To the editor:
We have read with particular interest the paper of Craig et al,1 describing that 6 of 7 human mucosa-associated lymphoid tissue (MALT) lymphoma–derived immunoglobulins (Igs) bound various antigens with intermediate affinity, thus implying that MALT lymphomas express polyreactive antigen receptors. We previously reported that MALT lymphomas frequently express Igs, which are homologous to canonical rheumatoid factors (RFs), encoded by V1-69-JH4/Vk3-20 and V3-7-JH3/Vk3-15 IgVH/IgVk rearrangements and that recombinant Igs derived of these MALT lymphomas indeed bound human IgG.2 Interestingly, we noticed that the panel of MALT lymphoma Igs produced by Craig et al also contained 3 cases with homology to V1-69 RFs (hu-1 and hu-7) and V3-7 RFs (hu-2), although it is noted that 2 of these MALT lymphoma RFs (ie hu-2 and hu-7) were not coexpressed with the canonical Vk3-20 and Vk3-15 genes as described in literature of 14 of 14 V1-69 RF and of 10 of 10 V3-7 RF cases, respectively.2-5
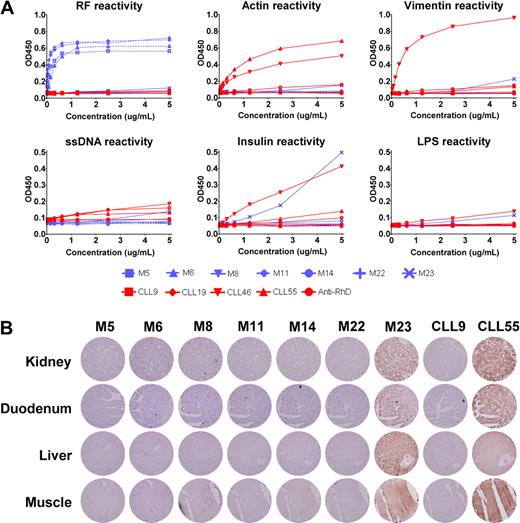
Prompted by the results by Craig et al, we tested the binding characteristics of 7 of our MALT lymphoma Igs, 4 of which were homologous to canonical RFs (ie, M5, M6, M11, and M222 ) in ELISAs. This study was conducted in accordance with the Declaration of Helsinki and the ethical standards of the research code committee on human experimentation of the Academic Medical Center of Amsterdam. As controls, we included 2 IgVH-unmutated and 2 IgVH-mutated B-cell chronic lymphocytic leukemia (CLL)–derived Igs, which are reported to be polyreactive and nonpolyreactive,6 respectively. In contrast to the data presented by Craig et al, 6 of 7 MALT lymphoma Igs, including the 4 RFs, reacted only with IgG or were nonreactive (Figure 1A), as may be expected from somatically mutated Igs. Only MALT lymphoma Ig M23 did bind several antigens. As expected, the 2 mutated CLLs were nonreactive whereas the 2 unmutated CLLs bound to essentially all antigens tested. In general, the M23 Ig showed a lower degree of polyreactivity compared with the unmutated CLL–derived Igs.
reactivity of MALT lymphoma–derived immunoglobulins. (A) Seven recombinant MALT lymphoma–derived IgMs (M5, M6, M8, M11, M14, M22, and M23), 2 unmutated CLL-derived IgMs (CLL46 and CLL55), 2 mutated CLL-derived IgMs (CLL9 and CLL19), and 1 IgM control anti-erythrocyte Rhesus D were tested for reactivity in ELISAs to IgG, ssDNA, insulin, lipopolysaccharide, actin, and vimentin, as described previously.2 The OD450 nm is plotted without subtraction of background OD450 nm. MALT lymphoma–derived Igs are represented by blue lines. Red lines represent control Igs. The 4 MALT lymphoma–derived RFs are represented by dashed blue lines. (B) Immunohistochemical stainings of human TMAs, containing 21 normal human tissues, with 7 recombinant MALT lymphoma–derived IgMs, one mutated CLL–derived IgM and one unmutated CLL–derived IgM. Displayed are kidney, duodenum, liver and muscle stained with 5 μg/mL of recombinant IgM, except for CLL55, which was used at 1 μg/mL. Staining was visualized using mouse anti–human IgM (clone MH15, Sanquin), followed by the Powervision+ detection system (ImmunoVision Technologies). Images were acquired with a Leica DM5000B microscope coupled to a Leica DFC500 camera (Leica Microsystems) at the original magnification of 200×.
reactivity of MALT lymphoma–derived immunoglobulins. (A) Seven recombinant MALT lymphoma–derived IgMs (M5, M6, M8, M11, M14, M22, and M23), 2 unmutated CLL-derived IgMs (CLL46 and CLL55), 2 mutated CLL-derived IgMs (CLL9 and CLL19), and 1 IgM control anti-erythrocyte Rhesus D were tested for reactivity in ELISAs to IgG, ssDNA, insulin, lipopolysaccharide, actin, and vimentin, as described previously.2 The OD450 nm is plotted without subtraction of background OD450 nm. MALT lymphoma–derived Igs are represented by blue lines. Red lines represent control Igs. The 4 MALT lymphoma–derived RFs are represented by dashed blue lines. (B) Immunohistochemical stainings of human TMAs, containing 21 normal human tissues, with 7 recombinant MALT lymphoma–derived IgMs, one mutated CLL–derived IgM and one unmutated CLL–derived IgM. Displayed are kidney, duodenum, liver and muscle stained with 5 μg/mL of recombinant IgM, except for CLL55, which was used at 1 μg/mL. Staining was visualized using mouse anti–human IgM (clone MH15, Sanquin), followed by the Powervision+ detection system (ImmunoVision Technologies). Images were acquired with a Leica DM5000B microscope coupled to a Leica DFC500 camera (Leica Microsystems) at the original magnification of 200×.
To further explore the reactivity of the MALT lymphoma Igs, we used the recombinant Igs in immunohistochemical stainings of tissue microarrays (TMAs) containing 21 paraffin-embedded normal human tissues. None of the MALT lymphoma Igs reacted with any of the tissues on the TMA, except for M23, which showed broad reactivity (Figure 1B). Similarly, the unmutated CLL Igs stained all the tissues tested, even at low concentrations. It is noted that the polyreactive MALT lymphoma M23 differs from the other MALT lymphomas in that it harbors a t(11;18)(API-MALT). This chromosomal translocation results in the constitutive activation of the nuclear factor κB (NF-κB) pathway, potentially rendering the cells independent of antigen receptor signals and thus disturbing the process of antigenic selection.
Our finding that MALT lymphoma RFs are monoreactive, is concordant with several papers in which V1-69/Vk3-20 RFs are compared with unmutated V1-69–encoded CLL Igs, demonstrating that mutated V1-69/Vk3-20 RFs are nonpolyreactive, whereas unmutated V1-69 CLL Igs show low-affinity binding to multiple antigens, including IgG.7,8
We conclude that V1-69/Vk3-20 and V3-7/Vk3-15 MALT lymphoma–derived Igs are ligand-selected, monospecific high-affinity antibodies.
Authorship
Acknowledgment: The authors thank A. R. Musler for generating the tissue microarrays.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carel J. M. van Noesel, MD, PhD, Department of Pathology, AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: c.j.vannoesel@amc.uva.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal