An elegant study by Sacharidou and colleagues in this issue of Blood provides fascinating insights into a signaling complex that is critical for endothelial cells to form lumen and tube structures in a 3-dimensional (3D) culture system, but not for their migration in 2D cultures.1
Endothelial cells are remarkably versatile entities that can invade the extracellular matrix to assemble the highly branched vasculature that reaches into all parts of an organism to supply vital oxygen and nutrients. The process of angiogenesis is critical for normal development, but also contributes to diseases such as cancer, proliferative retinopathies, and rheumatoid arthritis. So there is a significant interest in understanding the properties of endothelial cells and how they are able to assemble 3D vascular networks. Many key insights into the functions of endothelial cells and the underlying molecular mechanisms have come from studies in tissue culture. Endothelial cells respond to stimuli such as the vascular endothelial growth factor (VEGF-A) by migrating and proliferating and forming chordlike structures in a regular tissue culture plate (ie, 2D culture conditions). However, the true potential of these remarkable cells emerges when they are studied in a 3D culture system, such as the one pioneered by Davis and colleagues (for examples, see references2-5 ). In 3D culture, endothelial cells are placed within a collagen matrix that is deep enough for the cells to invade and form lumen and tube networks (see figure), allowing them to behave more as they would in an intact organism in vivo.
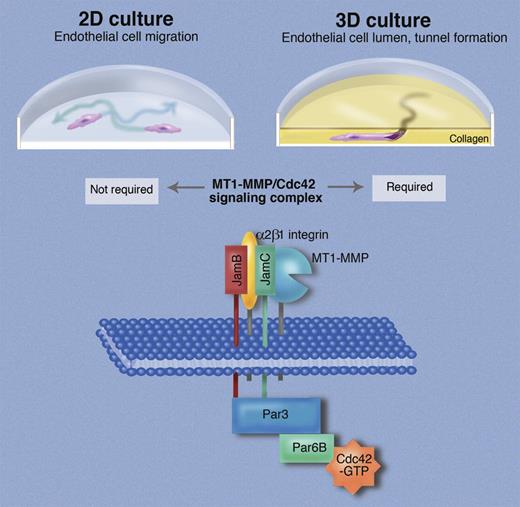
Endothelial cells in 2-dimensional tissue culture are able to migrate (indicated by arrows), proliferate, and form chordlike structures. When placed in 3-dimensional culture in a collagen matrix, endothelial cells form tunnels and generate a lumen, thereby displaying properties that are considered essential for assembling the vasculature in vivo. Sacharidou et al identify a signaling complex, termed “endothelial lumen signaling complex,” in which the membrane-anchored metalloproteinase MT1-MMP interacts with the integrin α2β1, and both bind to the junctional adhesion molecule JamC, which in turn interacts with the related JamB. The cytoplasmic domains of JamB and JamC then bind directly to Par3 to recruit the intracellular components of the signaling complex, which also include Par6B and Cdc42. Professional illustration by Marie Dauenheimer.
Endothelial cells in 2-dimensional tissue culture are able to migrate (indicated by arrows), proliferate, and form chordlike structures. When placed in 3-dimensional culture in a collagen matrix, endothelial cells form tunnels and generate a lumen, thereby displaying properties that are considered essential for assembling the vasculature in vivo. Sacharidou et al identify a signaling complex, termed “endothelial lumen signaling complex,” in which the membrane-anchored metalloproteinase MT1-MMP interacts with the integrin α2β1, and both bind to the junctional adhesion molecule JamC, which in turn interacts with the related JamB. The cytoplasmic domains of JamB and JamC then bind directly to Par3 to recruit the intracellular components of the signaling complex, which also include Par6B and Cdc42. Professional illustration by Marie Dauenheimer.
The advantages of studying endothelial cells in 3D culture over 2D cultures are driven home by Sacharidou et al's study in this issue. Their main goal is to provide a better understanding of how the membrane-anchored metalloproteinase MT1-MMP is regulated during matrix invasion and lumen formation of endothelial cells, as this enzyme is considered essential for invasion of a collagen matrix.6-9 The authors provide compelling functional and biochemical evidence that a signaling complex consisting of junctional adhesion molecules (termed JamB and JamC), an integrin (α2β1), proteins with roles in establishing cell polarity (Par3, Par6B), the GTP-ase Cdc42, and MT1-MMP is required for invasion of a 3D collagen matrix by endothelial cells, but not for their migration in 2D culture. The role of this complex and of its individual components is first assessed in endothelial tube morphogenesis assays using cells treated with siRNA to knock down expression of key components, or with soluble extracellular domains of Jams that exert a dominant negative function. Rescue experiments in which knocked-down proteins are replaced with mutant components of the signaling complex demonstrate that the catalytic activity of MT1-MMP is essential for complex assembly, pointing toward an interdependence between the enzymatic activity of MT1-MMP and Cdc42 signaling. Moreover, the cytoplasmic domains of JamB and JamC are required for complex formation and lumen formation. Evidently, these help link the extracellular components of this signaling complex (MT1-MMP, JamB, JamC, and α2β1 integrin) to their cytoplasmic partners (Par3, Par6B, CDC42; see figure). JamB and JamC were previously thought to mainly function in tight junctions in adherent cells, so Sacharidou et al uncover novel roles for these molecules as components of the lumen formation complex that is independent of their role in cell adhesion. Insights from functional studies are corroborated by coimmunoprecipitation experiments and immunofluorescence analysis that probe the assembly of the signaling complex and its localization under conditions where endothelial cells undergo tubular morphogenesis in a 3D matrix versus 2D culture conditions. Finally, an assessment of how knocking down individual components of the complex affects its integrity and phosphorylation status allows the authors to assemble a model of which components interact with one another directly and indirectly (see figure). Although the importance of some components of this complex for endothelial cells was previously known,3,5,8 the insights provided through the functional characterization of this signaling complex would probably not have emerged from studies using genetically modified mice, where one molecule is evaluated at a time. By turning to a 3D culture system, and combining this with the power of cell biology and biochemistry, and especially the ability to manipulate protein levels and analyze the consequences, Sacharidou et al have made critical contributions to our understanding of endothelial cell biology.
The current study raises several interesting questions for future research. What is the mechanism responsible for the functional interdependence between Cdc42 and MT1-MMP in 3D cellular morphogenic and invasive behavior of endothelial cells? The catalytic activity of MT1-MMP is required for activation and assembly of the Cdc42 GTPase into this signaling complex, yet how processing of extracellular molecules on endothelial cells trigger the assembly of the intracellular part of the complex is not clear. What are the functional consequences of the interaction of various components of this signaling complex in vivo, such as in mouse models of vasculogenesis, angiogenesis, and neovascularization? Sacharidou et al suggest that the 3D morphogenesis assay model is representative of vasculogenesis, a key de novo morphogenic assembly process during vascular development in which endothelial cells form the first vascular structures with lumens. But presumably, insights from their system are also pertinent for angiogenesis, something that must be addressed in vivo. Finally, it will be interesting to determine the potential clinical relevance of this signaling complex in pathologic neovascularization. The assays described by Sacharidou et al could be used to identify compounds that block the assembly of the lumen invasion complex, and such compounds could be tested for their ability to prevent invasion and lumen formation of endothelial cells in vitro and in vivo. Clearly, not only do the 3D endothelial cell assays used by Sacharidou et al provide a wealth of information on the mechanisms underlying the remarkable properties of endothelial cells, but they also serve as excellent hypothesis-building tools for further exploration of endothelial cell biology and pathology in the context of an intact organism.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal