Abstract
Activating alleles of Janus kinase 2 (JAK2) such as JAK2V617F are central to the pathogenesis of myeloproliferative neoplasms (MPN), suggesting that small molecule inhibitors targeting JAK2 may be therapeutically useful. We have identified an aminopyrimidine derivative (CYT387), which inhibits JAK1, JAK2, and tyrosine kinase 2 (TYK2) at low nanomolar concentrations, with few additional targets. Between 0.5 and 1.5μM CYT387 caused growth suppression and apoptosis in JAK2-dependent hematopoietic cell lines, while nonhematopoietic cell lines were unaffected. In a murine MPN model, CYT387 normalized white cell counts, hematocrit, spleen size, and restored physiologic levels of inflammatory cytokines. Despite the hematologic responses and reduction of the JAK2V617F allele burden, JAK2V617F cells persisted and MPN recurred upon cessation of treatment, suggesting that JAK2 inhibitors may be unable to eliminate JAK2V617F cells, consistent with preliminary results from clinical trials of JAK2 inhibitors in myelofibrosis. While the clinical benefit of JAK2 inhibitors may be substantial, not the least due to reduction of inflammatory cytokines and symptomatic improvement, our data add to increasing evidence that kinase inhibitor monotherapy of malignant disease is not curative, suggesting a need for drug combinations to optimally target the malignant cells.
Introduction
An activating mutation of Janus kinase 2 (JAK2; JAK2V617F) is present in almost all patients with polycythemia vera (PV), 30% to 50% of patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF), and smaller subsets of patients with other myeloproliferative neoplasms (MPN).1-5 JAK2V617F is thought to play a critical role in the pathogenesis of these disorders. Consistent with this, a PV-like disease with secondary myelofibrosis arises in mice that received transplants with bone marrow expressing JAK2V617F, and mice transgenic for JAK2V617F develop an ET- or PV-like MPN.6-9 Based on the success of the breakpoint cluster region–Abelson leukemia virus (BCR-ABL) inhibitor, imatinib, for the treatment of chronic myeloid leukemia (CML), there is considerable interest in the development of small molecule JAK2 kinase inhibitors for the treatment of MPN, and several compounds are currently in clinical trials of myelofibrosis.10-12 Here, we describe the in vitro and in vivo activity of CYT387, a specific small molecule inhibitor of wild-type and V617F mutant JAK2.
Methods
Expression vectors
For stable cell lines, the retroviral vector MSCV-IRES-GFP (MIG) was used containing wild-type or V617F alleles of murine JAK2 cDNA (kindly provided by Dr James Ihle, St Jude Children's Research Hospital, Memphis, TN) or the murine erythropoietin receptor (EPOR; kindly provided by Dr Dwayne Barber, Ontario Cancer Institute, Toronto, ON).
Retrovirus production, cell culture, and immunoblotting
Retrovirus production, cell culture, and immunoblotting were performed as described.6 For details, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mutagenesis screen
The selection for CYT387-resistant clones was performed as described.13 Details are provided in supplemental Methods.
Kinase assays
Glutathione S-transferase (GST)–tagged JAK kinase domains were cloned in gateway baculovirus vectors (Invitrogen) and expressed in SF9 insect cells. The fusion proteins were purified and used in a peptide substrate phosphorylation assay. Assays were performed in 384-well Optiplates using an Alphascreen Protein Tyrosine Kinase P100 detection kit (PerkinElmer) and a PerkinElmer Fusion Alpha instrument.
Bone marrow transplantation
Standard techniques were used. All mouse work was performed with approval from the Oregon Health & Science University (OHSU) Institutional Animal Care and Use Committee. For details, see supplemental Methods.
CYT387 administration
On day 32 after bone marrow transplantation (when all mice exhibited severe leukocytosis and erythrocytosis), mice were assigned to 3 groups such that each group had equivalent average body weight and blood counts (see Figure 2, supplemental Figure 2A). CYT387 was dissolved in NMP (120 mg/mL final; 1-methyl-2-pyrrolidinone, Chromasolv Plus; Sigma-Aldrich). Subsequently, the CYT387/NMP mix was diluted with 0.14M Captisol (Cydex Pharmaceuticals Inc) to a concentration of 6 mg/mL and further diluted with 0.1M Captisol to a final concentration of 4 mg/mL. All 3 groups of mice (n = 12 per group) were administered CYT387 by oral gavage twice daily at 10- to 12-hour intervals from day 34 after bone marrow transplantation to day 82 (end of experiment). Mice received NMP/Captisol without CYT387 (0 mg/kg group), 25 mg/kg CYT387, or 50 mg/kg CYT387. At day 82 after bone marrow transplantation, all mice were euthanized for analysis except for 2 mice each from the 50 mg/kg and 25 mg/kg groups, which were taken off CYT387 treatment and followed for 45 additional days. For assessment of the effects of CYT387 on normal blood counts, naive Balb/c mice were administered vehicle control, 50 mg/kg, or 100 mg/kg CYT387 in an identical fashion as described for the bone marrow transplant experimental mouse cohort. Peripheral blood was drawn at day 14, 28, 42, and 56 and levels of red cells, white cells, reticulocytes, granulocytes, lymphocytes, and monocytes were analyzed.
Pathologic examination
Blood was collected from anesthetized mice by lethal inferior vena cava bleeding. Blood was incubated at 5°C for 2 hours, centrifuged at 2000 rcf for 10 minutes, and serum was stored at −80°C. Organs were dissected and white blood cells from spleen and bone marrow were harvested by passing through a 70-μm nylon cell strainer (BD Biosciences), followed by red cell lysis.
Histologic analysis
Samples from liver, spleen, and humerus were fixed in 3.5% paraformaldehyde (Protocol) and embedded in paraffin. Sections of 5 μm were stained with hematoxylin/eosin (H&E). Decalcified sections of humerus were stained with H&E and reticulin stain (American Master*Tech Scientific Inc). Reticulin-stained humerus sections were scored in a blinded fashion on a scale of 0 to 3 for degree of fibrosis with 0 indicating normal tissue and 3 indicating extreme fibrosis.14
Flow cytometry
White cells from bone marrow and spleen were stained with conjugated monoclonal antibodies as described.6
Quantitative PCR for GFP (allele) burden
Genomic DNA was isolated from mouse splenocytes or Ba/F3 cells and standardized to 10 ng/μL. DNA was amplified with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) on an MJ Research Opticon 2 thermal cycler using primers specific for green fluorescent protein (GFP) or the genomic mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) locus: GFP forward (F): 5′-ACGTAAACGGCCACAAGTTC-3′; GFP reverse (R): 5′-AAGTCGTGCTGCTTCATGTG-3′; GAPDH F: 5′-AGCCTTAAAAGCCCTTGAGC-3′; GAPDH R: 5′-CTATAGGGCCTGGGTCAGTG-3′. On each plate, a standard curve of Ba/F3 cell genomic DNA was included that comprised 100%, 50%, 10%, 5%, 1%, 0.5%, 0.1%, and 0% JAK2V617F-GFP+ cells with the remainder of the cells being parental Ba/F3 (GFP−). The difference in cycle number between GFP and GAPDH (Δ cycle threshold [ΔCt]) was determined for each sample. Subsequently, the difference between the ΔCt of each well compared with the highest GFP value on the standard curve was calculated (ΔΔCt). The percent GFP by quantitative polymerase chain reaction (qPCR) was expressed as 2−ΔΔCt × 100, such that the well with highest GFP value on the standard curve is set at 100% and all other wells are expressed relative to that point.
Quantification of cytokines
Serum levels of 32 different cytokines were measured using a bead-based immunofluorescence assay (Luminex Inc), following the instructions of the manufacturer.
CYT387 pharmacokinetics
CYT387 (25 and 50 mg/kg) was administered to Balb/c mice as described under “CYT387 administration.” Groups of 4 mice were sacrificed after 1, 3, 6, 9 and 12 hours and plasma was collected and snap-frozen in liquid nitrogen. CYT387 plasma concentrations were determined by liquid chromatography (LC)/mass spectrometry (MS)/MS analysis of samples, referenced to an internal standard, using an Agilent liquid chromatograph (Agilent Technologies Inc) and an API3000 (triple-quadrupole) instrument from Applied Biosystems Inc with an electrospray ionization interface. Plasma samples (50.0 μL) were mixed with 10 μL of methanol and 250 μL of internal standard solution (100 ng/mL). After vortexing for 1 minute and centrifuging for 5 minutes at 15 000 rpm, 100-μL supernatants were analyzed by LC/MS/MS.
Statistics
The SPSS statistics package was used for all statistical analyses. Continuous variables were compared by pairwise t test for 2 independent samples. Nonlinear regression was used to determine a best-fit curve for the GFP qPCR standard curve and generate an r2 value.
Results
Profiling of a JAK2 inhibitor
CYT387 was discovered by high-throughput enzyme and cell-based screening of small molecule libraries, with subsequent optimization of the lead compounds using structure-guided medicinal chemistry (Figure 1A). Details of the identification process and the results of x-ray diffraction analysis of CYT387 in complex with the kinase domain of JAK2 will be published in a separate manuscript. In vitro kinase assays performed in the presence of 80μM adenosine triphosphate (ATP) revealed that CYT387 inhibited the activity of recombinant JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) kinase domains with 50% inhibition/inhibitory concentration (IC50) values of 11, 18, 155, and 17nM, respectively. To globally evaluate specificity, we tested CYT387 against a panel of 128 kinases, using the SelectScreen Profiling Service (Invitrogen). Significant activity (defined as an IC50 < 100nM) was detected against CDK2/cyclin A, MAPK8 (JNK1), PRKCN (PKD3), PRKD1 (PKCμ), ROCK2, and TBK1 (supplemental Table 1).
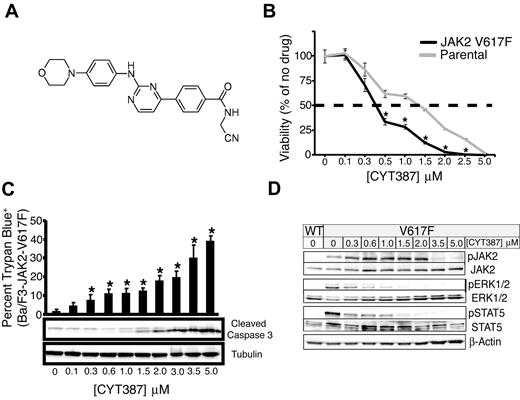
Efficacy of CYT387 against JAK2-dependent cell lines in vitro. (A) Chemical structure of CYT387. (B) Ba/F3-EpoR parental (cultured in media supplemented with 3 U/mL erythropoietin) or Ba/F3-EpoR-JAK2V617F cells (cultured in the absence of exogenous cytokines) were plated in 96-well plates over a dose gradient of CYT387 for 3 days at which point cell viability was measured with an MTS tetrazolium salt assay. Values represent mean ± SEM. n = 3; *P < .05. (C) Baf3-EpoR-JAK2V617F cells were plated in graded concentrations of CYT387 for 1 day (immunoblot) or 2 days (trypan blue exclusion) at which point cell death was measured by trypan blue exclusion and apoptosis was determined by immunoblot with antibodies specific for cleaved caspase 3 and tubulin. Values represent mean ± SEM. n = 3; *P < .05. (D) Ba/F3-EpoR-JAK2WT or Ba/F3-EpoR-JAK2V617F cells were serum-starved then plated in 6-well plates over a dose gradient of CYT387 for 16 hours at which point cells were lysed and whole-cell extracts were subjected to immunoblot analysis using antibodies specific for total or phospho-JAK2, total or phospho-ERK1/2, total or phospho-STAT5, or β-actin.
Efficacy of CYT387 against JAK2-dependent cell lines in vitro. (A) Chemical structure of CYT387. (B) Ba/F3-EpoR parental (cultured in media supplemented with 3 U/mL erythropoietin) or Ba/F3-EpoR-JAK2V617F cells (cultured in the absence of exogenous cytokines) were plated in 96-well plates over a dose gradient of CYT387 for 3 days at which point cell viability was measured with an MTS tetrazolium salt assay. Values represent mean ± SEM. n = 3; *P < .05. (C) Baf3-EpoR-JAK2V617F cells were plated in graded concentrations of CYT387 for 1 day (immunoblot) or 2 days (trypan blue exclusion) at which point cell death was measured by trypan blue exclusion and apoptosis was determined by immunoblot with antibodies specific for cleaved caspase 3 and tubulin. Values represent mean ± SEM. n = 3; *P < .05. (D) Ba/F3-EpoR-JAK2WT or Ba/F3-EpoR-JAK2V617F cells were serum-starved then plated in 6-well plates over a dose gradient of CYT387 for 16 hours at which point cells were lysed and whole-cell extracts were subjected to immunoblot analysis using antibodies specific for total or phospho-JAK2, total or phospho-ERK1/2, total or phospho-STAT5, or β-actin.
CYT387 selectively inhibits JAK2-dependent cell lines
We assessed the effects of graded CYT387 concentrations on a panel of cell lines, including hematopoietic lines transformed to growth factor independence by expression of JAK2V617F, HEL cells with naturally acquired JAK2V617F, and a variety of other leukemia and cancer cell lines (Table 1). Three groups of cell lines could be distinguished according to IC50 values and involvement of JAK2 in their survival signaling. The first group is entirely composed of cell lines known to be dependent on JAK2 signaling for viability and proliferation. This group encompasses Ba/F3 and 32D cells engineered to express JAK2V617F together with the EPOR, the parental lines transduced with empty vector or EPOR alone (which remain dependent on interleukin-3 [IL-3] or EPO), HEL cells, TF-1 cells (GM-CSF–dependent) and U266 myeloma cells (dependent on an autocrine IL-6 loop).15 The IC50 in these lines ranged between 500 and 1500nM. Although Ba/F3 cells expressing both JAK2V617F and EPOR were slightly more sensitive to CYT387 than parental controls (Figure 1B), sensitivity was not consistently higher in cells dependent on JAK2V617F versus wild-type JAK2. For a second group of cell lines, survival has not directly been linked to JAK2 signaling. Significant growth inhibition was observed in Molm14 cells, which carry an internal tandem duplication of FLT3. Furthermore, significant inhibition was observed in cell lines engineered to express BCR-ABL (Mo7e-p210BCR-ABL, Ba/F3-p210BCR-ABL-T315I, 32D-p210BCR-ABL, 32D-p190BCR-ABL). CMK cells, which are dependent on both JAK1 and JAK3 due to an activating mutation of JAK3 (JAK3A572V) that signals through wild-type JAK1,16 were also sensitive to CYT387. A third group of cell lines demonstrated higher IC50 values, in most cases exceeding 5000nM (the maximum concentration tested). This group encompasses all 4 nonhematopoietic cell lines tested. In aggregate, these data are consistent with relatively selective growth inhibition of JAK2 and possibly JAK1/TYK2-dependent cell lines. To assess whether CYT387 induces apoptosis in some of these JAK2-dependent cell lines, we performed trypan blue exclusion in conjunction with immunoblot for cleaved caspase 3 and found a dose-dependent increase in apoptosis (Figure 1C).
In vitro activity of CYT387 in 26 different hematopoietic and somatic cell lines
| Cell line . | Origin/phenotype . | Transforming kinase . | IC50, nM . |
|---|---|---|---|
| Ba/F3-EpoR parental control | Murine pre-B cell | IL-3–dependent growth | 1250 |
| Ba/F3-EpoR-JAK2-V617F | Murine pre-B cell | JAK2-V617F | 500 |
| 32D-EpoR parental | Murine myeloid precursor | IL-3–dependent growth | 1000 |
| 32D-EpoR-JAK2-WT | Murine myeloid precursor | IL-3–dependent growth | 1500 |
| 32D-EpoR-JAK2-V617F | Murine myeloid precursor | JAK2-V617F | 1500 |
| HEL | Acute myeloid leukemia | JAK2-V617F | 1500 |
| Ba/F3 | Murine pre-B cell | IL-3–dependent growth | 1750 |
| TF-1 | Acute myeloid leukemia | GM-CSF–dependent growth | 1250 |
| MO7e-p210 | Acute myeloid leukemia | BCR-ABL expression | 1500 |
| Ba/F3-p210 | Murine pre-B cell | BCR-ABL expression | 2000 |
| Ba/F3-p210-T315I | Murine pre-B cell | BCR-ABL expression | 1500 |
| 32D-p190 | Murine myeloid precursor | BCR-ABL expression | 1500 |
| KcL 22 | Chronic myeloid leukemia | BCR-ABL expression | 3000 |
| AR230-R1 | Chronic myeloid leukemia | BCR-ABL expression | 4000 |
| AR230-S | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| KBM5 | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| KU812 | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| Lama | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| Molm-14 | Acute myeloid leukemia | FLT3-ITD expression | 500 |
| CMK | Acute myeloid leukemia | JAK3-A572V | 1500 |
| REH | Acute lymphocytic leukemia | NN | 5000 |
| U266 | Multiple myeloma | IL-6–dependent growth | 750 |
| HeLa | Breast cancer | NN | 5000 |
| SKmI5 | Melanoma | NN | > 5000 |
| SKmI28 | Melanoma | NN | > 5000 |
| COS7 | Primate kidney | NN | > 5000 |
| Cell line . | Origin/phenotype . | Transforming kinase . | IC50, nM . |
|---|---|---|---|
| Ba/F3-EpoR parental control | Murine pre-B cell | IL-3–dependent growth | 1250 |
| Ba/F3-EpoR-JAK2-V617F | Murine pre-B cell | JAK2-V617F | 500 |
| 32D-EpoR parental | Murine myeloid precursor | IL-3–dependent growth | 1000 |
| 32D-EpoR-JAK2-WT | Murine myeloid precursor | IL-3–dependent growth | 1500 |
| 32D-EpoR-JAK2-V617F | Murine myeloid precursor | JAK2-V617F | 1500 |
| HEL | Acute myeloid leukemia | JAK2-V617F | 1500 |
| Ba/F3 | Murine pre-B cell | IL-3–dependent growth | 1750 |
| TF-1 | Acute myeloid leukemia | GM-CSF–dependent growth | 1250 |
| MO7e-p210 | Acute myeloid leukemia | BCR-ABL expression | 1500 |
| Ba/F3-p210 | Murine pre-B cell | BCR-ABL expression | 2000 |
| Ba/F3-p210-T315I | Murine pre-B cell | BCR-ABL expression | 1500 |
| 32D-p190 | Murine myeloid precursor | BCR-ABL expression | 1500 |
| KcL 22 | Chronic myeloid leukemia | BCR-ABL expression | 3000 |
| AR230-R1 | Chronic myeloid leukemia | BCR-ABL expression | 4000 |
| AR230-S | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| KBM5 | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| KU812 | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| Lama | Chronic myeloid leukemia | BCR-ABL expression | > 5000 |
| Molm-14 | Acute myeloid leukemia | FLT3-ITD expression | 500 |
| CMK | Acute myeloid leukemia | JAK3-A572V | 1500 |
| REH | Acute lymphocytic leukemia | NN | 5000 |
| U266 | Multiple myeloma | IL-6–dependent growth | 750 |
| HeLa | Breast cancer | NN | 5000 |
| SKmI5 | Melanoma | NN | > 5000 |
| SKmI28 | Melanoma | NN | > 5000 |
| COS7 | Primate kidney | NN | > 5000 |
NN indicates not known; GM-CSF, granulocyte monocyte–colony stimulation factor; BCR-ABL, breakpoint cluster region–Abelson leukemia virus; and FLT3-ITD, Fms-like tyrosine kinase 3–insert tandem duplication.
CYT387 inhibits JAK2 activity and signaling
To determine whether effects of CYT387 on proliferation and apoptosis correlate with inhibition of JAK2 signaling, we exposed Ba/F3 cells expressing JAK2V617F and EPOR to graded concentrations of CYT387 for 16 hours and analyzed phosphorylation of JAK2 signaling components by immunoblot (Figure 1D). While a significant reduction of phospho-JAK2 was evident only at relatively high concentrations of CYT387 (> 2μM), phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and signal transducer and activator of transcription 5 (STAT5) was inhibited at 0.3μM CYT387 and above.
CYT387 is effective in a murine model of MPNs
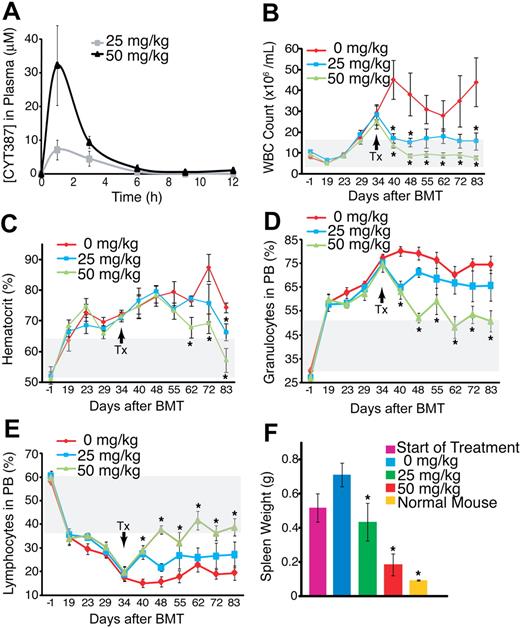
We next tested whether CYT387 is efficacious in an in vivo model of JAK2V617F-dependent MPN in which lethally irradiated Balb/c mice are transplanted with bone marrow transduced with a JAK2V617F retrovirus.6 Initially, we assessed the overall impact of this compound on the homeostasis of blood cells in naive mice. We found that CYT387 at twice the dose used in our subsequent disease model (50 and 100 mg/kg) had little to no effect on peripheral blood counts (supplemental Figure 1) over a period of 8 weeks. Next, we determined the plasma concentrations of CYT387 in Balb/c mice after a single dose of 25 and 50 mg/kg CYT387, the doses anticipated for use in our in vivo model. Median plasma peak concentrations were 7.1μM with the lower dose and 32.1μM with the higher dose, with a half-life of approximately 2 hours. Trough levels at 12 hours were 10nM for the 25 mg/kg and 900nM for the 50 mg/kg dose (Figure 2A, supplemental Table 2). In our murine MPN model, the animals develop PV-like MPN, with neutrophilic leukocytosis, erythrocytosis, and secondary myelofibrosis (Figure 2B-E). At day 34 after transplantation, the mean white blood cell counts and hematocrit values of the entire cohort exceeded the normal range for Balb/c mice by more than 1 SD. At this point, 6 mice were sacrificed and subjected to autopsy. In the remaining animals, treatment was initiated with 25 mg/kg CYT387, 50 mg/kg CYT387, or vehicle, administered twice daily by oral gavage (12 mice per treatment group). A rapid drop of the white cell counts was apparent in both dose cohorts as early as 6 days after initiation of treatment (Figure 2B) and a decline of the hematocrit was apparent after 20 days (Figure 2C). Complete normalization of hematocrit was achieved in the high-dose group, while slightly elevated values persisted in the low-dose group (Figure 2C). The drop in white blood cell counts was accompanied by a relative decrease in the granulocyte population and an increase to normal range of the lymphocyte cell population (Figure 2D-E, supplemental Figure 2C-D). In one single mouse in the high-dose group, both white blood cell count and hematocrit remained consistently above the normal range (data not shown). Thrombocytosis is not a feature of the murine MPN model used here, and platelet counts remained stable throughout the observation period (data not shown). No change in body weight was recorded in any of the 3 groups (supplemental Figure 2). In 4 mice (2 each in the low-dose and high-dose group), treatment was stopped on day 83 after transplantation. Repeat blood counts showed a recurrence of the elevated hematocrit in all mice and a recurrence of the elevated white blood cell counts in the 2 mice previously treated with 50 mg/kg CYT387 (supplemental Figure 3).
Efficacy of CYT387 against JAK2-dependent malignancy in vivo. (A) Balb/c mice were administered CYT387 by oral gavage and pharmacokinetics were measured by analysis of plasma concentrations at indicated time points. Values represent mean ± SEM. (n = 3) (B) Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). White blood cell (WBC) count of mice was monitored weekly for 83 days after bone marrow transplantation. (C) Mice were treated as in panel B and hematocrit levels (HCT) of mice were monitored weekly for 83 days after bone marrow transplantation. (D) Mice were treated as in panel B and the percentage of granulocytes in the peripheral blood of mice was monitored weekly for 83 days after bone marrow transplantation. (E) Mice were treated as in panel B and percentage of lymphocytes in the peripheral blood of mice was monitored weekly for 83 days after bone marrow transplantation. (F) Mice were treated as in panel B and the spleen weight of 3 mice was measured at the start of treatment and the spleen weight of all mice was measured at the end of treatment. For comparison purposes, spleen weight from normal mice is included. For panels B through F, values represent mean ± SEM, *P < .05 in a t test comparing 25 mg/kg or 50 mg/kg treatment groups with the 0 mg/kg vehicle control. The gray box indicates the normal range in mice. The arrow indicates initiation of CYT387 treatment.
Efficacy of CYT387 against JAK2-dependent malignancy in vivo. (A) Balb/c mice were administered CYT387 by oral gavage and pharmacokinetics were measured by analysis of plasma concentrations at indicated time points. Values represent mean ± SEM. (n = 3) (B) Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). White blood cell (WBC) count of mice was monitored weekly for 83 days after bone marrow transplantation. (C) Mice were treated as in panel B and hematocrit levels (HCT) of mice were monitored weekly for 83 days after bone marrow transplantation. (D) Mice were treated as in panel B and the percentage of granulocytes in the peripheral blood of mice was monitored weekly for 83 days after bone marrow transplantation. (E) Mice were treated as in panel B and percentage of lymphocytes in the peripheral blood of mice was monitored weekly for 83 days after bone marrow transplantation. (F) Mice were treated as in panel B and the spleen weight of 3 mice was measured at the start of treatment and the spleen weight of all mice was measured at the end of treatment. For comparison purposes, spleen weight from normal mice is included. For panels B through F, values represent mean ± SEM, *P < .05 in a t test comparing 25 mg/kg or 50 mg/kg treatment groups with the 0 mg/kg vehicle control. The gray box indicates the normal range in mice. The arrow indicates initiation of CYT387 treatment.
At day 83 after bone marrow transplantation, all mice were subjected to autopsy (with the exception of the 2 low-dose and 2 high-dose mice that were monitored after drug discontinuation). Our previous analysis of 6 mice at the start of CYT387 treatment (day 34 after bone marrow transplantation) had revealed that all 6 mice exhibited enlarged spleens compared with naive mice (Figure 2F). Mice that were subsequently treated with vehicle and sacrificed at day 83 after transplantation demonstrated gross splenomegaly, which had increased compared with the mice analyzed before therapy (Figure 2F). Spleen size was significantly reduced in both the 25 mg/kg and 50 mg/kg groups compared with vehicle mice. In the 50 mg/kg treatment group, spleen weights were not significantly different from weights of naive, age-matched controls. Liver weights were not significantly different among the cohorts of mice (data not shown). There was no macroscopic evidence for organ involvement other than spleen as assessed by examination of liver, lung, and kidney.
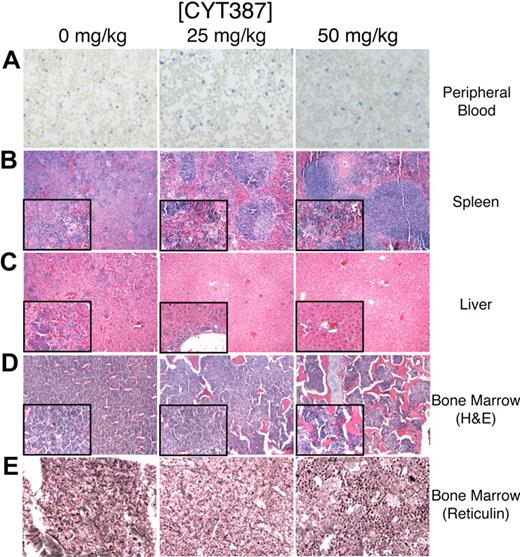
Staining of peripheral blood smears showed increased red blood cells and reticulocytes in the vehicle-treated controls. A significant reduction of reticulocytes in the low-dose (P = .007) and high-dose (P = .020) groups was observed compared with vehicle control (Figure 3A, supplemental Figure 2B). Histology of spleens from mice in the vehicle group showed extensive infiltration with extramedullary hematopoiesis and complete effacement of splenic architecture. Findings in mice from the low-dose group were similar, while there was partial restoration of splenic architecture in the high-dose group with intact follicles covering 30% to 50% of the area (Figure 3B). Liver histology revealed mild infiltration with extramedullary hematopoiesis, ranging from small periportal aggregates and scattered megakaryocytes to large perivascular and random aggregates of maturing granulocytes in vehicle and low-dose groups, but not in the high-dose group (Figure 3C). In all 3 groups, bone marrow histology was hypercellular with marked right-shifted granulocytic hyperplasia. Megakaryocytes were dysplastic in all samples with increases in cell and nuclear size and abnormal nuclear lobation and appeared to be substantially increased in the 50 mg/kg group (Figure 3D). Reticulin fibrosis was significantly reduced in high-dose–treated mice compared with the vehicle controls as determined by a blinded 0 to 3 scoring of femoral tissue sections of 6 animals from each group where 0 indicates normal tissue and 3 indicates extreme levels of fibrosis. The vehicle-control mice exhibited an average score of 1.5, while the 25 mg/kg and 50 mg/kg mouse group scores were reduced to an average of 1.0 and 0.5, respectively (Figure 3E, supplemental Table 3).
Histopathology after CYT387 treatment of JAK2-dependent malignancy in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were killed and representative histologic sections are shown from: (A) peripheral blood stained for reticulocytes, (B) spleen stained with H&E, (C) liver (H&E), (D) bone marrow (H&E), and (E) bone marrow (reticulin stain). The left column represents 0 mg/kg CYT387, the middle column represents 25 mg/kg CYT387, and the right column represents 50 mg/kg CYT387. Magnification is ×10 or ×40 for insets.
Histopathology after CYT387 treatment of JAK2-dependent malignancy in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were killed and representative histologic sections are shown from: (A) peripheral blood stained for reticulocytes, (B) spleen stained with H&E, (C) liver (H&E), (D) bone marrow (H&E), and (E) bone marrow (reticulin stain). The left column represents 0 mg/kg CYT387, the middle column represents 25 mg/kg CYT387, and the right column represents 50 mg/kg CYT387. Magnification is ×10 or ×40 for insets.
CYT387 restores hematopoietic lineage composition, but JAK2V617F-expressing cells persist
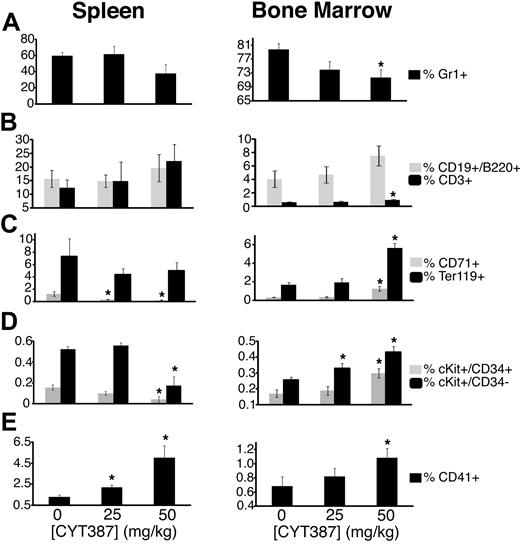
To determine the effects of CYT387 on the various hematopoietic cell compartments and differentiation stages, we performed fluorescence-activated cell sorter (FACS) analysis of bone marrow and spleen cells (Figure 4, supplemental Figure 4, and supplemental Table 4). CYT387 therapy induced a decrease in granulocytes (Gr1+) compared with vehicle-treated mice (Figure 4A), both in spleen (high-dose only) and bone marrow, which was accompanied by an increase of T cells (CD3+) and B cells (CD19+/B220+; Figure 4B). Cells of the erythroid lineage showed divergent behavior between spleen and marrow. Splenic early erythrocyte progenitors (CD71+) were significantly reduced in treated mice compared with vehicle-control mice, while mature erythrocyte progenitors (Ter119+) were reduced to a lesser extent. In contrast, both populations increased in the marrows of mice treated with 50 mg/kg (but not 25 mg/kg) CYT387 (Figure 4C). Similarly, we saw a relative reduction of splenic lineage-negative (Lin-neg) KIT+/CD34+ cells (representing progenitor cells) and Lin-neg KIT+/CD34− cells (containing hematopoietic stem cells and early progenitors) in the 50 mg/kg group versus vehicle-control group while both of these populations were increased in the marrow (Figure 4D). Megakaryocytic lineage cells (CD41+) were increased in spleen as well as bone marrow of treated mice (Figure 4E).
Effect of CYT387 on hematopoietic cell lineages in MPN in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were sacrificed and spleen and bone marrow cells were stained with fluorescently conjugated antibodies specific for: (A) Gr1 (Granulocytes), (B) CD19/B220 (B cells) and CD3 cells (T cells), (C) CD71 (early erythroid progenitors) and Ter119 (late erythroid progenitors), (D) cKit+CD34− (stem cells/early progenitors) and cKit+CD34+ (late progenitors), (E) CD41 (megakaryocytes). Values represent mean ± SEM and *P < .05 in a t test comparing 25 mg/kg or 50 mg/kg treatment groups with the 0 mg/kg vehicle control. A complete list of numerical values and statistical analyses is found in supplemental Table 2. Representative dot plots are found in supplemental Figure 4.
Effect of CYT387 on hematopoietic cell lineages in MPN in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were sacrificed and spleen and bone marrow cells were stained with fluorescently conjugated antibodies specific for: (A) Gr1 (Granulocytes), (B) CD19/B220 (B cells) and CD3 cells (T cells), (C) CD71 (early erythroid progenitors) and Ter119 (late erythroid progenitors), (D) cKit+CD34− (stem cells/early progenitors) and cKit+CD34+ (late progenitors), (E) CD41 (megakaryocytes). Values represent mean ± SEM and *P < .05 in a t test comparing 25 mg/kg or 50 mg/kg treatment groups with the 0 mg/kg vehicle control. A complete list of numerical values and statistical analyses is found in supplemental Table 2. Representative dot plots are found in supplemental Figure 4.
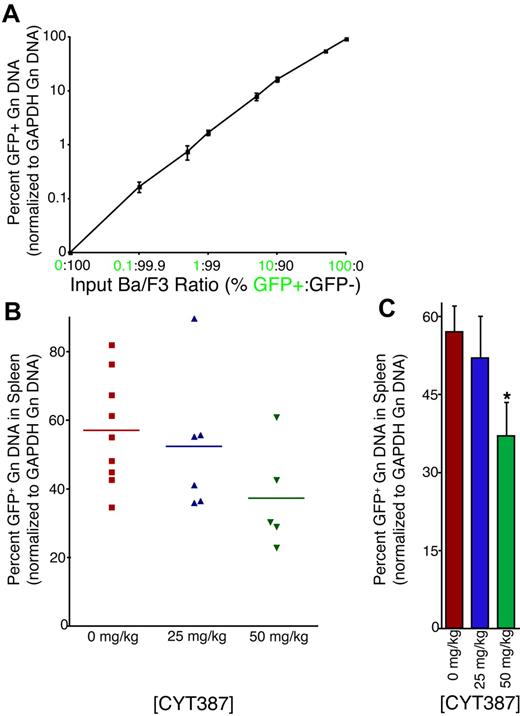
Unexpectedly, flow cytometric analysis revealed minimal expression of GFP (< 1% in many cell compartments; data not shown). As previous studies have shown that the internal ribosomal entry site (IRES)–GFP component of this transgene can become silenced and cells subsequently lose GFP expression,17 we developed a new qPCR assay for quantification of GFP (and thus allele burden) in genomic DNA of cells, using amplification of the genomic GAPDH locus for normalization. In multiple independently performed amplifications, using genomic DNA from a standard dilution of graded ratios of parental (GFP-negative) and JAK2V614F/GFP-positive Ba/F3 cells, we were able to demonstrate that this assay accurately quantifies GFP genomic DNA over a wide range (0.1%-100% GFP-positive cells) with an r2 value of 0.9999 (Figure 5A). Application of this technique on splenic genomic DNA isolated from mice treated with vehicle or CYT387 showed a high overall level of GFP with a range of 23% to 90% (Figure 5B). Interestingly, we observed a significant reduction in GFP levels (allelic burden) in mice treated with 50 mg/kg CYT387 (Figure 5C). These observations suggest that JAK2 inhibitor therapy results in a selective reduction of JAK2V617-expressing cells. However, even the high dose of CYT387 failed to eliminate the JAK2V617F/GFP-positive population, consistent with persistence of MPN. Similar observations have been made in early clinical trials with JAK2 inhibitors in myelofibrosis.18
Effect of CYT387 on JAK2 V617F+ allelic burden in MPN in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were sacrificed and genomic DNA was isolated from splenocytes. A qPCR assay was developed to assess the relative level of GFP, normalized to the genomic GAPDH locus. (A) Parental Ba/F3 (GFP-negative) were mixed at varying ratios with Ba/F3-JAK2V617F cells (GFP-positive). Genomic DNA was isolated and subjected to qPCR using primers specific for GFP or the GAPDH genomic locus. All values were normalized to GAPDH and then to the highest expressing well of genomic DNA from 100% GFP-positive cells. This value was set at 100%. Values represent mean ± SEM. (B) Genomic DNA from splenocytes of mice treated with vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 were subjected to qPCR using primers specific for GFP or the GAPDH genomic locus. The standard curve of Ba/F3 cells shown in panel A was amplified simultaneously, and all values were normalized to GAPDH and then to the highest expressing well of genomic DNA from 100% GFP-positive Ba/F3 cells. Each point represents the genomic GFP level in an individual mouse. (C) The values from panel B were averaged and presented in a bar graph. Values represent mean ± SEM and *P < .05 in a t test comparing 25 mg/kg or 50 mg/kg treatment groups with the 0 mg/kg vehicle control.
Effect of CYT387 on JAK2 V617F+ allelic burden in MPN in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were sacrificed and genomic DNA was isolated from splenocytes. A qPCR assay was developed to assess the relative level of GFP, normalized to the genomic GAPDH locus. (A) Parental Ba/F3 (GFP-negative) were mixed at varying ratios with Ba/F3-JAK2V617F cells (GFP-positive). Genomic DNA was isolated and subjected to qPCR using primers specific for GFP or the GAPDH genomic locus. All values were normalized to GAPDH and then to the highest expressing well of genomic DNA from 100% GFP-positive cells. This value was set at 100%. Values represent mean ± SEM. (B) Genomic DNA from splenocytes of mice treated with vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 were subjected to qPCR using primers specific for GFP or the GAPDH genomic locus. The standard curve of Ba/F3 cells shown in panel A was amplified simultaneously, and all values were normalized to GAPDH and then to the highest expressing well of genomic DNA from 100% GFP-positive Ba/F3 cells. Each point represents the genomic GFP level in an individual mouse. (C) The values from panel B were averaged and presented in a bar graph. Values represent mean ± SEM and *P < .05 in a t test comparing 25 mg/kg or 50 mg/kg treatment groups with the 0 mg/kg vehicle control.
Effects of CYT387 on plasma cytokines
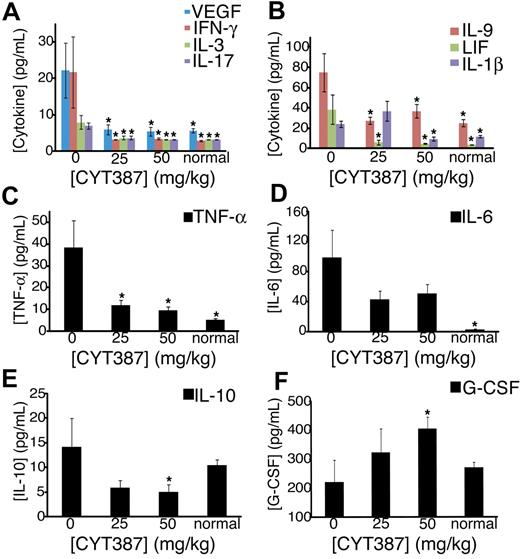
Patients with myelofibrosis exhibit increased plasma concentrations of inflammatory cytokines, such as IL-6, which are thought to be responsible for the constitutional symptoms that characterize the disease.19 We therefore hypothesized that responses to CYT387 may be associated with a normalization of abnormal cytokine expression. At the termination of the experiment, we collected plasma from the 2 CYT387 treatment groups, vehicle-treated controls (0 mg/kg) and untreated sex- and age-matched normal mice and measured the expression of a panel of 32 different cytokines and chemokines using a multiplex assay (supplemental Table 5). We found that the plasma levels of 13 pivotal inflammatory cytokines and chemokines, IL-1β, IL-3, IL-4, IL-6, IL-9, IL-17, IP-10, LIF, MCP-1, MIG, VEGF, TNF-α, and IFN-γ, were elevated in vehicle-treated MPN mice compared with normal naive mice (P ≤ .1). Most of these cytokines and chemokines showed a dose-dependent reduction in mice treated with CYT387 to normal or almost normal levels, although to a variable degree. For example, the concentrations of IFN-γ, IL-3, VEGF, IL-17, IL-9, LIF, and IL-1β were identical or almost identical in mice treated with 50 mg/kg CYT387 and normal mice (Figure 6A-B). In contrast, the concentrations of TNF-α (Figure 6C), IL-6 (Figure 6D), IL-10, IP-10, KC, and MCP-1 (supplemental Table 5) remained elevated compared with normal mice. By contrast, 3 cytokines (GM-CSF, IL-12p40, and IL-1α) were significantly reduced in vehicle-treated diseased mice compared with normal naive mice (supplemental Table 5). All 3 cytokines returned back to normal plasma levels with CYT387 treatment (supplemental Table 5). Interestingly, IL-10 and G-CSF concentrations were similar in vehicle-treated diseased mice compared with normal naive mice. However, IL-10 was significantly down-regulated with CYT387 treatment (Figure 6E) and G-CSF was significantly up-regulated compared with normal naive mice upon treatment with CYT387 (Figure 6F). Altogether, these data indicate that mice with JAK2V617F-induced disease exhibit a complex perturbation of cytokine and chemokine expression that is at least partially normalized by CYT387.
Effect of CYT387 on cytokine concentrations during MPN in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were sacrificed and serum was harvested from peripheral blood for cytokine analysis with a multiplexed 96-well ELISA-based assay. Bar graphs represent mean cytokine levels for: (A) VEGF (vascular endothelial growth factor), IFN-γ (interferon-γ), IL-3 and IL-17, (B) IL-9, LIF (leukocyte inhibitory factor) and IL-1β, (C) TNF-α (tumor necrosis factor-α), (D) IL-6, (E) IL-10, (F) G-CSF (granulocyte colony stimulation factor). Values represent mean ± SEM and *P ≤ .1 in a t test comparing normal mouse, 25 mg/kg, or 50 mg/kg treatment groups with the 0 mg/kg vehicle control. A complete list of numerical values and statistical analyses is found in supplemental Table 4.
Effect of CYT387 on cytokine concentrations during MPN in vivo. Balb/c mice were subjected to bone marrow transplantation with bone marrow donor cells retrovirally transduced to express JAK2V617F. Thirty-four days after transplantation, mice exhibited symptoms of MPN as measured by elevated white blood cell counts and hematocrit. Mice were divided into 3 groups and initiated on twice daily oral gavage administration of vehicle control, 25 mg/kg CYT387, or 50 mg/kg CYT387 (n = 12 per group). At day 83 after bone marrow transplantation, all mice were sacrificed and serum was harvested from peripheral blood for cytokine analysis with a multiplexed 96-well ELISA-based assay. Bar graphs represent mean cytokine levels for: (A) VEGF (vascular endothelial growth factor), IFN-γ (interferon-γ), IL-3 and IL-17, (B) IL-9, LIF (leukocyte inhibitory factor) and IL-1β, (C) TNF-α (tumor necrosis factor-α), (D) IL-6, (E) IL-10, (F) G-CSF (granulocyte colony stimulation factor). Values represent mean ± SEM and *P ≤ .1 in a t test comparing normal mouse, 25 mg/kg, or 50 mg/kg treatment groups with the 0 mg/kg vehicle control. A complete list of numerical values and statistical analyses is found in supplemental Table 4.
In vitro resistance to CYT387 by overexpression of JAK2V617F
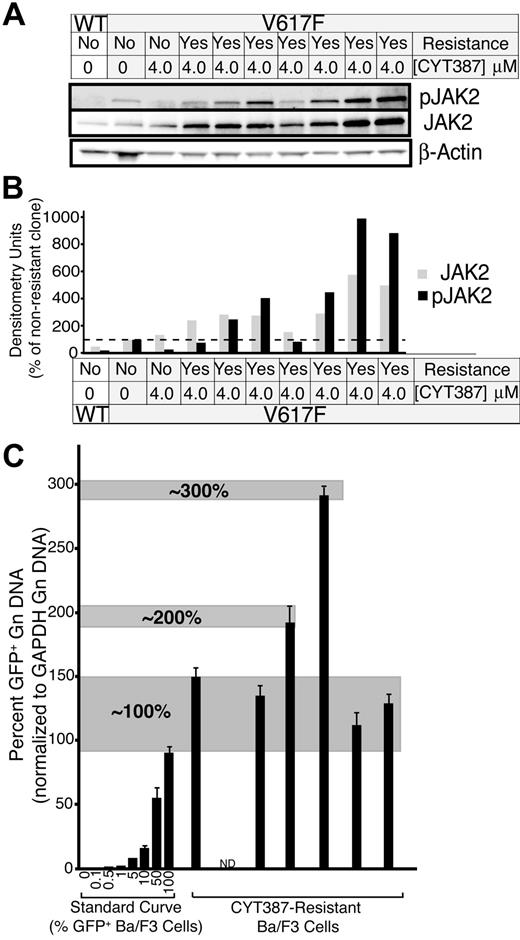
Resistance to tyrosine kinase inhibitors such as imatinib or dasatinib in CML, or gefitinib in non–small cell lung cancer is frequently due to acquired point mutations in the kinase domain of the target kinases.20,21 We have previously shown that resistance mutations in BCR-ABL are readily induced and selected by culture of ENU-treated Ba/F3 cells expressing BCR-ABL in the presence of inhibitors.13 To determine whether point mutations in the JAK2 kinase domain can confer resistance to CYT387, we treated Ba/F3-EPOR cells expressing JAK2V617F with ENU, followed by culture in the presence of 2 to 5μM CYT387. Cell clones emerged that were resistant to CYT387 up to a concentration of 4μM. Sequence analysis of the entire exogenous JAK2 DNA failed to reveal any mutations in over 200 single clones (data not shown). These data suggest that point mutations are an unlikely mechanism of clinical resistance to CYT387. Consistent with this, sequencing of spleen DNA from 1 mouse in the high-dose treatment group that failed to normalize the hematocrit showed only wild-type sequence (data not shown). However, 7 resistant cell clones derived from the 4-μM cultures expressed 2- to 6-fold higher levels of the JAK2 protein compared with the parental cells, with even greater differences in pJAK2 (Figure 7A-B). To investigate the possibility that this elevated expression of JAK2 may be due to selection for cells with extra copies of the JAK2V617F-IRES-GFP transgene, we applied our GFP qPCR technique to assess levels of GFP in the genomic DNA from these cells relative to a standard curve of Ba/F3 cells with a maximum of 100% (1 copy of the transgene). We investigated 6 of the 7 CYT387-resistant clones and found that 4 of 6 clones had levels of GFP equivalent to the top point on the curve (1 copy of the transgene), 1 of 6 clones exhibited 2-fold higher levels than the top point on the standard curve (2 copies of the transgene), and 1 of 6 clones exhibited GFP levels 3-fold of the top of the standard curve (3 copies of the transgene; Figure 7C). However, there was no correlation between the transgene copy number and the JAK2 protein expression. For example, the clone with 3 copies of the transgene did not exhibit the highest JAK2 expression. Hence, while one mechanism for increased JAK2 expression in this model may be selection for cells with extra JAK2V617F copies, this is not a consistent finding and additional mechanisms must govern JAK2 overexpression in this model system.
Resistant subclone evasion of CYT387 in vitro. (A) Baf3-EpoR cells expressing JAK2V617F were subjected to ENU mutagenesis in the presence of CYT387. Cells were plated at 2 × 105 cells per well of a 96 well plate in media with 4μM CYT387. Wells were examined every 5 days for colony outgrowth over a 38-day period. Colonies that grew out were expanded and subsequently serum-starved overnight in the presence of 4μM CYT387 and subjected to immunoblot analysis using antibodies specific for total or phospho-JAK2 or β-actin. (B) Densitometric analysis of immunoblots in panel A. Expression levels for total JAK2 ( ) and phospho JAK2 (■) were normalized to the levels observed in JAK2WT cells that are sensitive to CYT387. (C) Genomic DNA was isolated from CYT387-resistant Ba/F3 cells and levels of genomic GFP or GAPDH were assessed by quantitative PCR. A standard curve of varying ratios of GFP-positive/GFP-negative Ba/F3 cell mixtures was included as in Figure 5A. Levels of genomic GFP were normalized as in Figure 5 (normalized first to GAPDH, then to the highest value well on the standard curve) and are presented as percent GFP-positive. Values represent mean ± SEM (n = 3).
) and phospho JAK2 (■) were normalized to the levels observed in JAK2WT cells that are sensitive to CYT387. (C) Genomic DNA was isolated from CYT387-resistant Ba/F3 cells and levels of genomic GFP or GAPDH were assessed by quantitative PCR. A standard curve of varying ratios of GFP-positive/GFP-negative Ba/F3 cell mixtures was included as in Figure 5A. Levels of genomic GFP were normalized as in Figure 5 (normalized first to GAPDH, then to the highest value well on the standard curve) and are presented as percent GFP-positive. Values represent mean ± SEM (n = 3).
Resistant subclone evasion of CYT387 in vitro. (A) Baf3-EpoR cells expressing JAK2V617F were subjected to ENU mutagenesis in the presence of CYT387. Cells were plated at 2 × 105 cells per well of a 96 well plate in media with 4μM CYT387. Wells were examined every 5 days for colony outgrowth over a 38-day period. Colonies that grew out were expanded and subsequently serum-starved overnight in the presence of 4μM CYT387 and subjected to immunoblot analysis using antibodies specific for total or phospho-JAK2 or β-actin. (B) Densitometric analysis of immunoblots in panel A. Expression levels for total JAK2 ( ) and phospho JAK2 (■) were normalized to the levels observed in JAK2WT cells that are sensitive to CYT387. (C) Genomic DNA was isolated from CYT387-resistant Ba/F3 cells and levels of genomic GFP or GAPDH were assessed by quantitative PCR. A standard curve of varying ratios of GFP-positive/GFP-negative Ba/F3 cell mixtures was included as in Figure 5A. Levels of genomic GFP were normalized as in Figure 5 (normalized first to GAPDH, then to the highest value well on the standard curve) and are presented as percent GFP-positive. Values represent mean ± SEM (n = 3).
) and phospho JAK2 (■) were normalized to the levels observed in JAK2WT cells that are sensitive to CYT387. (C) Genomic DNA was isolated from CYT387-resistant Ba/F3 cells and levels of genomic GFP or GAPDH were assessed by quantitative PCR. A standard curve of varying ratios of GFP-positive/GFP-negative Ba/F3 cell mixtures was included as in Figure 5A. Levels of genomic GFP were normalized as in Figure 5 (normalized first to GAPDH, then to the highest value well on the standard curve) and are presented as percent GFP-positive. Values represent mean ± SEM (n = 3).
Discussion
More than 90% of patients with PV and 30% to 50% of patients with PMF and ET carry the JAK2V617F allele.22 Additional patients have activating mutations in exon 12 of JAK2 or in upstream signaling pathways, such as the thrombopoietin receptor (MPL), suggesting that JAK2 activation may be a universal feature of MPN, and as yet unknown mechanisms may be causal in patients without mutations in JAK2 or MPL.23,24 Given the efficacy of BCR-ABL inhibitors such as imatinib in patients with CML, there is considerable interest in the development of JAK2 inhibitors for the treatment of patients with MPN. Here, we characterized CYT387, a novel aminopyrimidine inhibitor of JAK2 in a murine model of JAK2V617F-driven MPN.
In vitro kinase assays revealed CYT387 as a low nanomolar inhibitor of JAK2 JAK1, and TYK2, with very limited off-target effects (supplemental Table 1). Proliferation of JAK2V617F cell lines was inhibited by CYT387, with IC50 values ranging between 500 and 1500nM (Figure 2A, Table 1), and this correlated with inhibition of downstream signaling (Figure 2C) and induction of apoptosis (Figure 2B). The differential (∼ 1.5-log) between the sensitivity in enzyme and cell proliferation assays presumably arises from the different concentrations of ATP in biochemical assays (typically 100μM or less) and cellular assays (> 1mM), though may also be related to drug transport mechanisms or protein binding. Ba/F3 cells expressing JAK2V617F and EPOR were slightly more sensitive to CYT387 than cells expressing EPOR alone (Figure 2A), but in general all cell lines dependent on JAK2 signaling were inhibited at CYT387 concentrations between 500 and 1500nM. Inhibition of JAK1 is the most likely explanation for the low IC50 in CMK cells, which express an activated JAK3 allele that is dependent on wild-type JAK1 for downstream signaling,16 whereas the growth inhibition of Molm14 cells (FLT3-ITD positive) remains mechanistically unexplained. Since the SelectScreen Profiling Service (Invitrogen) revealed the IC50 of CYT387 for the FLT3 kinase domain to be 200nM, it is unclear whether this effect on Molm14 cells is due to an off-target, direct effect of the inhibitor on FLT3 kinase, or whether this may indicate involvement of JAK2 family members in signaling downstream of FLT3-ITD, although a previous study found no evidence for this.25 In contrast, the IC50 values for BCR-ABL–expressing cell lines were in a range from 1.5 to 5μM. This may be explained by the fact that JAK2 is involved in signal transduction downstream of BCR-ABL.26 Since the SelectScreen Profiling Service (Invitrogen) showed an IC50 for ABL to be greater than 5000nM, this explanation of BCR-ABL signaling through JAK2 is a more likely explanation than direct effects of the inhibitor on BCR-ABL. Lastly, nonhematopoietic cell lines showed no significant inhibition at the highest concentration tested (5 μM). Together, these data show that CYT387 selectively inhibits the proliferation of JAK2 and possibly JAK1/TYK2-dependent cell lines, irrespective of whether they express wild-type or mutant JAK2.
Inhibition of STAT5 and ERK1/2 phosphorylation occurred at lower concentrations than of JAK2. This is presumably due to the binding of CYT387 to an open-phosphorylated form of JAK2 (C.J.B., E.F., manuscript in preparation). The competitive inhibition of ATP binding consequently blocks phosphorylation of multiple copies of STAT3 and ERK proteins, because JAK2 is a catalytic initiator of these signaling pathways.
CYT387 showed in vivo activity in a murine MPN model, with normalization of white blood cell counts, white cell differential counts and hematocrit in mice treated with 50 mg/kg CYT387 (Figure 3). As expected, effects on the hematocrit were apparent only after several weeks, reflecting the 23-day half-life of erythrocytes in Balb/c mice.27 Reticulocytes were reduced, however, elevated counts persisted despite CYT387 treatment. On autopsy, mice in the 50 mg/kg group showed almost normal spleen weight and greatly reduced extramedullary hematopoiesis, with a partial restoration of normal splenic architecture. In contrast, the effects of CYT387 on the bone marrow were less pronounced, with persistent hypercellularity even in the 50 mg/kg group.
FACS analysis of spleen and bone marrow according to hematopoietic lineage revealed differential effects. The decrease of Gr1+ cells in spleen and bone marrow, the most dominant cell population in these organs (Figure 5A), was in line with the observed decrease of granulocytes in the peripheral blood of CYT387-treated mice (Figure 3D). The relative increase of lymphoid cells (CD3+ and CD19+/B220+) in spleen and bone marrow of CYT387-treated mice is in line with the observed increase of intact lymphoid follicles (Figure 4B) and the increase of lymphoid cells in the peripheral blood of CYT387-treated mice (Figure 3E). The increase of the CD41+ cells (megakaryocytic lineage; Figure 5E), especially in the high-dose 50 mg/kg group, is supported by the histologic finding of increased megakaryocytes in the marrow of 50 mg/kg mice (Figure 4D) and likely represents a relative increase due to an overall decrease in the cellularity of the bone marrow and spleen. An additional level of complexity is added by the profound effects of CYT387 on the levels of inflammatory and hematopoietic cytokines, which may differentially affect JAK2WT versus JAK2V617F cells. For example, TNF-α is a negative regulator of erythropoiesis and the reduction of TNF-α in CYT387-treated mice may contribute to the relative increase of erythropoietic cells in the bone marrow of these mice,28 as observed by the increase of both early (CD71+) and late (Ter119+) erythroid progenitor cells in the bone marrow (of mice; Figure 5C).
Despite the profound reduction of overall cell numbers, evidenced by the normalization of blood counts and spleen size, the persistence of GFP in the splenic DNA clearly shows that CYT387 fails to eliminate JAK2V617F-expressing cells. Several explanations exist for this observation. First, while high peak levels are achieved, the drug half-life in mice is short, which is insufficient for continuously maintaining plasma concentrations above the IC50, even with the 50 mg/kg dose (Figure 3A). This may be sufficient for inhibition of proliferation, but insufficient for induction of apoptosis. Second, microenvironmental JAK2-independent signals may promote survival of JAK2V617F-expressing cells in the presence of CYT387. A third possibility is that the duration of therapy was insufficient and JAK2V617F-expressing cells would continue to decrease with extended duration of treatment.
Significant in vivo activity has also been observed with TG101348, another small molecule inhibitor of JAK2, in a murine model similar to the model used here as well as in xenografts.10-12 Because no mice were followed after discontinuation of TG101348, it is unknown whether this compound may induce more durable responses. However, the data from our mouse model are strikingly similar to early reports from studies of JAK2 inhibitors in patients with advanced myelofibrosis. While these drugs induce a rapid reduction of spleen size in practically all patients, there was an equally rapid recurrence of splenomegaly upon interruption of dosing for toxicity.18 All in all, these observations suggest that JAK2 inhibitors may be incapable of curing MPN, similar to imatinib's inability to eradicate CML.29 Although this appears to be common to both disease entities, there are also important differences. For example, MPN patients appear to have persistent clonal cells in all stages of maturity in contrast to CML where this is limited to early stem/progenitor cells. It will be interesting to determine the biologic basis for the different responses of these 2 oncogenic tyrosine kinases to kinase inhibitor therapy. Possible mechanisms may include differences in the normal biologic role for these proteins as well as potential differences in the way each oncogene may contribute to malignancy. Nonetheless, the dramatic reduction of splenomegaly and the normalization of cytokine concentrations suggest that CYT387 will be effective in human MPN. Phase 1 clinical trials were expected to begin in early 2010. Moreover, the profound effects of CYT387 on inflammatory cytokines suggest that potent JAK2 inhibitors may have therapeutic benefit in disorders like rheumatoid arthritis, where such cytokines play a major pathogenetic role.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dorian LaTocha and Jonathan Van Dyke, OHSU, for help with flow cytometry.
This work was supported in part by National Heart, Lung, and Blood Institute grant HL082978-01 (M.W.D.), National Institutes of Health nursing research grant 1R21NR010363 (L.W.), the Doris Duke Charitable Foundation (B.J.D.), the T. J. Martell Foundation (M.M.L.), and The Leukemia & Lymphoma Society (M.M.L., B.J.D., M.W.D.).
L.W. is a Research Scholar of the American Cancer Society; M.W.D. is a Scholar in Clinical Research of The Leukemia & Lymphoma Society; and K.J.A. is an Erwin Schroedinger Fellow of the Austrian Science Fund FWF (grant J2758-B12).
National Institutes of Health
Authorship
Contribution: J.W.T. and T.G.B. designed and performed research, analyzed data, and wrote the paper; J.D. designed and performed research, and analyzed data, L.W. performed research and contributed valuable reagents; K.J.A. and M.M.L. performed research and analyzed data; B.J.D. contributed valuable reagents; C.J.B. and E.F. designed and performed research, and contributed valuable reagents, and M.W.D. designed research, analyzed data, contributed valuable reagents, and wrote the paper.
Conflict-of-interest disclosure: C.J.B. and E.F. are employees of YM Biosciences Australia Pty Ltd. The remaining authors declare no competing financial interests.
Correspondence: Michael W. Deininger, Hematology and Medical Oncology, Oregon Health & Science University, BRB 553, Mailcode L592, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; e-mail: deininge@ohsu.edu.
References
Author notes
J.W.T., T.G.B., and J.D. contributed equally.