Abstract
Gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) arises against a background of chronic inflammation caused by persistent Helicobacter pylori infection. The clinical and histopathologic features of the human tumor can be reproduced by Helicobacter infection of BALB/c mice. In this study, we have analyzed the antibody sequences and antigen specificity of a panel of murine and human MALT lymphoma–derived antibodies. We find that a majority of tumors in patients as well as experimentally infected mice are monoclonal. The tumor immunoglobulin heavy chain genes have undergone somatic hypermutation, and approximately half of all tumors show evidence of intraclonal variation and positive and/or negative selective pressure. Recombinantly expressed MALT lymphoma antibodies bind with intermediate affinity to various unrelated self- and foreign antigens, including Helicobacter sonicate, immunoglobulin G (IgG), DNA, and stomach extract; antigen binding is blocked in a dose-dependent manner in competitive enzyme-linked immunosorbent assays. A strong bias toward the use of VH gene segments previously linked to autoantibodies and/or polyreactive antibodies in B-cell malignancies or autoimmune pathologies supports the experimental finding of polyreactivity. Our results suggest that MALT lymphoma development may be facilitated by an array of local self- and foreign antigens, providing direct antigenic stimulation of the tumor cells via their B-cell receptor.
Introduction
Gastric mucosa-associated lymphoid tissue (MALT) B-cell lymphoma develops in the context of long-term infection with the Gram-negative gastric bacterium Helicobacter pylori.1-3 Persistent infection with H pylori causes chronic gastritis that, in some people, can develop into more organized gastric mucosa-associated lymphoid tissue (MALT) with histologic similarity to the Peyer patches of the small intestine.4 The disease progresses when individual malignant clones grow out, displace the benign lymphoid tissue, and ultimately form the lymphoepithelial lesions that are a hallmark of MALT lymphoma.5,6 In its early stages, gastric MALT lymphoma is believed to be an antigen-dependent disease; H pylori infection is detectable in a large majority of cases.1,3,7 Eradication therapy induces tumor regression in approximately 75% of patients at this stage.8,9 Early-stage low-grade MALT lymphoma is generally considered an indolent tumor due to its slow growth, low proliferation rates, and minimal propensity for spreading. At later stages, however, the tumors can eventually undergo high-grade transformation or acquire one of several known characteristic chromosomal translocations, thereby rendering the lymphoma independent of antigen exposure and refractory to H pylori eradication therapy.
In contrast to cases with chromosomal rearrangements, which grow autonomously due to constitutive activation of the nuclear factor κB (NF-κB) signaling pathway brought about by overexpression of MALT-1, B-cell leukemia 10 (Bcl-10), or production of the Baculovirus IAP repeat-containing 3 (API2)–MALT1 fusion protein (reviewed in Isaacson and Du),5 little is known about the pathogenesis of early MALT lymphoma. Several studies have implicated the abundant population of tumor-infiltrating T cells in providing growth signals to tumor B cells.10-12 In one study, depletion of T cells was shown to abrogate the proliferation of explanted MALT lymphoma cultures.12 We and others have reported that MALT lymphomas express high levels of interleukin-4 (IL-4) and other T helper 2 cytokines in vivo, supporting a role for T helper 2–polarized T-helper responses in early MALT lymphomagenesis.10,11
An alternative possibility is that early stage MALT lymphoma B cells receive signals via antigenic stimulation through their B-cell receptor (BCR), which would lead to NF-κB activation, survival, and proliferation. Indeed, MALT lymphoma cells carry functional, rearranged, and somatically mutated immunoglobulin genes on their surface.13,14 Sequence analysis of the VH genes suggests that the tumor cells have undergone positive selection in germinal centers.14-16 Intraclonal variation caused by ongoing somatic mutation and/or replacement of a part of the variable heavy segment (receptor revision) has been reported.15-17 Despite these clear results, the search for a target antigen has proven difficult and has yielded controversial results, either providing evidence for reactivity toward certain structures of normal human tissues (follicular dendritic cells, venules, epithelial cells, connective tissue)18,19 or human immunoglobulin G (IgG),13 or not identifying any target antigens at all.20
We and others have shown earlier that long-term, persistent infection of the BALB/c strain of mice with H pylori or its close relatives Helicobacter felis and Helicobacter heilmannii results in the reproducible induction of lesions that clinically and histologically resemble human MALT lymphoma.10,21-23 These lesions are slow growing, spread only infrequently (usually to the spleen), and rarely transform to high-grade lymphoma.10,22 The tumors further recapitulate the human disease in that they regress in a majority of mice upon eradication of the infection,22 but recur rapidly upon reintroduction of the bacteria, revealing gastric persistence of the tumor clone despite complete histologic remission.10
Using this experimental mouse model as well as human MALT lymphoma biopsy material, we deduced and compared the immunoglobulin sequences of 20 murine and 7 human tumors. Based on the monoclonal Ig sequences, we generated a comprehensive panel of recombinant antibodies expressed as soluble IgG of matching heavy and light chains and tested their reactivity toward various self- and foreign antigens. The tumor antibodies, which were functional and somatically hypermutated and in some cases showed evidence of positive and/or negative selection, surprisingly reacted with roughly equal affinity with both gastric self- and Helicobacter-derived foreign antigens. This pattern of reactivity is consistent with a diagnosis of polyreactivity, and could further be linked to a strongly biased use of specific VH family gene segments in both the murine and the human system.
Methods
Animal experimentation, tumor cell cultures, and immunophenotyping
Specific pathogen-free female BALB/c mice were infected orally at 6 weeks of age with 3 consecutive doses of approximately 5 × 107H felis (CS1, ATCC 49179). All animal experiments were approved by Stanford University's Institutional Animal Care and Use Committee. Upon killing, the stomachs were removed and opened along the lesser curvature; macroscopically visible tumors were dissected. Single-cell suspensions were generated and cultured in RPMI supplemented with 10% fetal calf serum (FCS) and antibiotics. Where appropriate, 10 μg/mL Helicobacter lysate was used. Tumor cell proliferation was quantified by [3H]-thymidine incorporation assay, or by bromodeoxyuridine (BrdU) incorporation followed by fluorescence-activated cell sorting analysis using a fluorescent in situ cell proliferation kit (Roche Diagnostics) The following antibodies were used for immunophenotyping: anti-CD19 (monoclonal antibody [mAb] clone 6D5; Abcam), anti-B220 (mAb clone RA3-6B2, BD Pharmingen), anti-IgM (polyclonal goat, no. 1020; Southern Biotech), anti-IgG (polyclonal goat, no. 1030; Southern Biotech), anti-CD3 (mAb clone 145-2C11; BD Pharmingen), anti-CD11c (mAb clone N418; AbD Serotec), anti–proliferating cell nuclear antigen (PCNA; mAb clone PC10; Zymed Labs), and anti-CD11b (mAb clone M1/70.15; AbD Serotec). Flow cytometry was performed on a CyanADP instrument (Dako).
Patient material
Human patient material was obtained from 7 patients with gastric MALT lymphoma who were part of a previously published study conducted at Philipps-University Marburg.24 All tumors were diagnosed as H pylori–positive low-grade gastric MALT lymphomas and all were negative for the translocation t(11;18)(q21:q21). The 5 cases of chronic lymphocytic leukemia (CLL), diagnosed according to standard criteria, were described in previous studies25,26 ; all 5 tumor-derived IgVH sequences had been determined previously to be somatically mutated.
RNA extraction, VH and VL gene amplification, and cloning
RNA was isolated from the murine tumor cell suspensions and from patient biopsy material using the RNeasy kit (QIAGEN) according to the manufacturer's instructions. cDNA was synthesized using oligo-dT primers and SuperScript III reverse transcriptase (Invitrogen). The human Ig heavy and light chain sequences were obtained using Ig framework-specific primers for all human variable heavy and light chain framework 1 families in combination with primers specific for all human J segments, as specified by Marks et al.27 The obtained variable heavy and light chain products were cloned into expression vectors in frame with a 5′ leader peptide and a 3′ human IgG1 or kappa light chain constant domain. The amplification and cloning of murine Ig variable heavy and light chain sequences was performed accordingly.
Sequence analysis
Sequencing of heavy and light chain variable regions was performed by Microsynth. The obtained sequences were aligned to germline sequences from the IGMT database (international ImMunoGeneTics database, http://www.imgt.org).28 Tumors were defined as clonal if identical or near identical VH sequences were obtained from 2 independent polymerase chain reactions. Somatic hypermutation and intraclonal variation of IgVH genes was assessed as described by Lossos et al29 ; VH gene sequences that deviated by more than 2% from the corresponding germline gene sequence were defined as somatically hypermutated. The degree of intraclonal diversity of IgVH genes was defined as follows: an unconfirmed mutation is a substitution mutation observed in only one of the VH gene clones from the same tumor specimen; a confirmed mutation is a mutation observed in more than one VH clone from the same tumor specimen.29 The role of antigen selection in shaping MALT lymphoma immunoglobulins was assessed by calculating and comparing the ratios of replacement (R) to silent (S) mutations in the framework (FR) and complementary determining regions (CDRs) of the somatically mutated Ig heavy chain genes. Positive selection was diagnosed if the R/S ratios of mutations in the CDRs were greater than 2.9; negative selection pressure by antigen was postulated if the R/S ratios in the FR regions were lower than 2.9.30 The probability that an excess or scarcity of replacement mutations in VH CDRs or framework regions occurred by chance was calculated by a multinomial distribution model.31 A P value less than .05 was considered statistically significant.
Expression and purification of recombinant antibodies
293T cells were cultured in Opti-MEM I reduced Serum Media (Invitrogen) supplemented with GlutaMAX (Invitrogen). Cells were transfected by calcium phosphate coprecipitation with equimass amounts of the heavy and light chain vector and the supernatant was harvested after 7 days. Recombinant antibodies were purified from culture supernatants on a 1-mL HiTrap Protein G Sepharose column (Amersham Pharmacia Biotech) and dialyzed against phosphate-buffered saline (PBS).
Preparation of H felis antigens, murine stomach extract, and AGS cell extract
H felis and H pylori were grown as described.21 Sonicate was prepared by harvesting cells in PBS and the cells were disrupted by sonication (Bandelin Sonopuls GM 70; Bandelin). For preparation of gastric mucosal extract, the mucosa was scraped from wild-type BALB/c mice and lysed in lysis buffer (10% glycerol, 1% Triton X-100, 100 nM NaCl, 20 mM N-2-hydroxyethylpiperazine-N′;-2-ethanesulfonic acid [pH 7.4], and 1mM sodium vanadate). For preparation of human stomach cell extract, human gastric carcinoma cells (AGS) were lysed with the same buffer. The protein content of all antigen preparations was determined by Bicinchoninic acid protein assay (Thermo Scientific) and aliquots were stored at −70°C until used.
Enzyme-linked immunosorbent assays
Duplicate wells of Maxisorp micotiter plates (Nunc) were coated with various antigens. Single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA) were coated at 100 μg/mL in PBS. All other antigens tested were coated at 5 μg/mL each in PBS. After washing with PBS-0.5% (vol/vol) Tween-20, the wells were blocked with 2% bovine serum albumin (BSA) in PBS for 2 hours. All antibodies were incubated overnight at 0.1, 1, and 10 μg/mL at 4°C. After washing, wells were incubated for 1 hour with either peroxidase-conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) or goat peroxidase-conjugated anti–human IgG (Abcam). Enzyme activity was detected by incubation with tetramethylbenzidine (Sigma-Aldrich). Rheumatoid factor activity of murine and human antibodies was determined using the mouse rheumatoid factor Ig enzyme-linked immunosorbent assay (ELISA) kit (Alpha Diagnostic International) and the Aeskulisa Human Rf-AGM kit (Aesku Diagnostics), respectively. The dissociation constants (Kd) were calculated according to Friguet et al.32
Results
Induction of Helicobacter-dependent gastric MALT lymphoma in a BALB/c mouse model and ex vivo culturing of tumor cell suspensions
To induce the formation of Helicobacter-dependent gastric MALT lymphoma, 18 female BALB/c mice were infected with H felis for 18 months; 7 additional age-matched females served as uninfected controls. Sixteen of the 18 infected, but none of the uninfected mice, had developed gastric MALT lymphoma at the time of killing. The tumors were macroscopically visible as nodules ranging from 1 to 2 mm in diameter. They were positive for the B-cell marker B220 and had proliferative indices of approximately 30% as determined by PCNA staining (Figure 1A-B). The tumors had formed predominantly at the forestomach/corpus junction. A total of 20 macroscopically discernible tumors were dissected and single-cell suspensions were generated of individual tumors. These were subjected to Ig sequence analysis (all 20 tumors; isolated from 10 individual mice), immunophenotyping (a subset of 14 tumors, from the same 10 mice), and/or ex vivo culturing (9 tumors from 8 mice).
Murine MALT lymphoma cells proliferate in response to Helicobacter antigen. (A-B) Histopathology of murine mucosa-associated lymphoid tissue (MALT) lymphoma. Consecutive paraffin sections were stained with hematoxylin and eosin (H&E), the B-cell marker B220, and the proliferation marker PCNA; representative sections are shown for an unaffected area (A) and a lymphoma (B) from the same stomach. (C-F) Single-cell suspensions of 9 murine MALT lymphomas were cultured in the presence or absence of 10 μg/mL Helicobacter felis lysate. The proportion of CD19+ B cells and CD3+ T cells was determined flow cytometrically for each culture (C). Proliferation was determined by [3H]-thymidine incorporation (D) or BrdU incorporation (E) after 5 days of culturing. Individual cultures and/or averages of all cultures are shown. Error bars represent SD. (F) The BrdU+ cells are mostly B and T cells.
Murine MALT lymphoma cells proliferate in response to Helicobacter antigen. (A-B) Histopathology of murine mucosa-associated lymphoid tissue (MALT) lymphoma. Consecutive paraffin sections were stained with hematoxylin and eosin (H&E), the B-cell marker B220, and the proliferation marker PCNA; representative sections are shown for an unaffected area (A) and a lymphoma (B) from the same stomach. (C-F) Single-cell suspensions of 9 murine MALT lymphomas were cultured in the presence or absence of 10 μg/mL Helicobacter felis lysate. The proportion of CD19+ B cells and CD3+ T cells was determined flow cytometrically for each culture (C). Proliferation was determined by [3H]-thymidine incorporation (D) or BrdU incorporation (E) after 5 days of culturing. Individual cultures and/or averages of all cultures are shown. Error bars represent SD. (F) The BrdU+ cells are mostly B and T cells.
In the tumor cell suspension cultures, an average of 64% of all leukocytes were CD19+ B cells (Figure 1C); most of the remaining leukocytes stained positive for CD3 (Figure 1C). Only a minor fraction was positive for CD11b or CD11c (1.5% on average; data not shown). The suspensions generally contained less than 15% nonleukocyte cells. Although all tumor cell suspensions could be kept alive for 5 days in standard cell culture media, only cultures to which Helicobacter lysate had been added proliferated within this time frame, as determined by [3H]-thymidine (Figure 1D) or BrdU incorporation (Figure 1E). Addition of Helicobacter lysate induced an on average 4-fold increase in [3H]-thymidine incorporation compared with the corresponding unstimulated culture (Figure 1D); BrdU-positive cells increased from approximately 2.5% in unstimulated cultures to approximately 3.8% in stimulated cultures (Figure 1E). Immunophenotyping of BrdU-positive cells further revealed that the proliferating population in the cultures consisted predominantly of CD19+ B cells (average: 83%; Figure 1F), with T cells accounting for the remaining proliferative subset. In conclusion, murine MALT lymphoma B cells not only are antigen dependent in vivo as we and others have shown previously by inducing tumor regression through Helicobacter eradication therapy,10,22 but also retain their dependence on Helicobacter antigen ex vivo.
Immunophenotyping of murine MALT lymphomas
Human MALT lymphomas are known to express surface IgM and pan-B-cell markers (CD19, CD20, CD79a) and the marginal zone markers CD35 and CD21, although are negative for CD5, CD10, CD23, and cyclin D1.33 Because we aimed to analyze the antibody sequences and specificity of our murine tumors, we flow cytometrically determined the surface IgM and IgG expression of 14 tumor cell suspensions (Figure 2). Indeed, the majority of CD19+, B220+ B cells in every tumor showed high expression of IgM (average: 90%); a minority expressed high IgG (average: 1.5%) and the rest expressed only low levels of either Ig (Figure 2).
Murine MALT lymphoma cells express B220 and CD19 and are surface IgM positive. Fourteen independent single-cell suspension cultures of murine MALT lymphomas were analyzed flow cytometrically with respect to B220, CD19, and IgM/IgG expression. Results from a representative culture as well as the averages of all 14 cultures are shown. Error bars represent SEM.
Murine MALT lymphoma cells express B220 and CD19 and are surface IgM positive. Fourteen independent single-cell suspension cultures of murine MALT lymphomas were analyzed flow cytometrically with respect to B220, CD19, and IgM/IgG expression. Results from a representative culture as well as the averages of all 14 cultures are shown. Error bars represent SEM.
Sequence analysis of murine MALT lymphoma immunoglobulins
To determine the clonal status of a panel of 20 murine MALT lymphomas, the corresponding cDNAs were generated and subjected to several rounds of cloning and sequencing of the IgVH genes. (All sequences reported in this paper have been deposited in the GenBank database; accession nos. GQ856044–GQ856073.34 ) Tumors were pronounced monoclonal if more than half of all obtained IgVH sequences were identical or near identical in 2 independent rounds of sequencing. Clear monoclonal status was demonstrated for 11 lymphomas (55% of cases), whereas 1 lymphoma appeared biclonal and the remaining 8 tumors were classified as polyclonal. None of the monoclonal sequences harbored internal stop codons, suggesting that murine MALT lymphoma B cells express potentially functional surface IgM.
The 11 clonal IgVH sequences were further analyzed for evidence of somatic hypermutation and intraclonal variation (Table 1). Germline genes with the highest homology to the consensus tumor IgVH sequence were identified (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article); with the exception of 2 cases, all IgVH genes analyzed displayed somatic mutation, that is, they deviated from the most closely related germline sequence in more than 2% of positions, with the average homology being 92.2% (Table 1, supplemental Figure 1). This result is in line with previous studies reporting somatic hypermutation of human MALT lymphoma IgVH.14-16 Four IgVH sequences were found to be positively selected by antigen, that is, the ratios of replacement/silent mutations in their CDRs were higher than would be expected if mutations had occurred by chance alone without selective forces (based on a cutoff ratio of > 2.930 ; Table 1). Six sequences demonstrated the presence of negative selection pressure by antigen, that is, the replacement/silent mutation ratios in their FR regions were lower than expected by chance alone. Two sequences showed evidence of both negative and positive selection. Analysis of antigen selection using the more stringent multinomial model29,31 identified only 3 sequences with significant positive selection and one with significant negative selection (Table 1). Previous studies examining antigenic selection of human IgVH have mostly reported evidence of positive selective pressure,14-16 suggesting that MALT lymphoma B cells have undergone germinal center reactions and affinity maturation.
VH gene analysis of murine gastric MALT lymphoma cases
| Case . | VH segment . | Homology % . | Clones sequenced . | Somatic* mutation . | FR/CDR . | R . | S . | Antigen selection† . | P‡ . | Intraclonal variation . | CDR3 length . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mu-1 | IGHV1-S130*01 | 93.06 | 11 | + | FR | 42 | 1 | − | .490 | + | 11 |
| CDR | 29 | 0 | + | .000 | |||||||
| mu-2 | IGHV14-3*02 | 92.36 | 10 | + | FR | 13 | 3 | − | .391 | + | 15 |
| CDR | 6 | 0 | + | .059 | |||||||
| mu-3 | IGHV1-S81*02 | 97.92 | 13 | + | FR | 4 | 2 | + | .562 | − | 13 |
| CDR | 2 | 0 | − | .801 | |||||||
| mu-4 | IGHV14-3*02 | 99.31 | 10 | − | FR | 1 | 1 | + | .000 | + | 13 |
| CDR | 0 | 0 | − | .621 | |||||||
| mu-5 | IGHV1-69*02 | 86.46 | 8 | + | FR | 24 | 6 | − | .491 | − | 10 |
| CDR | 5 | 4 | − | .601 | |||||||
| mu-6 | IGHV1-67*1 | 80.90 | 10 | + | FR | 8 | 5 | + | .03 | − | 12 |
| CDR | 5 | 2 | − | .110 | |||||||
| mu-7 | IGHV1-S22*01 | 94.62 | 22 | + | FR | 7 | 2 | − | .112 | − | 15 |
| CDR | 2 | 0 | − | .009 | |||||||
| mu-8 | IGHV14-3*02 | 98.61 | 9 | − | FR | 2 | 1 | + | .329 | + | 11 |
| CDR | 1 | 0 | − | 1.278 | |||||||
| mu-9 | IGHV1-18*02 | 88.19 | 8 | + | FR | 18 | 10 | + | .107 | + | 12 |
| CDR | 5 | 1 | + | .369 | |||||||
| mu-10 | IGHV1-S81*02 | 95.83 | 10 | + | FR | 4 | 1 | − | .23 | + | 14 |
| CDR | 3 | 3 | − | .156 | |||||||
| mu-11 | IGHV1-74*01 | 86.81 | 10 | + | FR | 17 | 9 | + | .12 | − | 12 |
| CDR | 10 | 2 | + | .034 |
| Case . | VH segment . | Homology % . | Clones sequenced . | Somatic* mutation . | FR/CDR . | R . | S . | Antigen selection† . | P‡ . | Intraclonal variation . | CDR3 length . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mu-1 | IGHV1-S130*01 | 93.06 | 11 | + | FR | 42 | 1 | − | .490 | + | 11 |
| CDR | 29 | 0 | + | .000 | |||||||
| mu-2 | IGHV14-3*02 | 92.36 | 10 | + | FR | 13 | 3 | − | .391 | + | 15 |
| CDR | 6 | 0 | + | .059 | |||||||
| mu-3 | IGHV1-S81*02 | 97.92 | 13 | + | FR | 4 | 2 | + | .562 | − | 13 |
| CDR | 2 | 0 | − | .801 | |||||||
| mu-4 | IGHV14-3*02 | 99.31 | 10 | − | FR | 1 | 1 | + | .000 | + | 13 |
| CDR | 0 | 0 | − | .621 | |||||||
| mu-5 | IGHV1-69*02 | 86.46 | 8 | + | FR | 24 | 6 | − | .491 | − | 10 |
| CDR | 5 | 4 | − | .601 | |||||||
| mu-6 | IGHV1-67*1 | 80.90 | 10 | + | FR | 8 | 5 | + | .03 | − | 12 |
| CDR | 5 | 2 | − | .110 | |||||||
| mu-7 | IGHV1-S22*01 | 94.62 | 22 | + | FR | 7 | 2 | − | .112 | − | 15 |
| CDR | 2 | 0 | − | .009 | |||||||
| mu-8 | IGHV14-3*02 | 98.61 | 9 | − | FR | 2 | 1 | + | .329 | + | 11 |
| CDR | 1 | 0 | − | 1.278 | |||||||
| mu-9 | IGHV1-18*02 | 88.19 | 8 | + | FR | 18 | 10 | + | .107 | + | 12 |
| CDR | 5 | 1 | + | .369 | |||||||
| mu-10 | IGHV1-S81*02 | 95.83 | 10 | + | FR | 4 | 1 | − | .23 | + | 14 |
| CDR | 3 | 3 | − | .156 | |||||||
| mu-11 | IGHV1-74*01 | 86.81 | 10 | + | FR | 17 | 9 | + | .12 | − | 12 |
| CDR | 10 | 2 | + | .034 |
MALT indicates mucosa-associated lymphoid tissue; FR, framework region; CDR, complementary determining region; R, replacement; S, silent; and mu, murine.
VH gene sequences deviating more than 2% from the corresponding germline gene were defined as somatically mutated.
Presence or absence of positive selection by antigen in the CDR is denoted by + and −, respectively. Presence or absence of negative selection by antigen in the FR is denoted by + and −, respectively, based on the cutoff ratio of 2.9 for replacement to silent mutations.
The P value was calculated based on the multinomial distribution model and is the probability that excess (for CDR) or scarcity (for FR) of mutations occurred by chance.
To evaluate the presence of ongoing mutation, the 11 clonal sequences were examined by comparing at least 8 and up to 22 molecular clones from each tumor (supplemental Figure 2). Five of the somatically mutated clonal VH gene isolates did not show intraclonal heterogeneity (Table 1), whereas the remaining 6 tumors, including the 2 with unmutated IgVH sequences, harbored confirmed mutations and therefore showed evidence of intraclonal variation (Table 1, supplemental Figure 2). The average length of the CDR3 region for the 11 monoclonal tumor immunoglobulins was 12.5 amino acids, which is longer than the average length of 8.5 amino acids usually found in a normal murine splenic B-cell repertoire (Table 1).35 VH gene family use was found to be strongly biased toward VH1 (n = 7) and VH14 (n = 4). Of the 12 known murine VH gene families, the VH1 gene family accounts for 28% of the antibody repertoire in BALB/c splenocytes; the VH14 family in contrast is rarely used in BALB/c mice.
Antigen reactivity of recombinant murine MALT lymphoma–derived antibodies
To investigate the antigen specificity of murine MALT lymphoma Ig, the corresponding light chains of 5 of the clonal heavy chains were identified. In cases with ongoing mutation, the IgVH sequence most closely matching the consensus sequence was chosen. Paired heavy and light chains were expressed recombinantly in 293T cells and purified by affinity chromatography. We deliberately selected a mixed panel of mutated and unmutated, antigen selected and unselected IgVH sequences (mu Abs 2, 3 8, 10, and 11). A series of ELISAs were performed to systematically screen Ig reactivity toward a broad range of antigens. Possible autoreactivity was assessed using murine stomach extract; antinuclear activity was examined using both ssDNA and dsDNA, and rheumatoid factor activity was assessed by IgG binding; the foreign antigens tested included H felis and Escherichia coli sonicate and purified E coli lipopolysaccharide (LPS). BSA was used as a negative control. The combined results of all ELISAs revealed quite unequivocally that all antibodies analyzed bound to a surprising variety of antigens in a dose-dependent manner (Figure 3). An overall trend of higher affinity binding toward more complex antigen compositions (extracts of H felis and of murine stomach) was noted; no preference for either Helicobacter-only reactivity or autoreactivity-only could be detected in any of the antibodies' ELISA profiles. A monoclonal, isotype-matched murine control antibody failed to bind any of the antigens tested (Figure 3). Somatically mutated antibodies did not differ from unmutated antibodies, and evidence of antigen selection in the IgVH sequence also did not seem to correlate with an increased overall or antigen-specific affinity.
Recombinant murine MALT lymphoma–derived antibodies are polyreactive. Three concentrations (0.1, 1, and 10 μg/mL) of 5 murine antibodies cloned from the tumors of 5 independent mice and recombinantly expressed in 293T cells were tested by ELISA for reactivity toward BSA, LPS, IgG, dsDNA, ssDNA, Escherichia coli, or Helicobacter felis sonicate and protein that was extracted from the mucosa of BALB/c stomachs (either infected with H felis or uninfected). Antibodies 2, 3, 8, and 11 display strong reactivity toward the majority of targets, whereas Ab10 has comparatively low affinity. A monoreactive antibody (mu AbGR1) was used as a control.
Recombinant murine MALT lymphoma–derived antibodies are polyreactive. Three concentrations (0.1, 1, and 10 μg/mL) of 5 murine antibodies cloned from the tumors of 5 independent mice and recombinantly expressed in 293T cells were tested by ELISA for reactivity toward BSA, LPS, IgG, dsDNA, ssDNA, Escherichia coli, or Helicobacter felis sonicate and protein that was extracted from the mucosa of BALB/c stomachs (either infected with H felis or uninfected). Antibodies 2, 3, 8, and 11 display strong reactivity toward the majority of targets, whereas Ab10 has comparatively low affinity. A monoreactive antibody (mu AbGR1) was used as a control.
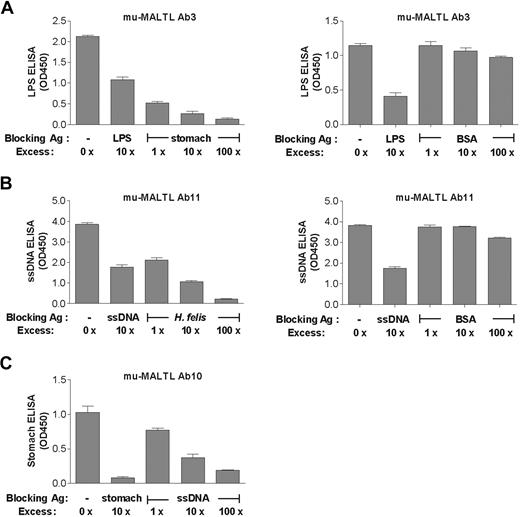
Taken together, our results support a diagnosis of polyreactivity. To confirm the specificity of the assay, we performed a series of competitive inhibition ELISAs (Figure 4A-C). To this end, binding of the antibody of interest to one of its targets (immobilized on the ELISA plate) was competitively blocked with increasing concentrations of soluble target of the same or an alternative specificity. In all combinations tested, the soluble-phase antigens were able to competitively block binding to the immobilized target in a dose-dependent manner (Figure 4A-C). As would be expected, high-affinity soluble antigens blocked binding to low-affinity immobilized targets with a higher efficiency than vice versa (Figure 4A-C). BSA, which was used as a negative control, did not block binding even at 100× excess (Figure 4A-B). Overall, the competition results confirmed the specificity of the ELISAs as well as our general observation that complex antigen mixtures constitute higher affinity targets than relatively simple structures such as DNA or LPS.
Competitive inhibition ELISAs confirm polyreactive binding patterns of murine MALT lymphoma antibodies. (A-C) A single concentration of the indicated recombinant murine antibodies 3, 11, and 10 (10 μg/mL) was incubated with plate-coated (solid-phase) antigen in the presence of a 10× excess of the same antigen in soluble form or increasing concentrations of an alternative soluble antigen. Three representative inhibition assays are shown and demonstrate the ability of different soluble-phase antigens to inhibit the binding of the antibody to solid-phase antigens. In contrast, BSA did not block binding of Abs 3 and 11 even at 100× excess concentrations (A-B right panels). Error bars represent SEM.
Competitive inhibition ELISAs confirm polyreactive binding patterns of murine MALT lymphoma antibodies. (A-C) A single concentration of the indicated recombinant murine antibodies 3, 11, and 10 (10 μg/mL) was incubated with plate-coated (solid-phase) antigen in the presence of a 10× excess of the same antigen in soluble form or increasing concentrations of an alternative soluble antigen. Three representative inhibition assays are shown and demonstrate the ability of different soluble-phase antigens to inhibit the binding of the antibody to solid-phase antigens. In contrast, BSA did not block binding of Abs 3 and 11 even at 100× excess concentrations (A-B right panels). Error bars represent SEM.
Sequence analysis of human MALT lymphoma immunoglobulins
To be able to compare the sequence characteristics and specificity of the murine tumor immunoglobulins to their human counterparts, we cloned, sequenced, and recombinantly expressed the antibodies of 7 human low-grade MALT lymphomas.24 All 7 cases were clonal. Five of the 7 cases showed evidence of somatic hypermutation (Table 2, supplemental Figure 3). Interestingly, we found a bias toward use of the VH gene segment 1-69*01, which was used in 4 of the 7 cases (Table 2), and has been reported previously for MALT lymphomas of the parotid gland, stomach, and lung.13 No bias was detected in J and D segment use, but all matching light chains were of the κ isotype (Table 2), another seemingly common bias in MALT lymphoma antibodies.13,20 Three of 7 cases further showed evidence of intraclonal variation (Table 2, supplemental Figure 4). Interestingly, the CDR3 length of the heavy chainswas higher than average (15.3 amino acids as opposed to the 12.7 amino acids usually found in human blood B-cell antibodies),35 confirming our observation from the murine model. Overall, the sequence analysis of our panel of 7 antibodies confirmed several observations reported previously for human gastric MALT lymphoma and the panel was therefore considered suitable for antigen binding studies.
Immunoglobulin variable heavy and light chain genes of 7 human MALT lymphoma cases
| Case . | VH segment . | Homology % . | Clones sequenced . | Somatic mutation . | Intraclonal variation . | JH segment . | D segment . | CDR3 length . | VL segment . | JL segment . |
|---|---|---|---|---|---|---|---|---|---|---|
| hu-1 | IGHV1-69*01 | 88.19 | 8 | + | − | IGHJ4*02 | IGHD4-17*01 | 14 | IGKV3-20*01 | IGKJ4*01 |
| hu-2 | IGHV3-7*01 | 98.61 | 6 | − | − | IGHJ3*02 | IGHD3-10*01 | 17 | IGKV1-9*01 | IGKJ4*01 |
| hu-3 | IGHV3-66*02 | 93.33 | 7 | + | + | IGHJ4*02 | IGHD3-3*01 | 14 | IGKV1-5*03 | IGKJ2*01 |
| hu-4 | IGHV1-69*01 | 94.10 | 6 | + | − | IGHJ3*02 | IGHD1-1*01 | 18 | IGKV3-20*01 | IGKJ4*01 |
| hu-5 | IGHV3-33*01 | 98.61 | 5 | − | + | IGHJ3*02 | IGHD5-24*01 | 12 | IGKV1-5*03 | IGKJ1*01 |
| hu-6 | IGHV1-69*01 | 96.88 | 6 | + | + | IGHJ5*02 | IGHD6-19*01 | 17 | IGKV3-20*01 | IGKJ4*01 |
| hu-7 | IGHV1-69*05 | 85.07 | 8 | + | − | IGHJ1*01 | IGHD4-23*01 | 15 | IGKV1-9*01 | IGKJ5*01 |
| Case . | VH segment . | Homology % . | Clones sequenced . | Somatic mutation . | Intraclonal variation . | JH segment . | D segment . | CDR3 length . | VL segment . | JL segment . |
|---|---|---|---|---|---|---|---|---|---|---|
| hu-1 | IGHV1-69*01 | 88.19 | 8 | + | − | IGHJ4*02 | IGHD4-17*01 | 14 | IGKV3-20*01 | IGKJ4*01 |
| hu-2 | IGHV3-7*01 | 98.61 | 6 | − | − | IGHJ3*02 | IGHD3-10*01 | 17 | IGKV1-9*01 | IGKJ4*01 |
| hu-3 | IGHV3-66*02 | 93.33 | 7 | + | + | IGHJ4*02 | IGHD3-3*01 | 14 | IGKV1-5*03 | IGKJ2*01 |
| hu-4 | IGHV1-69*01 | 94.10 | 6 | + | − | IGHJ3*02 | IGHD1-1*01 | 18 | IGKV3-20*01 | IGKJ4*01 |
| hu-5 | IGHV3-33*01 | 98.61 | 5 | − | + | IGHJ3*02 | IGHD5-24*01 | 12 | IGKV1-5*03 | IGKJ1*01 |
| hu-6 | IGHV1-69*01 | 96.88 | 6 | + | + | IGHJ5*02 | IGHD6-19*01 | 17 | IGKV3-20*01 | IGKJ4*01 |
| hu-7 | IGHV1-69*05 | 85.07 | 8 | + | − | IGHJ1*01 | IGHD4-23*01 | 15 | IGKV1-9*01 | IGKJ5*01 |
MALT indicates mucosa-associated lymphoid tissue; and hu, human.
Antigen reactivity of recombinant human MALT lymphoma–derived antibodies
To determine whether human MALT lymphoma Ig also follows a polyspecific binding pattern, matched heavy and light chains of all 7 human MALT lymphoma cases were screened for reactivity toward BSA, H pylori sonicate, LPS, IgG, ssDNA, and AGS cell extract (Figure 5A). All but 1 (hu Ab2) of the human MALT lymphoma antibodies exhibited a polyreactive binding profile very similar to the patterns observed with the murine tumor Igs. Interestingly, hu Ab2 was the only human tumor antibody on the panel to not have undergone either somatic hypermutation or ongoing mutation. As observed for the murine polyreactive MALT lymphoma antibodies, a trend of higher affinity binding toward more complex antigen compositions was evident (Figure 5A). To properly control for the validity of our ELISA readout in the human system, we chose 5 additional non-MALT lymphomas for amplification of their immunoglobulin genes, sequence analysis, and recombinant expression in 293T cells and subsequent ELISA analysis. We selected cases of “mutated” chronic lymphocytic leukemia (CLL; ie, cases expressing somatically mutated Ig), as these are known to be monoreactive in contrast to their “unmutated” CLL counterparts.36 All 5 cases were clonal and showed on average 93% homology with the most closely related germline sequence. None of the antibodies displayed a polyreactive binding pattern similar to the one we observed for 6 of the 7 MALT lymphoma Igs. In fact, all but 1 of the 5 CLL antibodies failed to show any affinity for any of the targets of our ELISAs (one—Ab3—bound to ssDNA, but not to any of the other antigens). This result showed that MALT lymphoma antibodies have fundamentally different antigen-binding properties than monoreactive CLL antibodies, and confirms the validity of our ELISA.
Recombinant human MALT lymphoma–derived antibodies are polyreactive. Three concentrations (0.1, 1, and 10 μg/mL) of 7 recombinant human MALT lymphoma antibodies (A) and 5 recombinant human CLL antibodies (B) were tested by ELISA for reactivity with the antigens indicated in the legends. All MALT lymphoma antibodies, with the exception of Ab2, but none of the CLL antibodies, exhibit a polyreactive binding pattern.
Recombinant human MALT lymphoma–derived antibodies are polyreactive. Three concentrations (0.1, 1, and 10 μg/mL) of 7 recombinant human MALT lymphoma antibodies (A) and 5 recombinant human CLL antibodies (B) were tested by ELISA for reactivity with the antigens indicated in the legends. All MALT lymphoma antibodies, with the exception of Ab2, but none of the CLL antibodies, exhibit a polyreactive binding pattern.
Finally, the antigen specificity of the recombinant human MALT lymphoma antibodies was confirmed by a series of competitive inhibition ELISAs (Figure 6A-C); as observed for the murine antibodies, soluble-phase antigens were able to competitively block binding to the immobilized target in a dose-dependent manner, whereas BSA did not block binding even at 100× excess (Figure 6A-C).
Competitive inhibition ELISAs confirm polyreactive binding patterns of human MALT lymphoma antibodies. (A-C) Three representative competition ELISAs performed as described in the legend to Figure 4 are shown and demonstrate the ability of different soluble targets to inhibit the binding of the recombinant antibody of interest (5, 4, and 1; used at 10 μg/mL) to solid-phase antigens. BSA did not block binding of Abs 5 and 4 even at 100× excess concentrations (A-B right panels). Error bars represent SEM.
Competitive inhibition ELISAs confirm polyreactive binding patterns of human MALT lymphoma antibodies. (A-C) Three representative competition ELISAs performed as described in the legend to Figure 4 are shown and demonstrate the ability of different soluble targets to inhibit the binding of the recombinant antibody of interest (5, 4, and 1; used at 10 μg/mL) to solid-phase antigens. BSA did not block binding of Abs 5 and 4 even at 100× excess concentrations (A-B right panels). Error bars represent SEM.
To assess the binding affinity of the various tumor Igs, we calculated Kd values for a select group of 6 tumor antibody-antigen complexes using a curve-fitting approach as described.32 The Kd values ranged from 1.1 × 10−7 M (hu Ab5 reactivity toward LPS) to 6.3 × 10−8 M (hu Ab4 toward H pylori extract). Murine tumor antibodies showed a similar range of affinity (eg, Kd = 2 × 10−8 M for mu Ab11 toward ssDNA; Kd = 5 × 10−8 M for mu Ab11 toward stomach extract). Overall, the binding affinity of polyspecific MALT lymphoma–derived antibodies is clearly lower than what is typically observed for monoreactive antibodies (which have Kds of 10−8 -10−11 M), but higher than the typical affinity of polyreactive, unmutated IgM expressed by immature B cells (Kds of 10−4-10−7 M).37
Discussion
Persistent infection of BALB/c mice with various Helicobacter species results in the development of gastric MALT lymphoma in a majority of mice, with “clinical” features that are strikingly similar to the human form of the malignancy: late onset of disease, dependence on active infection with the organisms, and a low propensity for spreading.10,21-23 We show here that several molecular parameters of the murine tumors also mimic the human disease well: (1) the monoclonal status of more than half of all individually dissected tumors, (2) the antigen dependence of tumor cell proliferation in vitro, (3) the surface exposure of IgM and apparent lack of class switch recombination, (4) the somatic hypermutation of antibody sequences, accompanied in several cases by positive and/or negative antigen selection, and (5) the intraclonal variability that is a hallmark of continuing antigen exposure and, consequently, ongoing somatic mutation. We further demonstrate that both the murine tumor-derived antibodies as well as all but 1 of the antibodies derived from MALT lymphoma patient biopsies show a pattern of polyreactivity, displaying equally strong affinity toward a diverse panel of foreign but also self-antigens. Finally, this result is corroborated by our observation of a strong bias toward the use of VH gene segments that have previously been associated with autoantibodies or polyreactive antibodies in other B-cell malignancies or autoimmune pathologies.
In the murine setting, we often find that more than 1 tumor arises per stomach, that is, the lymphoma presents as a multifocal disease (as is the case in humans).38 In those cases in which we were able to dissect multiple monoclonal tumors from the same stomach, they were usually derived from independent clones, confirming that MALT lymphoma is a highly localized disease with little tendency to spread, even within the same organ. The monoclonal status of 55% of individually dissected tumors confirms the validity of our model; clonality rates of human MALT lymphomas range from 63% to 92% of histologically confirmed cases.20,39 The lower rates of clonality in the murine model may be due to the tiny size and multifocal nature of the murine tumors, which are difficult to dissect without contamination from surrounding normal tissue as well as neighboring tumor material.
Gastric MALT lymphomas are antigen dependent in their early stages, that is, they require the constant presence of as yet unknown antigen(s) for growth in vivo.9,10,22 Interestingly, this requirement for antigen is retained in ex vivo cultures of single-cell suspensions derived from individual murine tumors, which proliferate only in the presence of Helicobacter extract. Despite the apparent complexity of tumor cell suspensions, which contained a significant proportion of admixed T cells and other leukocytes, the proliferating population consisted predominantly of (tumor) B cells.
The immunoglobulin genes of murine as well as human gastric MALT lymphomas are subject to somatic hypermutation but fail to undergo class switch recombination. Indeed, expression of the enzyme responsible for both processes, activation-induced cytidine deaminase, could be detected by reverse-transcription–polymerase chain reaction in material generated from both murine tumor and human MALT lymphomas (data not shown). Although the 2 processes are known to be linked and usually happen simultaneously during germinal center reactions, it is now widely accepted that a fraction of post–germinal center memory cells are IgM+.40 In particular, this seeming contradiction has been described multiple times in B-cell malignancies such as Burkitt lymphoma and CLL as well as in extracerebral and central nervous system diffuse large B-cell lymphoma.41,42 In MALT lymphoma, switch recombination is often “illegitimate,” that is, involving only 1 rather than 2 switch sites and leading to an aberrant rearrangement of the IgH switch region not actually accompanied by isotope switching.42 The fact that the IgVH genes of both our panels of murine and human tumor antibodies are somatically hypermutated and, at least in approximately 50% of cases, are subject to ongoing mutation suggests that antigen plays a role during the initiation and the progression of the tumor.
To examine the reactivity of 5 murine and 7 human tumor immunoglobulins with various autoantigens and foreign antigens, we recombinantly expressed them as murine and human IgG, respectively; this approach ensured that the specificity of the recombinant antibodies indeed corresponded with that of the tumor while maintaining sufficiently high avidity. Previous efforts, with the exception of one report,20 have identified a multitude of alternative targets with the shared characteristic of being autoantigens.18,19,43 For example, Bende et al found that 7 of 10 gastric and nongastric MALT lymphoma antibodies had rheumatoid factor activity, that is, they bound to auto-IgG.13
Our study confirms binding of MALT lymphoma antibodies to IgG. However, with roughly the same or an even higher affinity, our panel of antibodies also bound to a variety of other targets, including Helicobacter-derived antigens and stomach extract. The reactivity was nevertheless “specific” in the sense that binding could be blocked to a solid-phase target by an excess of an alternative, soluble target in a concentration-dependent manner. The prevalence of polyreactivity in our panel of antibodies is clearly higher than that of normal peripheral mature B cells, in which the polyreactivity rate is estimated to be less than 5%.44
Polyreactivity is not a new phenomenon in the context of B-cell malignancies. It has been described in detail for CLL, which, like MALT lymphoma, is believed to be an antigen-driven malignancy. A majority of unmutated, but not of mutated, CLL-derived antibodies tested with respect to their specificity have revealed polyreactive patterns in previous studies, binding to DNA, IgG, insulin, and LPS.36,45 In contrast, mutated CLL-derived antibodies are typically monoreactive.36,45 Indeed, none of the mutated 5 CLL antibodies we included as controls in our analysis showed evidence of polyreactivity. With respect to the link between mutational status and antigen reactivity, CLL-derived antibodies thus differ clearly from MALT lymphoma–derived antibodies. In fact, we found that the only human MALT lymphoma Ig that failed to exhibit polyreactivity was also the only unmutated antibody among those analyzed. It will be interesting to see whether the link between mutation status and polyreactive binding profile holds true in larger studies that include more of the rare unmutated cases.
Both polyreactive CLL46 and the MALT tumor immunoglobulins reported here share a strongly biased use of the IgVH1-69 gene segment; 4 of 7 of our panel of human MALT lymphoma antibodies use this segment and, of these, 3 combine the IgVH1-69 heavy chain with the same light chain VL segment IGKV3-20*01. This exact combination is typical of rheumatoid factors, with IgVH1-69 being preferentially used in autoimmune gammopathies reactive toward human IgG such as cryoglobulinemia and Waldenstrom macroglobulinemia.47,48 IgVH1-69 was also found to be used in a biased fashion in nongastric MALT lymphomas, including those of the parotid gland, tonsil, and lung.13 Overall, the strongly biased use of IgVH1-69 in MALT lymphoma and CLL is consistent with their shared polyreactive antibody specificity, as well as their shared dependence on antigenic stimulation.
Polyreactivity is thought to be caused by a special flexibility of the antigen-binding pocket, with multiple configurations or “isomers” of an antibody existing even before exposure to antigen49,50 ; some evidence suggests that a longer CDR3 region of polyreactive antibodies might be underlying this flexibility.51 Indeed, we find that our human as well as our mouse MALT lymphoma antibodies possess longer than average CDR3 regions. In conclusion, many of the known molecular and structural hallmarks of polyreactive antibodies such as IgM class, CDR3 length, and preferential VH gene use are detected in MALT lymphoma antibodies and are consistent with our experimental finding of polyreactivity, suggesting a role for antigenic stimulation in MALT lymphoma pathogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Nadal and Joe Jiricny for helpful discussions. We are grateful to Raquel Malumbres for help with calculating the probability of selective pressure using a multinomial model. Stephan Stilgenbauer is thanked for CLL patient material.
This study was funded by grants from Schweizer Nationalfonds (3100A0-113452), UBS Foundation (BA29 S8Q7-DZZ 969/A), Oncosuisse (OCS-02099-08-2007), and the Nils and Desiree Yde Stiftung (A.M.). Additional funding was supplied by the University Research Priority Program in Systems Biology/Functional Genomics and the Institute of Molecular Cancer Research at the University of Zürich (A.M.). This study was further supported by the Loewe research grant “Tumor and Inflammation,” A1 (A.N.).
Authorship
Contribution: V.J.C. designed and performed research, analyzed data, and wrote the paper; I.A. and C.G. collected experimental animal material and performed fluorescence-activated cell sorting analyses; M.Q.H., T.W., A.N., and C.R. collected patient material and extracted mRNA; S.F. contributed vital tools and helped analyze data; and A.M. designed research, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Mueller, Institute of Molecular Cancer Research, University of Zürich, Winterthurerstr 190, 8057 Zürich, Switzerland; e-mail: mueller@imcr.unizh.ch.

![Figure 1. Murine MALT lymphoma cells proliferate in response to Helicobacter antigen. (A-B) Histopathology of murine mucosa-associated lymphoid tissue (MALT) lymphoma. Consecutive paraffin sections were stained with hematoxylin and eosin (H&E), the B-cell marker B220, and the proliferation marker PCNA; representative sections are shown for an unaffected area (A) and a lymphoma (B) from the same stomach. (C-F) Single-cell suspensions of 9 murine MALT lymphomas were cultured in the presence or absence of 10 μg/mL Helicobacter felis lysate. The proportion of CD19+ B cells and CD3+ T cells was determined flow cytometrically for each culture (C). Proliferation was determined by [3H]-thymidine incorporation (D) or BrdU incorporation (E) after 5 days of culturing. Individual cultures and/or averages of all cultures are shown. Error bars represent SD. (F) The BrdU+ cells are mostly B and T cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2009-06-228015/4/m_zh89990947390001.jpeg?Expires=1767732066&Signature=zwLMthNhCyqrhMYnRuATj9Lc10LdA4H6PvmoQkb7tjQ-34XATMFwiEDIf4C5AytFzUHEDiyvJ~V3Tb~ZkRFTCpr9I0zj6E5O6r-u~Hs7SqvV91z1k~y~Eb3m~mKbSoDkDpyLzdOWD-n3Rz5M5QKonq~PxVDE7U-1ziOP6h6~ur-SC-CipDG9HClfEC7LzEuAMwvS9wHu1csd9B2aoXpC~czKPtY1gUBR5rHytsXJy7OScwlTvmI74QUK1S0oDPxBAcQ3zJs8DkJiH4oMvLM2CSYBcZeCUEEsqHA8gMeeXTTKkZhir1KHKwo0syZKHBhS-CdLQmWIUX4ow6zy1xSBeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)