In this issue of Blood, Byrd and colleagues report on the outcomes of a phase 1/2 study of anti-CD23 monoclonal antibody lumiliximab combined with FCR in patients with refractory/relapsed CLL.
For the past 20 years, significant progress in molecular and cellular biology has resulted in a better characterization and understanding of the biology and prognosis of chronic lymphocytic leukemia (CLL). These achievements have provided new opportunities for the development of innovative, more effective therapies in this disease. These treatments include purine nucleoside analogs, monoclonal antibodies, agents targeting the antiapoptotic bcl-2 family of proteins, immunomodulating drugs, and other agents. In recent years, chemoimmunotherapy combining rituximab with fludarabine and cyclophosphamide (FCR) has become the first-line choice for CLL patients. Investigators at the M. D. Anderson Cancer Center conducted a single-arm study of FCR in 177 patients with previously treated CLL.1 Complete response (CR) was achieved in 25% and overall response (OR) in 73% of the patients (see figure). Even more encouraging results were reported by Keating et al2 in 224 treatment-naive CLL patients treated with this regimen; the CR rate was 70% and OR rate 95%. Two-thirds of patients evaluated with flow cytometry had less than 1% CD5+/CD19+ cells in bone marrow after therapy. These studies indicate that an FCR regimen has extraordinary clinical activity in previously untreated and pretreated patients with CLL. Two recent randomized clinical trials have confirmed these results and indicated that in patients with CLL, FCR can increase the OR and CR rate and prolong progression-free survival (PFS), compared with FC in previously untreated and refractory/relapsed patients.3,4 FCR is becoming the first-line choice for younger patients.
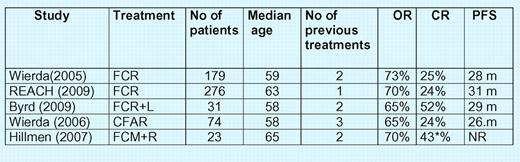
Studies evaluating FCR alone and in combination with other agents in refractory/relapsed CLL. NR indicates not reported; F, fludarabine; C, cyclophosphamide; R, rituximab; L, lumiliximab; A, alemtuzumab; M, mitoxantrone; and m, months. *CR + CR unconfirmed.
Studies evaluating FCR alone and in combination with other agents in refractory/relapsed CLL. NR indicates not reported; F, fludarabine; C, cyclophosphamide; R, rituximab; L, lumiliximab; A, alemtuzumab; M, mitoxantrone; and m, months. *CR + CR unconfirmed.
Lumiliximab is a genetically primatized, macaque-human chimeric IgG1κ antibody investigated for the treatment of refractory and relapsed CLL. This agent binds specifically to CD23, a glycoprotein expressed on the majority of CLL cells.5 The primary mechanism of action of lumiliximab in both CD23+ lymphoma B cells and CLL cells is sensitization to apoptotic cell death. It also induces antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. In addition, lumiliximab enhances apoptosis in vivo when combined with CLL therapies including chlorambucil, fludarabine, alemtuzumab, and rituximab. In a phase 1 study of 46 patients with refractory/relapsed CLL, lumiliximab was given as 6 different dosing regimens, the lowest dose being 125 mg/m2/wk for 4 weeks, and the highest dose being 500 mg/m2 3 times a week for 4 weeks.6 Reductions in absolute lymphocyte count and lymphadenopathy were observed in 42 of 46 patients (91%).
In this issue of Blood, Byrd et al7 report the results of a phase 1/2 study, testing the FCR regimen combined with lumiliximab in 31 patients with refractory/relapsed CLL. The patients had 1 to 10 (median, 2) previous therapies and 61% had previously received fludarabine.7 The OR rate was 65%, including 52% CRs. The median PFS was 30.4 months (range, 9.8-47.7 months). In terms of CR rate, FCR plus lumiliximab in this study compared favorably with the activity of FCR in a similar patient population (25% CRs) previously reported by Wierda et al.1 The PFS was similar in both trials: 29 months and 28 months, respectively. Importantly, the addition of lumiliximab to FCR treatment did not increase its toxicity. A 2-fold increase in CR rate in patients treated with FCR and lumiliximab is of importance as it can potentially lead to longer PFS and overall survival. Although it is not a randomized comparison and the patients were treated in different centers, both groups have similar demographics as well as clinical and laboratory characteristics. The OR rates and safety profiles were similar in both studies. A large global, randomized study comparing FCR with FCR plus lumiliximab in previously treated CLL patients is ongoing (the LUCID [Lumiliximab With Fludarabine, Cyclophosphamide, and Rituximab Versus FCR Alone in Subjects With Relapsed Chronic Lymphocytic Leukemia] study).8 If the results of this pivotal trial confirm the findings of Byrd et al,7 it will have an important influence on the treatment strategy of patients with CLL.
In recent years, 4 drug combination therapies with FCR and alemtuzumab or FCR and mitoxantrone in refractory/relapsed CLL patients were also investigated9,10 (see figure). However, the results were less impressive than those reported in the present study of Byrd et al7 CFAR (FCR + alemtuzumab) immunochemotherapy was evaluated in heavily pretreated patients with up to 14 previous therapies.9 Of the 74 patients, 18 (24%) achieved CR, 2 nodular partial response, and 28 partial response. The OR was 65%. In another randomized phase 2 study, Hillmen et al10 compared an FCM (fludarabine + cyclophosphamide + mitoxantrone) regimen with FCM plus rituximab in previously treated CLL. In this study, a 4-drug regimen induced a higher CR and CR(i) rate (43%) than FCM alone (13%) (see figure). However, the study design did not allow for a statistical comparison of the 2 combinations.
In conclusion, despite the significant progress made in recent years, available therapies for refractory/relapsed CLL are only partially effective, and there is an obvious need to develop better strategies and new, more specific and active drugs.
Conflict-of-interest disclosure: The author received research grants from Hoffman-LaRoche and Biogen Idec, and serves/has served as a consultant with Hoffman-LaRoche and Biogen Idec. ■