narrowed down the list of candidates to 2. Testing for recognition of peptides with HLA-B8 binding properties using the SYFPEITHI peptide binding prediction software,8 they found one HLA-B8 binding peptide sequence ISKERAEAL in the HEATR1 gene, which encodes a protein of unknown function. The clone recognized this peptide and using gene-specific primers, the donor was found to be homozygous for the alternative allele differing by one amino acid: ISKERAGAL. The HEATR1 mHAg fits many of the criteria for an ideal mHAg—it is expressed by the leukemia stem cells, and there is a calculated donor-recipient disparity of the gene of about 20% for HLA-B8 persons or 3% to 5% for the entire transplantation population. The HEATR1-specific T-cell clone does not lyse patient fibroblasts, and gene expression analysis by quantitative RT-PCR confirmed the very low expression levels of this mHAg in nonhematopoietic cells.
The ability to readily clone mHAg-specific T cells in pretransplantation material has 2 implications. First, it will accelerate the discovery of new mHAg and the exploration of GVHD and GVL reactions at the molecular level. While the technical steps in using T-cell clones to find and name the antigens seem daunting to the nonexpert, it should be noted that much of the searching is carried out using genetically well-characterized, publically available immortalized cell lines that can be modified to express the HLA restriction element of choice. Second, the technical breakthrough of the reliable generation of mHAg-specific T cells paves the way for the expansion of mHAg-specific T-cell lines for treatment, with a view to delivering expanded numbers of GVL-specific T cells. One important step before this becomes a reality is the perfection of reliable ways to select only hematopoietic or leukemia-restricted mHAg-specific T cells. Bleakley et al use the classical method of selecting clones that only see recipient B-LCL and not fibroblasts.9 However, whether nonhematopoietic cells will remain undamaged in a posttransplantation milieu of inflammatory cytokines is unclear from this study.10 Warren et al have already described unwanted side effects from infusion of mHAg-specific T cells.11 As we move on to the next stage, the careful monitoring of unwanted alloreactions from such powerful cytotoxic T cells will be required. Perhaps the HEATR1 gene may harbor more useful polymorphisms. Meanwhile, many more mHAgs remain to be discovered before mHAg-based treatments can be applied to every donor-recipient pair.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal