Abstract
Few techniques are available to characterize in vivo the early cellular dynamics of long-term reconstitution of hematopoiesis after transplantation of hematopoietic stem cells (HSCs) after lethal irradiation. Using a fiber-optic imaging system, we track the early steps of in vivo recruitment and proliferation of Lin−Sca-1+c-Kit+CD34− (LSKCD34−) HSCs highly enriched in HSCs and transplanted into lethally irradiated mice. Recruitment of the transplanted LSKCD34− hematopoietic cells first occurs in the femoral head and is continuous during 24 hours. Quantification of the fluorescence emitted by the transplanted hematopoietic cells shows that proliferation of LSKCD34− hematopoietic cells in the femoral head was potent 3 days after transplantation. Using a development of this fiber-optic imaging system, we show that the transplanted LSKCD34− hematopoietic cells are associated with vascularized structures as early as 5 hours after transplantation. This early association is dependent on reactive oxygen species (ROS) partly through the regulation of vascular cell adhesion molecule-1 expression on endothelial cells and is followed by a ROS-dependent proliferation of LSKCD34− hematopoietic cells. This new in vivo imaging technique permits the observation of the early steps of hematopoietic reconstitution by HSCs in long bones and shows a new role of ROS in the recruitment of HSCs by bone marrow endothelial cells.
Introduction
One of the aims of regenerative medicine is to repair or restore functional organs by engrafting adult or fetal somatic stem cells into a damaged tissue. These cells, present in most self-renewing tissues, such as skin, intestine, and the hematopoietic system, can be purified, expanded ex vivo, and then used for reconstitution of damaged tissues.1,2 Many major advances have been achieved in purification and ex vivo amplification of somatic stem cells,1,3 but few data are available on the prerequisites that will enhance their in vivo biologic activities. New methods to improve the efficiency of bone marrow transplantation and, more generally, reconstitution of damaged tissues by somatic stem cells, depend on tracking of stem cells injected into the animal and thus on the development of imaging strategies that reveal the recruitment, homing, and initial proliferation of these injected somatic stem cells in the context of a living body.
The best-characterized mammalian adult somatic stem cells are the hematopoietic stem cells (HSCs)4,5 whose maintenance and development in the bone marrow are dependent on the HSC niche through niche-regulating pathways.6 HSCs can be purified close to homogeneity,7 and a single HSC can produce lifelong complete hematopoietic reconstitution of a lethally irradiated recipient mouse.4 Many adhesion molecules, signaling pathways, and transcription factors that regulate hematopoietic reconstitution have been characterized, and the roles of these regulatory factors have been shown in vivo using overexpression or genetic inactivation. Yet, the critical early events of recruitment to the bone marrow, homing in the bone marrow microenvironment, and initial proliferation of HSCs after transplantation into lethally irradiated mice are poorly characterized because few methods are available to study these dynamics processes at the cellular level.
The early cellular events that precede hematopoietic reconstitution from a small number of HSCs cannot be studied in vitro on hematopoietic cells recovered from recipient animals and presents a demanding challenge for imaging studies as the initial signals that can be detected are very weak. Among imaging techniques, 4 can presently be used to follow the reconstitution of the hematopoietic system at the cellular level. The first technique combines local surgery for placement of a bone window that is used for fluorescent microscopy, but this technique is invasive and limited by the size of the window.8 The second technique combines high-resolution confocal microscopy and 2-photon video imaging and has greatly improved detection of multiple fluorescent signals in a living animal. This intravital microscopy permits high-resolution imaging of small tissue volume but, in the case of hematopoietic reconstitution, is limited by the thickness of the bone.9-12 It cannot be used to study hematopoietic reconstitution in long bones and has been used to image hematopoietic reconstitution in mouse calvarium bone marrow. The third technique used time-lapse multiphoton intravital microscopy to quantify the dynamics of young and aged HSCs inside bone marrow of long bones and is dedicated to study distinct locations of hematopoietic cells relative to the endosteum.13 The fourth technique used an ex vivo real-time imaging technology and immunoassaying to trace the homing of HSCs in the femoral bone marrow but cannot be used to follow the homing of HSCs in vivo.14 These 4 techniques have improved our knowledge of the early steps of hematopoietic reconstitution, but new imaging techniques are needed to study the regulation of recruitment and initial proliferation of HSCs in long bones in vivo.
To track fluorescent cell HSCs in the femur of living animals, we have developed an application of the fiber-optic imaging. This application consists of a fiber-optic imaging probe that can navigate inside the femoral cavity from the knee to the femoral head. Imaging is done in living mice, does not alter their viability, does not severely interfere with hematopoietic reconstitution, and can easily be used to follow the temporal dynamics of hematopoietic reconstitution in a living mouse. Using this imaging system, we studied the topologic and temporal patterns of recruitment, proliferation, and seeding of hematopoietic cells enriched in HSCs in the femoral bone marrow after lethal irradiation and defined reactive oxygen species (ROS) as one of the critical parameters that regulate the recruitment and initial proliferation of hematopoietic cells.
Methods
Detailed methods are available in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article). Approval for animal care was received from Services Vétérinaires de la Santé et de la Production Animale delivered by the Ministère de l'Agriculture, France.
In vivo imaging
The knee of anesthetized transplanted mice was flexed and superficially pierced with a 23-gauge needle through the joint. Through this route, the S300 flexible microprobe (Mauna Kea Technologies) was entered and moved slowly inside the femoral cavity all the way up to the femoral head.
To validate the imaging system used, 2 kinds of probe were used to perform imaging acquisition. The first probe, a flat S300 flexible microprobe that covered a 300-μm circular diameter in a focal plane, has been used to analyze the central sinus from the knee to the femoral head. The second probe, a 45-degree beveled S300 flexible microprobe, has been used to analyze cells located near the endosteum. In addition, the screwing or spiral movement applied to the microprobe during the femoral endoscopy did increase by 3.5 times the field of observation covering 77% of the femoral volume.
To assess the GFP+ cell distribution within the femur, (1) some of the transplanted mice were killed, and the endoscope was introduced near the femoral head of a broken femur; and (2) multiple fluorescence-activated cell sorter (FACS) analyses from cutting parts (knee's epiphysis, diaphysis, and femoral head's epiphysis) of the femur were performed.
For imaging of vascular networks, we used the 45-degree beveled S300 flexible microprobe. Video data acquisitions and analyses were performed with the CellVizio 488 and Image Cell software (Mauna Kea Technologies).
Finally, if not specified, videos were done in the femurs of 3 different mice, and no repeated imaging was done in the same femur.
In vivo immunofluorescence imaging
Anti–platelet endothelial cell adhesion molecule-1 (PECAM-1; BD Biosciences), anti–vascular cell adhesion molecule-1 (VCAM-1; Santa Cruz Biotechnology) antibodies, and control anti-IgG isotype antibodies were purchased as phycoerythrin-fluorescent conjugate and injected using a retro-orbital injection mode into mice at doses of 0.5 to 1 mg/kg (30 μg/mouse). To maximize antibody binding and clearance of unbound label from the circulation, fluorescent antibodies were injected just after irradiation, that is, 24 hours before 5-carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled LSKCD34− hematopoietic cell transplantation. In vivo confocal imaging was then performed as described, and image analyses were performed with ImageJ software (Image Processing and Analysis in Java; public domain).
Imaging and statistical analysis
Before video acquisition, the S300 microprobe was calibrated with a fluorescence calibration kit (Mauna Kea Technologies) to homogenize individual fiber signal by addressing the same value of background and giving the same potential for quantitative detection of fluorescence intensity to all fibers. Image Cell acquisition software addresses colors and relative fluorescence units (RFU) according to values of signal intensity without affecting intrinsic data. Fluorescence signal intensity of one spot is comparable with another on the same picture and from one experiment to another. Positive fluorescence signal is set more than 60 RFU. For in vivo immunofluorescence imaging, technical developments have been done to address an 80- to 100-RFU signal for the fluorescent antibodies and more than 125 RFU for CFSE. Statistical analysis was performed with a 1-way analysis of variance test (inStat Version 3.0; GraphPad Software).
Results
Cellular imaging of femoral bone marrow hematopoietic reconstitution after transplantation of Lin−Sca-1+c-Kit+CD34− hematopoietic cells into lethally irradiated mice
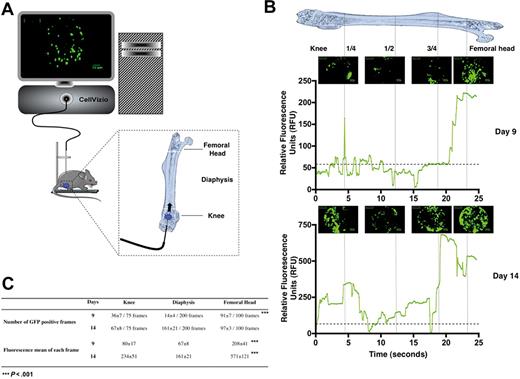
We first used the β-actin GFP transgenic mice as a source of donor cells15 and transplanted 1000 GFP+ Lin−Sca-1+c-Kit+CD34− (LSKCD34−) hematopoietic cells enriched in HSCs16 into lethally irradiated congenic wild-type recipient mice. This cell number was chosen according to previous reports17 showing that 500 LSKCD34− hematopoietic cells are sufficient for long-term reconstitution of hematopoiesis of lethally irradiated mice. At selected time intervals after transplantation of GFP+ LSKCD34− hematopoietic cells, cellular imaging was performed within the femoral bone marrow of living mice using a flexible microprobe containing 10 000 optical fibers that carry light from a continuous laser source at 488 nm to the living tissue. Fluorescence emitted by excitation of the GFP protein is carried back by optical fibers to the apparatus where a dedicated set of algorithms reconstructs images in real time. The rate of acquisition is 12 frames per second, and the field of view and spatial resolution depend on the flexible probe used. With the S300 flexible microprobe used here, the field of the reconstituted image covered a 300-μm circular diameter in a focal plane, 0 to 15 μm away from the probe's optical window with a spatial resolution in the plane of 3.3 μm. This spatial resolution is rather low but compatible with the size of single cells (8-10 μm), indicating that we could observe these cells individually even if they could appear blurred.18 The flexible probe was entered at the level of the knee and then moved slowly (0.3-0.6 mm/s) inside the femoral cavity, all the way up to the femoral head. The complete femoral canal of a mouse was scanned in 30 to 50 seconds at a constant displacement speed. The flexible probe was then moved backward along the same path out of the femur, and the mouse was allowed to recover (Figure 1A).
In vivo monitoring of hematopoietic reconstitution. (A) Schematic drawing represents the fluorescence confocal endoscope system (CellVizio, MKT) equipped with the S300 flexible microprobe containing 10 000 optical fibers that carry light from a continuous laser source at 488 nm to the living tissue. The distribution of transplanted fluorescent hematopoietic cells was monitored through the femur of living mice from the knee to the femoral head using video data acquisition software (IC-Viewer, MKT). (B) At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 GFP+ LSKCD34− sorted cells. Nine (top panel) and 14 (bottom panel) days after transplantation, the GFP+ hematopoietic cell tracking was performed. Pictures representative of GFP+ hematopoietic cells present in the knee, diaphysis, and femoral head areas are shown. These pictures represented frames of 30 to 50 seconds acquired videos over the length of the femur. Scale bar represents 50 μm. The graphics shown below the pictures represent the mean of the relative fluorescence units detected over the time in the femur by the 10 000 optical fibers per individual video frame. The vertical strokes indicate different positions of the microprobe tip in the femoral cavity. Only relative fluorescence units in excess of a value of 60 is considered a positive signal. Note the scale difference between the relative fluorescence units detected 9 and 14 days after transplantation. These data are representative of 5 to 10 independent experiments. (C) Quantification and fluorescence mean of the images obtained 9 and 14 days after transplantation of 1000 GFP+ LSKCD34− hematopoietic cells. Seventy-five, 200, and 100 video frames were studied in the knee, the diaphysis, and the femoral head, respectively. The number of GFP+ frames and the fluorescence mean of each frame are indicated for each femoral compartment. These data are representative of 5 to 10 independent experiments. Statistical analysis was performed with the 1-way analysis of variance test.
In vivo monitoring of hematopoietic reconstitution. (A) Schematic drawing represents the fluorescence confocal endoscope system (CellVizio, MKT) equipped with the S300 flexible microprobe containing 10 000 optical fibers that carry light from a continuous laser source at 488 nm to the living tissue. The distribution of transplanted fluorescent hematopoietic cells was monitored through the femur of living mice from the knee to the femoral head using video data acquisition software (IC-Viewer, MKT). (B) At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 GFP+ LSKCD34− sorted cells. Nine (top panel) and 14 (bottom panel) days after transplantation, the GFP+ hematopoietic cell tracking was performed. Pictures representative of GFP+ hematopoietic cells present in the knee, diaphysis, and femoral head areas are shown. These pictures represented frames of 30 to 50 seconds acquired videos over the length of the femur. Scale bar represents 50 μm. The graphics shown below the pictures represent the mean of the relative fluorescence units detected over the time in the femur by the 10 000 optical fibers per individual video frame. The vertical strokes indicate different positions of the microprobe tip in the femoral cavity. Only relative fluorescence units in excess of a value of 60 is considered a positive signal. Note the scale difference between the relative fluorescence units detected 9 and 14 days after transplantation. These data are representative of 5 to 10 independent experiments. (C) Quantification and fluorescence mean of the images obtained 9 and 14 days after transplantation of 1000 GFP+ LSKCD34− hematopoietic cells. Seventy-five, 200, and 100 video frames were studied in the knee, the diaphysis, and the femoral head, respectively. The number of GFP+ frames and the fluorescence mean of each frame are indicated for each femoral compartment. These data are representative of 5 to 10 independent experiments. Statistical analysis was performed with the 1-way analysis of variance test.
Nine days after transplantation, numerous GFP+ hematopoietic cells were visualized in the knee and the femoral head, whereas fewer GFP+ cells were detected in the diaphysis (Figure 1B top panel and supplemental Video 1). Indeed, no signal was detected in the femur of an animal not transplanted or transplanted with nonfluorescent LSKCD34− hematopoietic cells (data not shown; and supplemental Video 2). The same videos were obtained when the flexible microprobe was introduced near the femoral head of a mouse killed for the experiment (data not shown). This result indicated that the different densities of GFP+ cells detected in the diaphysis and in the femoral head are not accounted for by GFP+ cells being pushed during the progression of microprobe inside the femoral cavity and that the apparent dislodgment of some cells observed in supplemental Video 1 might be explained by change of focal plane (maximum, 15 μm). Quantification of the frames that contained GFP+ cells 9 days after transplantation shows a higher number of frames containing GFP+ cells in the femoral head and knee areas (91% and 48%, respectively) than in the diaphysis area (7%; Figure 1C). To further validate these imaging data, we purified, 9 days after transplantation, the GFP+ cells from the femoral head, the diaphysis, or the knee of 6 lethally irradiated mice each transplanted with 1000 GFP+ LSKCD34− cells and found that the 2 epiphyses contained 2.3 more GFP+ cells than the diaphysis and that the GFP+ cell density in the femoral head was 5-fold higher than in the diaphysis (Table 1).19 These results correlated with the imaging data and indicate the potential of the cellular imaging system used to follow the femoral bone marrow hematopoietic reconstitution after transplantation of hematopoietic stem/progenitor cells into lethally irradiated mice.
Distribution of the GFP+ hematopoietic cells in the 3 compartments of the femoral bone marrow 9 days after transplantation
| . | Femoral head . | Diaphysis . | Knee . |
|---|---|---|---|
| No. of GFP+ cells | 4600 ± 250 | 2000 ± 200 | 4000 ± 250 |
| Cellular density, C/mm3 | 11 500 ± 250 | 2310 ± 250 | ND |
| . | Femoral head . | Diaphysis . | Knee . |
|---|---|---|---|
| No. of GFP+ cells | 4600 ± 250 | 2000 ± 200 | 4000 ± 250 |
| Cellular density, C/mm3 | 11 500 ± 250 | 2310 ± 250 | ND |
Data are total number and cellular density of GFP+ cells isolated from the femoral head, the diaphysis, and the knee 9 days after transplantation of 1000 LSKCD34−GFP+ cells into a lethally irradiated mouse. Data represent mean ± SEM of GFP+ cells purified from the 2 femurs of 6 different transplanted mice. The cellular density was calculated using the published volumes of the femoral head and of the diaphysis.19
ND indicates not done.
This imaging system can quantify the fluorescence emitted by all the cells located in a frame by transforming, in real time, the optical signal into a relative fluorescence unit. More than 10 fibers analyze the fluorescence signal emitted by one fluorescent cell in the focal plane and, as the microprobe is calibrated by equalizing all fibers signals with fluorescein, the signal intensity obtained is dependent on the fluorescence emitted by all fluorescent cells detected by all the fibers in a frame. When the fluorescent signal comes from clusters of cells and cannot be distinguished from a high fluorescent signal emitted by only one fluorescent cell, a semiquantitative representation can be done by addressing relative fluorescence units to the fluorescence mean of all fibers for each frame. Such a quantification of fluorescence is shown in Figure 1B for GFP+ and will also be used to monitor the cellular density and proliferation of hematopoietic cells labeled with a fluorescent dye.
A cellular imaging study 14 days after transplantation revealed a higher colonization of the femur with a fluorescence intensity that was 3 to 8 times higher than it was 9 days after transplantation as numerous clusters of GFP+ cells were detected (Figure 1B bottom panel, supplemental Video 3). Quantification of the frames that contained GFP+ cells 14 days after transplantation shows a similar number of frames containing GFP+ cells in the femoral head and knee areas and in the diaphysis area (Figure 1C). Altogether, these results show the usefulness of this imaging system to follow hematopoietic reconstitution in the long bones after transplantation of a small number of HSCs/progenitor cells into lethally irradiated mice and indicate a primary engraftment of GFP+ hematopoietic cells in the 2 epiphyses followed by secondary seeding in the diaphysis.
Hematopoietic reconstitution is not impaired by a femoral endoscopy performed 24 hours after transplantation
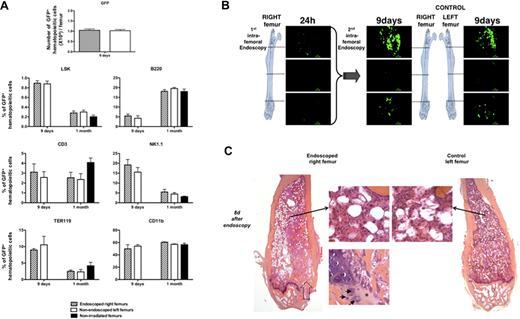
To determine whether a confocal endoscopy performed in a femur 24 hours after transplantation of 1000 GFP+ LSKCD34− cells into lethally irradiated mice alters the hematopoietic reconstitution in this femur, we designed 3 types of experiments. First, we counted and performed a phenotype analysis of GFP+ hematopoietic cells present in the endoscoped (right) and nonendoscoped (left) femurs 8 days and 1 month after endoscopy and found no quantitative or qualitative difference between the GFP+ hematopoietic cells present in the 2 femurs (Figure 2A). Second, endoscopies of the endoscoped (right) and nonendoscoped (left) femurs 8 days after endoscopy of the right femur did not show any difference (Figure 2B, supplemental Video 4). Third, 8 days after endoscopy of the right femur, we killed the mice and compared the histology of the right and left femurs using hematoxylin-eosin-safran coloration. Histologic analysis of serial sections of both endoscoped and control bones performed showed no difference in organization of bone trabeculae or in medullar cellularity. Only a fibrous scar and a beginning of restoration of the endochondreal ossification could be observed at the entry point of the needle protecting the probe (Figure 2C). Altogether, these results showed that a femoral confocal endoscopy performed early after transplantation did not severely interfere with the ongoing hematopoietic reconstitution.
Analysis of femoral hematopoietic reconstitution after endoscopy. At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 LSKCD34− GFP+; and 1 day later, an endoscopy was performed in the right femur of the recipient mice. (A) Eight days and 1 month after this first endoscopy, mice were killed, bone marrow from right and left femurs was flushed, and the GFP+ cells were counted by FACS analysis (8 days after endoscopy) and phenotyped by FACS analysis (8 days and 1 month after endoscopy). Graphics show the number of GFP+ cells present in right and left femurs 8 days after the endoscopy of the right femur and the percentage of immature hematopoietic cells (LSK), lymphocytes (B220, CD3, and NK1.1), myelocytes (CD11b), and erythrocytes (Ter119) found in the bone marrow of the right and left femurs of mice 8 days and 1 month after endoscopy. The percentage of GFP+ cells found in the different hematopoietic populations of a femur from nonirradiated and nonendoscoped mice is also shown (n = 5 for each group of mice). Error bars represent SEM. (B) Eight days after the first endoscopy, endoscopies were performed in the right and the left femurs of the same mouse. Pictures representative of GFP+ hematopoietic cells present in the knee, diaphysis, and femoral head areas are shown. These pictures represent frames of 30 to 50 seconds acquired videos over the length of the femur. Scale bar represents 50 μm. No difference could be detected in the imaging of double-scanned right femurs and simple-scanned left femurs. These data are representative of 3 independent experiments. (C) Eight days after this endoscopy, single-scanned right femur and intact left femur from transplanted mice were removed, included in paraffin, and cut longitudinally into 4-μm-depth slices for histologic analysis with hematoxylin-eosin-safran coloration. → indicates the path of the needle in the femoral cavity (left panel). Compared with control femur (right panel), endoscoped femur (left panel) demonstrated a similar organization of bone trabeculae. In both femurs, identical cellularity of hematopoietic and stromal cells was observed (2 adjacent top middle panels). Finally, the beginning of restoration of the endochondreal ossification was evidenced in the right endoscoped femur (bottom left panel): fibrous scar resulting from the endoscopic procedure is replaced by chondroid matrix (*) inhabited by few chondrocytes (→).
Analysis of femoral hematopoietic reconstitution after endoscopy. At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 LSKCD34− GFP+; and 1 day later, an endoscopy was performed in the right femur of the recipient mice. (A) Eight days and 1 month after this first endoscopy, mice were killed, bone marrow from right and left femurs was flushed, and the GFP+ cells were counted by FACS analysis (8 days after endoscopy) and phenotyped by FACS analysis (8 days and 1 month after endoscopy). Graphics show the number of GFP+ cells present in right and left femurs 8 days after the endoscopy of the right femur and the percentage of immature hematopoietic cells (LSK), lymphocytes (B220, CD3, and NK1.1), myelocytes (CD11b), and erythrocytes (Ter119) found in the bone marrow of the right and left femurs of mice 8 days and 1 month after endoscopy. The percentage of GFP+ cells found in the different hematopoietic populations of a femur from nonirradiated and nonendoscoped mice is also shown (n = 5 for each group of mice). Error bars represent SEM. (B) Eight days after the first endoscopy, endoscopies were performed in the right and the left femurs of the same mouse. Pictures representative of GFP+ hematopoietic cells present in the knee, diaphysis, and femoral head areas are shown. These pictures represent frames of 30 to 50 seconds acquired videos over the length of the femur. Scale bar represents 50 μm. No difference could be detected in the imaging of double-scanned right femurs and simple-scanned left femurs. These data are representative of 3 independent experiments. (C) Eight days after this endoscopy, single-scanned right femur and intact left femur from transplanted mice were removed, included in paraffin, and cut longitudinally into 4-μm-depth slices for histologic analysis with hematoxylin-eosin-safran coloration. → indicates the path of the needle in the femoral cavity (left panel). Compared with control femur (right panel), endoscoped femur (left panel) demonstrated a similar organization of bone trabeculae. In both femurs, identical cellularity of hematopoietic and stromal cells was observed (2 adjacent top middle panels). Finally, the beginning of restoration of the endochondreal ossification was evidenced in the right endoscoped femur (bottom left panel): fibrous scar resulting from the endoscopic procedure is replaced by chondroid matrix (*) inhabited by few chondrocytes (→).
Imaging the in vivo association of transplanted LSKCD34− hematopoietic cells with vascularized structures in the femoral head
Because the GFP-expressing HSCs will give rise to GFP progeny, it is difficult to use this experimental system to study the early steps of the development of the transplanted LSKCD34− in the femoral bone marrow. Thus, to study the initial recruitment and proliferation of HSCs into the femur, we applied the imaging system to CFSE-labeled LSKCD34− hematopoietic cells. CFSE is a dye that diffuses into cells and is diluted among daughter cells. CFSE staining did not impair the viability of LSKCD34− hematopoietic cells or their capacity to reconstitute hematopoiesis (data not shown). One day after transplantation, more than 90% of the CFSE-labeled cells present in the femoral bone marrow were detected in the femoral head, approximately 10% of fluorescent cells were detected in the knee, and no fluorescent cells could be detected in the diaphysis (data not shown). We thus monitored the initial events that followed the transplantation of LSKCD34− hematopoietic cells by performing, in the femoral head, cellular imaging at 1, 3, 5, and 15 hours after transplantation of CFSE-labeled cells. As early as 1 hour after transplantation of 1000 CFSE-labeled LSKCD34− hematopoietic cells, single CFSE-labeled cells could be detected in the femoral head. The number of the CFSE-labeled single LSKCD34− hematopoietic cells increased 3, 5, and 15 hours after transplantation without any change in the fluorescence intensity (Figure 3 left panel; and data not shown), indicating a continuous recruitment of the LSKCD34− hematopoietic cells during this early phase. To localize the cells that might recruit these hematopoietic cells in the bone marrow, we injected intravenously into mice fluorescent anti–PECAM-1 antibodies 24 hours before transplantation10 or fluorescent anti-IgG isotype antibodies. The fibered confocal fluorescence imaging system that we used cannot allow concurrent imaging of 2 colors simultaneously but can quantitatively discriminate signal intensities (Figure 1B). Thus, we took advantage of the high fluorescence of the CFSE-labeled LSKCD34− hematopoietic cells and the low fluorescence of PECAM-1–labeled endothelial cells to detect any association between endothelial and hematopoietic cells. A 45-degree beveled S300 flexible microprobe was entered at the level of the knee, and the femoral cavity was scanned during 35 seconds (420 frames; 12 frames per second). From frame 340, the microprobe, located into the femoral head, was stabilized and rotated through a 360-degree angle in 4 seconds (60 frames). Only CFSE-labeled LSKCD34− hematopoietic cells could be detected when fluorescent anti-IgG isotype antibodies were injected (data not shown), but associations between CFSE-labeled LSKCD34− hematopoietic cells and vascularized structures were brought into prominence when anti–PECAM-1 antibodies were injected (Figure 3 right panel; and supplemental Video 5), indicating that these vascularized structures might be the recipient of the circulating transplanted LSKCD34− hematopoietic cells.
Early association of transplanted LSKCD34− hematopoietic cells with vascularized structures. (Left panel) At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 LSKCD34− CFSE-labeled cells. One, 3, 5, and 15 hours after transplantation, videos of the hematopoietic reconstitution were performed on the femurs of 3 different mice. Pictures taken over time are representative of the very few frames of the femoral head that contain single cells. Scale bar represents 50 μm. White arrows indicate individual cells, and the fluorescence intensity of these single cells is shown. (Right panel) At the time of irradiation, 30 μg of fluorescent anti–PECAM-1 or anti-IgG isotype antibodies was injected into recipient mice; and 24 hours later, these mice were transplanted with 1000 LSKCD34− CFSE-labeled cells. Five hours after transplantation, videos of the hematopoietic reconstitution were performed on the femurs of 3 different mice. Pictures obtained with the fluorescent anti–PECAM-1 antibodies and the LSKCD34− CFSE-labeled cells represent selected frames (from 349 and 359) of the femoral head. The top window represents a 2-fold enlargement of the indicated femoral area showing CFSE-labeled hematopoietic cells in the vicinity of PECAM-1+ cells.
Early association of transplanted LSKCD34− hematopoietic cells with vascularized structures. (Left panel) At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 LSKCD34− CFSE-labeled cells. One, 3, 5, and 15 hours after transplantation, videos of the hematopoietic reconstitution were performed on the femurs of 3 different mice. Pictures taken over time are representative of the very few frames of the femoral head that contain single cells. Scale bar represents 50 μm. White arrows indicate individual cells, and the fluorescence intensity of these single cells is shown. (Right panel) At the time of irradiation, 30 μg of fluorescent anti–PECAM-1 or anti-IgG isotype antibodies was injected into recipient mice; and 24 hours later, these mice were transplanted with 1000 LSKCD34− CFSE-labeled cells. Five hours after transplantation, videos of the hematopoietic reconstitution were performed on the femurs of 3 different mice. Pictures obtained with the fluorescent anti–PECAM-1 antibodies and the LSKCD34− CFSE-labeled cells represent selected frames (from 349 and 359) of the femoral head. The top window represents a 2-fold enlargement of the indicated femoral area showing CFSE-labeled hematopoietic cells in the vicinity of PECAM-1+ cells.
Cellular dynamics of the initiation of femoral bone marrow hematopoietic reconstitution
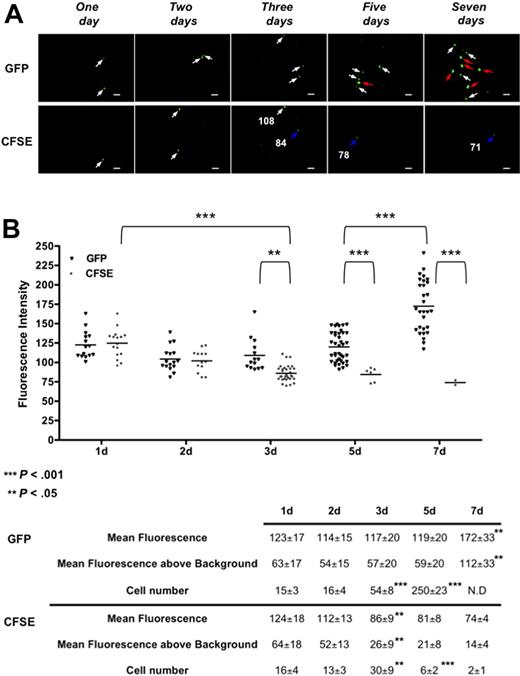
The initial proliferation of transplanted LSKCD34− hematopoietic cells was followed, in the femoral head, after injection of 1000 GFP+ or CFSE-labeled LSKCD34− hematopoietic cells harboring comparable fluorescence intensities (supplemental Figure 1). One, 2, 3, 5, and 7 days after transplantation, imaging was performed in the femurs (Figure 4A). The fluorescence intensities of each GFP+ or CFSE-labeled LSKCD34− hematopoietic cell in all the frames that contain labeled cells (every day for CFSE-labeled cells and days 1, 2, and 3 for GFP+ cells) or in an average of 25 frames that are representative of all the frames collected in the femoral head (days 5 and 7 for GFP+ cells) were quantified. First, we repeatedly found that approximately 1.5% of the transplanted LSKCD34− hematopoietic cells were recruited in one femur 1 day after transplantation. Second, a decreased fluorescence intensity of the CFSE-labeled or GFP+ hematopoietic cells between day 1 and day 2 was detected. As this decrease was independent of the HSC labeling, it might reflect quenching of the fluorescence resulting from a different cellular environment of these cells between day 1 and day 2. Two days after transplantation, the same numbers of CFSE-labeled or GFP+ hematopoietic cells with similar fluorescence intensity could be detected, indicating almost no proliferation of the transplanted hematopoietic cells detected in the femoral head (Figure 4B). Between 2 and 3 days after transplantation, the average fluorescence intensity of CFSE-labeled LSKCD34− hematopoietic cells decreased 2-fold and the number of GFP+ cells increased 3.3-fold (Figure 4A day 3; Figure 4B). Together, these results indicate proliferation of the transplanted LSKCD34− hematopoietic cells 2 days after transplantation. Five and 7 days after transplantation, very few, if any, CFSE-labeled hematopoietic cells with fluorescence intensity just above the background could be detected, whereas GFP+ hematopoietic cells could be detected mostly as single cells 5 days after transplantation or, 7 days after transplantation, as single cells and cluster, for example, association of 2 or more GFP+ hematopoietic cells in the same focal plane characterized by a higher GFP fluorescence intensity (Figure 4A days 5 and 7; and supplemental Video 6). As GFP expression level in CD3+, NK1.1+, CD11b+, or B220+ bone marrow cells 7 days after transplantation is similar to the GFP expression level of the transplanted LSKCD34− hematopoietic cells (data not shown and supplemental Figure 1), this increased GFP level might be the result of the multiplicity of cells in the focal plane. Altogether, these results showed that the imaging system we have developed can be used to follow proliferation of CFSE-labeled hematopoietic cells in vivo and that initial hematopoietic reconstitution in the femoral head bone marrow is temporally divided into an initial phase with little proliferation followed by a second phase where proliferation occurs.
Kinetics of the initiation of proliferation of transplanted LSKCD34− hematopoietic cells. (A) One, 2, 3, 5, and 7 days after transplantation of 1000 LSKCD34− GFP+ or CFSE-labeled cells, videos of the hematopoietic reconstitution were performed in the femurs of 3 different mice. Pictures acquired from videos of the femoral head show one of the few frames that contain single GFP+ or CFSE-labeled cells 1 and 2 days after transplantation, a frame that contains 2 CFSE-labeled cells with different fluorescent intensities 3 days after transplantation and frames that contain the numerous single GFP+ cells 5 days after transplantation or clusters of GFP+ cells 7 days after transplantation cells. Scale bar represents 50 μm. White, blue, and red arrows, respectively, indicate cells with fluorescence intensities more than 100, approximately 80, and more than 150. The fluorescence intensity (relative fluorescence units) of some single CFSE+ labeled cells is shown. (B) Relative fluorescence units of individual cells were quantified in all frames (150) of the femoral head 1, 2, 3, 5, and 7 days after transplantation for CFSE-labeled cells and in all the frames (150) of the femoral head 1, 2, and 3 days after transplantation or in an average of 25 frames that are representative of all the frames collected in the femoral head 5 and 7 days after transplantation for GFP+ cells. **P < .05; ***P < .001. Graphics show the distribution of relative fluorescent units of each cell present in the analyzed frames and the means of these individual relative fluorescent units. These values, together with the cell number detected and the mean fluorescence above background (which is 60, as shown in Figure 1B), are shown below graphics. For GFP+ hematopoietic cells, the indicated cell number corresponds to the number of cells detected in the 25 frames analyzed at day 5 multiplied by 6 as 150 frames were analyzed at days 1, 2, and 3. At day 7, the relative fluorescent units were determined in individual GFP+ cells from 25 frames. N.D. indicates that clustering of cells impairs the determination of the total cell number at day 7. The data are representative of those obtained with 3 different mice. Statistical analysis was performed with the 1-way analysis of variance test.
Kinetics of the initiation of proliferation of transplanted LSKCD34− hematopoietic cells. (A) One, 2, 3, 5, and 7 days after transplantation of 1000 LSKCD34− GFP+ or CFSE-labeled cells, videos of the hematopoietic reconstitution were performed in the femurs of 3 different mice. Pictures acquired from videos of the femoral head show one of the few frames that contain single GFP+ or CFSE-labeled cells 1 and 2 days after transplantation, a frame that contains 2 CFSE-labeled cells with different fluorescent intensities 3 days after transplantation and frames that contain the numerous single GFP+ cells 5 days after transplantation or clusters of GFP+ cells 7 days after transplantation cells. Scale bar represents 50 μm. White, blue, and red arrows, respectively, indicate cells with fluorescence intensities more than 100, approximately 80, and more than 150. The fluorescence intensity (relative fluorescence units) of some single CFSE+ labeled cells is shown. (B) Relative fluorescence units of individual cells were quantified in all frames (150) of the femoral head 1, 2, 3, 5, and 7 days after transplantation for CFSE-labeled cells and in all the frames (150) of the femoral head 1, 2, and 3 days after transplantation or in an average of 25 frames that are representative of all the frames collected in the femoral head 5 and 7 days after transplantation for GFP+ cells. **P < .05; ***P < .001. Graphics show the distribution of relative fluorescent units of each cell present in the analyzed frames and the means of these individual relative fluorescent units. These values, together with the cell number detected and the mean fluorescence above background (which is 60, as shown in Figure 1B), are shown below graphics. For GFP+ hematopoietic cells, the indicated cell number corresponds to the number of cells detected in the 25 frames analyzed at day 5 multiplied by 6 as 150 frames were analyzed at days 1, 2, and 3. At day 7, the relative fluorescent units were determined in individual GFP+ cells from 25 frames. N.D. indicates that clustering of cells impairs the determination of the total cell number at day 7. The data are representative of those obtained with 3 different mice. Statistical analysis was performed with the 1-way analysis of variance test.
ROS regulate the kinetics of hematopoietic reconstitution
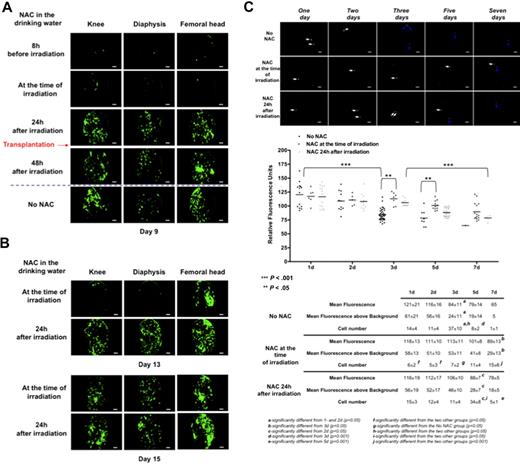
Total body irradiation leads to oxidative stress and ROS production in the bone marrow.20 ROS have been shown to regulate the fate of HSCs,21 but the effects of ROS on the initial events that follow transplantation of HSCs after lethal irradiation are not known. To study these effects, we treated recipient mice with the antioxidant N-acetylcysteine (NAC)22 or control water for 2 months starting 8 hours before irradiation or 5 minutes, 24 hours, and 48 hours after irradiation. All the mice transplanted with 1000 GFP+ LSKCD34− hematopoietic cells 24 hours after irradiation and treated with NAC reconstituted their hematopoiesis as control mice and survived (data not shown), indicating that ROS have no effect on long-term hematopoietic reconstitution after lethal irradiation. To analyze whether ROS might regulate the kinetics of hematopoietic reconstitution, we performed videos at different times. Nine days after transplantation, a weak engraftment could be detected in the mice treated with NAC 8 hours before irradiation or just after irradiation, whereas an engraftment similar to the one observed in control mice occurred when mice were treated with NAC 24 or 48 hours after irradiation (Figure 5A; supplemental Videos 7-8). These results suggest that ROS produced after irradiation could regulate the femoral recruitment and/or homing and/or proliferation of LSKCD34− hematopoietic cells. Thirteen days after transplantation, most GFP+ hematopoietic cells were found in the femoral head when the mice were treated with NAC before or just after irradiation, whereas normal femoral engraftment was obtained when the mice were treated with NAC 24 or 48 hours after irradiation (Figure 5B; and data not shown). Fifteen days after irradiation, no difference in the topology or in the intensity of GFP+ hematopoietic cells could be detected among the different NAC treatment schedules (Figure 5B; and data not shown). Nine, 13, and 15 days after transplantation, quantification by flow cytometry, of the number of GFP+ hematopoietic cells present in the femurs of mice treated or not treated with NAC, showed kinetics of proliferation of GFP+ hematopoietic cells that correlated with the imaging data (supplemental Figure 2 top panel). Qualitative analysis of the different hematopoietic populations present in the femoral bone marrow of mice treated with NAC at the time of irradiation showed that, 13 days after transplantation, these mice have hematopoietic populations similar to the one of nontreated mice 9 days after transplantation (compare supplemental Figure 2 bottom panel; and Figure 2A). Two months after hematopoietic reconstitution, secondary transplantations were performed using 1000 LSKCD34− GFP+ hematopoietic cells from mice not treated or treated with NAC at the time of irradiation or at 24 hours after irradiation. No difference was observed in the kinetics of hematopoietic reconstitution (data not shown) or in cell distribution 3 months after this secondary transplantation (supplemental Figure 3), indicating that the kinetics of NAC treatment did not modify the biologic properties of the LSKCD34− hematopoietic cells. These results indicate the usefulness of this new imaging system to study the parameters that regulate the early events of hematopoietic reconstitution and show that ROS produced after irradiation modulate the temporal kinetics of femoral hematopoietic reconstitution but are not necessary for long-term hematopoietic reconstitution.
ROS levels modulated the temporal kinetics of femoral hematopoietic reconstitution. C57BL/6 recipient mice (10 mice in each group) were orally treated with NAC 8 hours before irradiation, at the time of irradiation, and 24 or 48 hours after irradiation until 1 month after transplantation. At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 LSKCD34− GFP+ hematopoietic cells. Pictures in panels A and B represent selected frames (knee, diaphysis, and femoral head areas) of 30 seconds to 50 seconds acquired videos (scale bar represents 50 μm) obtained 9 (A) or 13 and 15 (B) days after transplantation for each mouse group. (A) Only transplanted mice treated with NAC 24 hours and 48 hours after irradiation displayed the same density of GFP+ hematopoietic cells as untreated transplanted mice. (B) The delay in the cellular density of GFP+ hematopoietic cells of mice treated with NAC at the time of irradiation was diminished 13 days after transplantation and was recovered 15 days after transplantation. (C top panel) Relative fluorescence units of individual cells detected in all frames of the femoral head 1, 2, 3, 5, and 7 days after transplantation of 1000 CFSE-labeled LSKCD34− cells into mice not treated or treated with NAC at the time of irradiation or 24 hours after irradiation was quantified. One, 2, 3, 5, and 7 days after transplantation, videos of the hematopoietic reconstitution were recorded in the 2 femurs of 3 different mice. Pictures acquired from videos of the femoral head show one of the few frames that contain single CFSE-labeled cells. White and blue arrows, respectively, indicate cells with fluorescence intensities more than 100 or approximately 80. **P < .05; ***P < .001. (Bottom panel) Graphics show the distribution of relative fluorescence units of each cell present in the analyzed frames and the means of these individual relative fluorescence units. These values, together with the cell number detected and the mean fluorescence above background (which is 60, as shown in Figure 1B), are shown below graphics. The data are representative of those obtained in 3 different mice. Statistical analysis was performed with the 1-way analysis of variance test.
ROS levels modulated the temporal kinetics of femoral hematopoietic reconstitution. C57BL/6 recipient mice (10 mice in each group) were orally treated with NAC 8 hours before irradiation, at the time of irradiation, and 24 or 48 hours after irradiation until 1 month after transplantation. At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice were transplanted with 1000 LSKCD34− GFP+ hematopoietic cells. Pictures in panels A and B represent selected frames (knee, diaphysis, and femoral head areas) of 30 seconds to 50 seconds acquired videos (scale bar represents 50 μm) obtained 9 (A) or 13 and 15 (B) days after transplantation for each mouse group. (A) Only transplanted mice treated with NAC 24 hours and 48 hours after irradiation displayed the same density of GFP+ hematopoietic cells as untreated transplanted mice. (B) The delay in the cellular density of GFP+ hematopoietic cells of mice treated with NAC at the time of irradiation was diminished 13 days after transplantation and was recovered 15 days after transplantation. (C top panel) Relative fluorescence units of individual cells detected in all frames of the femoral head 1, 2, 3, 5, and 7 days after transplantation of 1000 CFSE-labeled LSKCD34− cells into mice not treated or treated with NAC at the time of irradiation or 24 hours after irradiation was quantified. One, 2, 3, 5, and 7 days after transplantation, videos of the hematopoietic reconstitution were recorded in the 2 femurs of 3 different mice. Pictures acquired from videos of the femoral head show one of the few frames that contain single CFSE-labeled cells. White and blue arrows, respectively, indicate cells with fluorescence intensities more than 100 or approximately 80. **P < .05; ***P < .001. (Bottom panel) Graphics show the distribution of relative fluorescence units of each cell present in the analyzed frames and the means of these individual relative fluorescence units. These values, together with the cell number detected and the mean fluorescence above background (which is 60, as shown in Figure 1B), are shown below graphics. The data are representative of those obtained in 3 different mice. Statistical analysis was performed with the 1-way analysis of variance test.
To monitor the effects of ROS in the early development of the transplanted LSKCD34− hematopoietic cells, we labeled these cells with CFSE and followed their development in the femoral head. Twenty-four hours after transplantation, mice treated with NAC 24 hours after irradiation displayed a similar recruitment of LSKCD34− hematopoietic cells than control mice, whereas a 2.5-fold decrease of CFSE-labeled LSKCD34− hematopoietic cells could be detected in the femoral head when mice were treated with NAC 5 minutes after irradiation (Figure 5C). During 5 days after transplantation, in the mice treated with NAC 5 minutes after irradiation, the number of the CFSE-labeled LSKCD34− hematopoietic cells increased slowly and the fluorescence intensity of the recruited CFSE-labeled LSKCD34− hematopoietic cells remained similar (Figure 5C). These results indicate slow recruitment and little proliferation of LSKCD34− hematopoietic cells into the femoral head when mice are treated with NAC 5 minutes after irradiation. To analyze the effects of ROS on proliferation of LSKCD34− hematopoietic cells, we monitored the fluorescence intensities of the CFSE-labeled LSKCD34− hematopoietic cells. Mice treated with NAC 24 hours after transplantation showed a delay in fluorescence decrease that started 3 to 5 days after transplantation compared with 2 days for control mice (Figure 5C). In mice treated with NAC 5 minutes after irradiation, the fluorescence intensity of the transplanted hematopoietic cells started to decrease 5 to 7 days after transplantation (Figure 5C). These results indicate that ROS may regulate both the recruitment of LSKCD34− hematopoietic cells in the femoral head and the proliferation of these cells once they are within the femoral head bone marrow.
VCAM-1 expression on endothelial cells is regulated by ROS and determined the recruitment of LSKCD34− hematopoietic cells in the bone marrow early after transplantation
As ROS have an effect on the kinetics of recruitment of HSCs in the femoral head, we studied the effects of ROS on the initial association of the LSKCD34− hematopoietic cells with endothelial cells and used transplanted mice not treated or treated with NAC 5 minutes after irradiation. Five hours after transplantation, NAC treatment did not affect the expression of PECAM-1 in the femoral head (Figure 6A top panel) but diminished the number of CFSE-labeled LSKCD34− hematopoietic cells (Figure 6A bottom panel). As the expression of VCAM-1, which is a major receptor of LSKCD34− hematopoietic cells on endothelial cells,23,24 is positively regulated by ROS in vitro25 and is up-regulated in vivo after total body irradiation,26 we studied the effects of NAC on VCAM-1 expression in the femoral head 5 hours after transplantation. We found that (1) CFSE-labeled LSKCD34− hematopoietic cells were associated with VCAM-1–expressing cells, (2) the number of cells that expressed VCAM-1 diminished in the presence of NAC (Figure 6B top panel), and (3) the number of CFSE-labeled LSKCD34− hematopoietic cells detected decreased in parallel with the diminished number of VCAM-1–expressing cells, but the proportion of CFSE-labeled hematopoietic cells associated with VCAM-1 was similar in the presence or in the absence of NAC (Figure 6B bottom panel). To validate the VCAM-1–regulated expression by ROS in vivo, we performed 2 types of experiments. First, we performed immunohistochemistry analysis of VCAM-1 expression in irradiated bone marrow of the femoral head of mice treated or not with NAC and found fewer sinusoids containing labeled endothelial cells when the mice were treated with NAC (Figure 6C). Second, we analyzed, by flow cytometry, VCAM-1 expression at the surface of CD45−Hoechst−MECA32+ cells from mice not treated or treated with NAC at the time of irradiation or at 24 hours after irradiation. One day after irradiation, no difference was observed in the level of VCAM-1 expression at the surface of endothelial cells between mouse groups, but the number of CD45−Hoechst−MECA32+ cells expressing VCAM-1 was 2-fold decreased when the mice are treated with NAC at the time of irradiation (Figure 6D). Nine days after irradiation, the percentage of CD45−Hoechst−MECA32+ cells expressing VCAM-1 was similar in mice treated or not treated with NAC at the time of irradiation, suggesting that NAC treatment at the time of irradiation might block the early up-regulation of VCAM-1 expression induced by ROS and could delay the hematopoietic cell recruitment. Altogether, these results suggest that a ROS-dependent expression of VCAM-1 on endothelial cells promotes efficient recruitment of LSKCD34− hematopoietic cells.
VCAM-1 expression on endothelial cells is dependent on ROS and regulates the recruitment of LSKCD34− hematopoietic cells early after transplantation. C57BL/6 recipient mice were treated or not with the antioxidant agent NAC at the time of irradiation. Five minutes after irradiation at 10 Gy, 30 μg of fluorescent anti–PECAM-1 (A), anti–VCAM-1 (B), or anti-IgG isotype antibodies (data not shown) was injected into recipient mice. Twenty-four hours later, mice were not transplanted (A-B top panels) or transplanted (A-B bottom panels) with 1000 LSKCD34− CFSE-labeled cells; and 5 hours after transplantation, videos were performed in the femurs. No signal could be detected with the anti-IgG isotype antibodies (not shown). (A-B top panels) Pictures represent the expression of PECAM-1 (A) or VCAM-1 (B) in selected frames from femoral heads of mice treated or not with NAC. The histogram shows the number of PECAM-1 (A) or VCAM-1 (B) positive frames in the 150 frames that covered femoral heads of mice treated with or without NAC (n = 3 mice). Error bars represent SEM. Statistical analysis was performed with the 1-way analysis of variance test. (A bottom panel) Pictures represent selected frames that show associations of CFSE-labeled LSKCD34− hematopoietic cells and PECAM-1–stained structures in the femoral head. The histogram shows the number of positive CFSE cells in the 150 frames that covered femoral heads of mice treated with or without NAC (n = 3 mice). Error bars represent SEM. Statistical analysis was performed with the 1-way analysis of variance test. (B bottom panel) Pictures represent selected frames that show associations between LSKCD34− hematopoietic cells and VCAM-1–stained structures in the femoral head. The histograms show the number of positive CFSE cells in the 150 frames that covered femoral heads of mice treated with or without NAC (top histogram) and the percentage of CFSE+ cells associated with PECAM-1- or VCAM-1–positive frames (bottom histogram; n = 3 mice). Error bars represent SEM. Statistical analysis was performed with the 1-way analysis of variance test. (C) Immunohistochemistry analysis of VCAM-1 expression in irradiated bone marrow of femoral heads of mice treated or not with NAC. Note that fewer sinusoids with intense VCAM-1–labeled cells are present in mice treated with NAC. (D top panel) At 24 hours after a 10-Gy irradiation, FACS analysis of VCAM-1 expression by MECA32+CD45− live (Hoechst−) cells from femoral heads of mice treated or not with NAC. Note that the fluorescence mean of VCAM-1 expression is similar in the presence or in the absence of NAC but that the number of VCAM-1+ cells is different. (D bottom panel) At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice, treated or not with NAC at the time of irradiation or 24 hours after irradiation, were transplanted with 1000 LSKCD34− cells. One and 9 days after transplantation, FACS analyses were performed and the percentage of MECA32+CD45− live (Hoechst−) cells expressing VCAM-1 is shown. Statistical analysis was performed with the 1-way analysis of variance test.
VCAM-1 expression on endothelial cells is dependent on ROS and regulates the recruitment of LSKCD34− hematopoietic cells early after transplantation. C57BL/6 recipient mice were treated or not with the antioxidant agent NAC at the time of irradiation. Five minutes after irradiation at 10 Gy, 30 μg of fluorescent anti–PECAM-1 (A), anti–VCAM-1 (B), or anti-IgG isotype antibodies (data not shown) was injected into recipient mice. Twenty-four hours later, mice were not transplanted (A-B top panels) or transplanted (A-B bottom panels) with 1000 LSKCD34− CFSE-labeled cells; and 5 hours after transplantation, videos were performed in the femurs. No signal could be detected with the anti-IgG isotype antibodies (not shown). (A-B top panels) Pictures represent the expression of PECAM-1 (A) or VCAM-1 (B) in selected frames from femoral heads of mice treated or not with NAC. The histogram shows the number of PECAM-1 (A) or VCAM-1 (B) positive frames in the 150 frames that covered femoral heads of mice treated with or without NAC (n = 3 mice). Error bars represent SEM. Statistical analysis was performed with the 1-way analysis of variance test. (A bottom panel) Pictures represent selected frames that show associations of CFSE-labeled LSKCD34− hematopoietic cells and PECAM-1–stained structures in the femoral head. The histogram shows the number of positive CFSE cells in the 150 frames that covered femoral heads of mice treated with or without NAC (n = 3 mice). Error bars represent SEM. Statistical analysis was performed with the 1-way analysis of variance test. (B bottom panel) Pictures represent selected frames that show associations between LSKCD34− hematopoietic cells and VCAM-1–stained structures in the femoral head. The histograms show the number of positive CFSE cells in the 150 frames that covered femoral heads of mice treated with or without NAC (top histogram) and the percentage of CFSE+ cells associated with PECAM-1- or VCAM-1–positive frames (bottom histogram; n = 3 mice). Error bars represent SEM. Statistical analysis was performed with the 1-way analysis of variance test. (C) Immunohistochemistry analysis of VCAM-1 expression in irradiated bone marrow of femoral heads of mice treated or not with NAC. Note that fewer sinusoids with intense VCAM-1–labeled cells are present in mice treated with NAC. (D top panel) At 24 hours after a 10-Gy irradiation, FACS analysis of VCAM-1 expression by MECA32+CD45− live (Hoechst−) cells from femoral heads of mice treated or not with NAC. Note that the fluorescence mean of VCAM-1 expression is similar in the presence or in the absence of NAC but that the number of VCAM-1+ cells is different. (D bottom panel) At 24 hours after irradiation at 10 Gy, C57BL/6 recipient mice, treated or not with NAC at the time of irradiation or 24 hours after irradiation, were transplanted with 1000 LSKCD34− cells. One and 9 days after transplantation, FACS analyses were performed and the percentage of MECA32+CD45− live (Hoechst−) cells expressing VCAM-1 is shown. Statistical analysis was performed with the 1-way analysis of variance test.
Discussion
Bone marrow or CD34+ hematopoietic cell transplantation is a common treatment for patients with chronic viral infection or with nonhematopoietic and hematopoietic malignancies. In these clinical settings, the patient is often preconditioned by total body irradiation before donor HSCs contained within bone marrow or CD34+ hematopoietic cells are injected intravenously into the recipient. The prerequisite for efficient reconstitution of the recipient's blood system after transplantation is that the HSCs contained in the injected cells are efficiently recruited in the bone marrow, home, and engraft within HSC niches that regulate maintenance, proliferation, and determination of these HSCs. Very few methods can presently be used to study the early steps of HSCs recruitment and proliferation in vivo, and most of our knowledge comes, until recently, from studies that use total bone marrow or more than 104 precursor hematopoietic cells containing less than 1% of HSCs.27-30 Recently, several studies were performed using bioluminescence or fluorescence imaging to follow the hematopoietic reconstitution after injection of hematopoietic cell populations highly enriched in HSCs.9,10,12,31 When 10, 50, or 250 HSCs containing a luciferase transgene were injected with unlabeled whole bone marrow, luciferase activity was used to detect foci initiated from HSCs, but this detection can be done only several days after HSC injection and thus cannot be used to define the initial steps of HSC homing.32 Intravital microscopy can detect single cells in a living animal, has been used to follow HSCs homing and engraftment in mouse calvarium, but cannot presently be applied to long bones as they are too thick.9 To get insights into hematopoietic reconstitution in long bones, we have now developed a new technique that can track individual GFP+ or CFSE-labeled cells in the femoral bone marrow of living mice and showed that this technique can be used to define the early recruitment and proliferation in the femoral bone marrow of injected LSKCD34− hematopoietic cells after lethal irradiation.
During the first 15 hours after transplantation, recruitment of LSKCD34− hematopoietic cells occurs in the femoral head but not in the femoral diaphysis. Using PECAM-1 labeling, we found that injected LSKCD34− hematopoietic cells are associated, 5 hours after injection, with vascular structures, an association also detected 1 hour after injection of CD34+ human hematopoietic cells in an SCID mouse.33 As the vascular network is in close proximity to the endosteal surface in the femoral head but not in the femoral diaphysis,14 this result extended the recent in vivo imaging of homing of transplanted HSCs in perivascular/periendosteal niches of the mouse calvarium9 to long bones. Proliferation of transplanted LSKCD34− hematopoietic cells could be detected 2 days after injection as measured by the decreased of CFSE fluorescence of these cells. This result is in accordance with the observed BrdU incorporation of HSCs 2 days after transplantation9 and indicates a possible homing without any proliferation of stem/progenitor cells during the first 12 to 24 hours that followed their recruitment in the femoral head. Finally, as we observed a continuous recruitment of HSCs that starts 1 hour after transplantation and lasts at least 15 hours, our results are also in accordance with the proliferation of few progenitor/stem cells observed 12 hours after transplantation.14 In the future, we need to isolate and characterize the hematopoietic cells that home and start to proliferate early in the femoral head to evaluate their long-term repopulating capacity and to determine the signaling pathways and the cell-cell interactions that initiate their proliferation.
We then explored the potential of this cellular imaging system to follow the in vivo effects of modulators of hematopoietic reconstitution. Using the antioxidant agent NAC, we showed that ROS acted as positive regulators of the initial steps of hematopoietic reconstitution because treatment with the antioxidant agent NAC before transplantation delayed the kinetics of bone marrow repopulation without affecting long-term hematopoietic reconstitution of the mice, indicating that other signaling pathways can compensate ROS functions. Elevation of ROS in HSCs leads to defective cell-cycle quiescence34,35 and negatively affects HSC-osteoblastic niche interactions.36,37 We found that NAC treatment of transplanted mice results in a delay in LSKCD34− hematopoietic cell proliferation in vivo, indicating a positive effect of ROS on hematopoietic reconstitution. In addition, our results suggest that elevation of ROS after lethal irradiation also positively affects stem/progenitor cell–endothelial cell interactions in vivo by modulating VCAM-1 expression on endothelial cells. Together with the previously described induced activation of VCAM-1 in the endothelial cells of irradiated bone marrow26,38 and the involvement of VCAM-1 in the transendothelial migration of hematopoietic cells,25 these data indicate how ROS positively regulate the initial homing and proliferation of the transplanted HSCs in the femoral head of lethally irradiated mice.
In conclusion, we have visualized transplanted HSCs in the femoral bone marrow within a living animal and have defined the kinetics and the regulation by ROS of recruitment and homing of HSCs in long bones. The combination of this technique and molecular and cellular biology will be useful to indicate the role of specific transcription factors or signaling pathways in homing, engraftment, and repopulation of bone marrow by HSCs and to study the effects of drugs on the dynamic localization of HSCs.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Gisselbrecht and F. Pflumio for critical reading of the manuscript, A. Le Nevez (Mauna Kea Technologies) and J. Rebouillat for technical support, the staff of the institut de Radiobiologie Cellulaire et Moléculaire animal colony for excellent support in mouse studies, and P. Opolon and O. Bawa for histology.
D.L. and V.B. are supported by fellowships from the CEA. This project was supported by grants from La Ligue de Paris contre le Cancer (RS 07/75-20), ARC (3527), ANR (NT05-2-44232, 06-TecScan-019-04, 06-EMPB-002), Inserm, CEA/DSV, and the European Molecular Imaging Laboratories European Network of Excellence, and the European Union (contract LSH-2004-503569).
Authorship
Contribution: D.L., V.B., F.D., J.B., J.T.V.N., C.P., and P.F. performed research and analyzed data; B.T. contributed to the development of this new imaging system; and D.L. and P.-H.R. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul-Henri Roméo, CEA, Institut de Radiobiologie Cellulaire et Moléculaire, Laboratoire de recherche sur la Réparation et la Transcription dans les cellules Souches, 18 route du Panorama, BP6, 92265 Fontenay-aux-Roses cedex, France; e-mail: paul-henri.romeo@cea.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal