Abstract
The tumor suppressor gene phosphatase and tensin homolog (PTEN) is inactivated in many human cancers. However, it is unknown whether PTEN functions as a tumor suppressor in human Philadelphia chromosome–positive leukemia that includes chronic myeloid leukemia (CML) and B-cell acute lymphoblastic leukemia (B-ALL) and is induced by the BCR-ABL oncogene. By using our mouse model of BCR-ABL–induced leukemias, we show that Pten is down-regulated by BCR-ABL in leukemia stem cells in CML and that PTEN deletion causes acceleration of CML development. In addition, overexpression of PTEN delays the development of CML and B-ALL and prolongs survival of leukemia mice. PTEN suppresses leukemia stem cells and induces cell-cycle arrest of leukemia cells. Moreover, PTEN suppresses B-ALL development through regulating its downstream gene Akt1. These results demonstrate a critical role of PTEN in BCR-ABL–induced leukemias and suggest a potential strategy for the treatment of Philadelphia chromosome–positive leukemia.
Introduction
The human Philadelphia chromosome (Ph) arises from a reciprocal translocation between chromosome 9 and 22, resulting in the formation of chimeric BCR-ABL oncogene. BCR-ABL encodes a constitutively activated, oncogenic tyrosine kinase.1 Ph+ leukemia induced by BCR-ABL includes chronic myeloid leukemia (CML) and B-cell acute lymphoid leukemia (B-ALL). The BCR-ABL kinase inhibitor imatinib mesylate induces a complete hematologic and cytogenetic response in the majority of chronic-phase CML patients2 but is unable to completely eradicate BCR-ABL–expressing leukemic cells,3,4 suggesting that leukemia stem cells are not eliminated. Over time, patients frequently become drug-resistant and develop progressive disease despite continued treatment.5-7 Moreover, B-ALL is less sensitive to imatinib, suggesting that inhibition of BCR-ABL kinase activity is not enough to suppress B-ALL development. New therapeutic strategies need to be developed for Ph+ leukemia.
Tumors progress to more advanced stages after acquiring additional genetic alterations, and inactivation of tumor suppressor genes are common in human cancers. Phosphatase and tensin homolog (PTEN)8 is often deleted or inactivated in many tumor types, including glioblastoma,9 endometrial carcinoma,10 and lymphoid malignancies.11 PTEN is a phosphatase that dephosphorylates phosphatidylinositol-3-trisphosphate.12,13 Phosphatidylinositol-3-trisphosphate is a direct product of phosphoinositide 3-kinase (PI3K) activity and plays a critical role in the regulation of cell survival and growth by activating the Ser/Thr protein kinase PDK1 and its downstream target Akt.14,15 Activated Akt mediates several well-described PI3K responses that include cell survival and growth, cellular metabolism, angiogenesis, and cell migration.
Mice with a complete null mutation of Pten develop early embryonic lethality at E9.5.16-18 Pten-heterozygous mice die within 1 year after birth, and survivors develop a broad range of tumors, including mammary, thyroid, endometrial, and prostate cancers,16-18 as well as autoimmunity related to Fas-mediated response.19 Mice with the tissue-specific deletion of Pten using the Cre-loxP system have become available for studying physiologic functions of Pten in adult tissues and organs.20,21 For example, mice with Pten deletion in adult hematopoietic cells develop and die of acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).22
Akt1 is a major downstream signaling molecule of PTEN and is activated after PTEN is mutated in human cancers. Investigators in a recent study23 showed that the deficiency of Akt1 is sufficient to suppress the development of several types of tumors in Pten-heterozygous mice, including prostate cancer, endometrial carcinoma, thyroid neoplasia, intestinal polyps, and lymphoid hyperplasia. Moreover, rapamycin, which directly inhibits the Akt downstream molecule mammalian target of rapamycin (mTOR), effectively inhibits survival and proliferation of AML cells from PTENfl/fl;Mx-1-Cre AML mice and prolongs the survival of these diseased mice.24 Together, these results demonstrate a crucial role of the PI3K-PTEN-Akt pathway in cancer development. In this study, we investigated the role of Pten in the development of BCR-ABL–induced CML and B-ALL in mice. We also tested the effect of Pten on leukemia stem cells (LSCs) and studied the role of Akt1 as a Pten downstream signaling molecule in B-ALL development. Furthermore, we evaluated the potential role of targeting the Akt1-mTOR pathway in the treatment of BCR-ABL–induced leukemia.
Methods
Cell lines
Ba/F3 pre-B cells were grown in RPMI 1640 medium containing 10% fetal calf serum (FCS), 10% WEHI medium, and 50μM 2-mercaptoethanol. Ba/F3-BCR-ABL, parental ENU, and ENU-BCR-ABL cells were grown in RPMI 1640 medium containing 10% FCS, and 50μM 2-mercaptoethanol.
Mice
C57BL/6J, B6.129S4-Ptentm1Hwu/J (Ptenfl/fl) and B6.129P2-Akt1tm1Mbb/J (Akt1−/−) mice were obtained from The Jackson Laboratory. Mice were maintained in a temperature- and humidity-controlled environment and given unrestricted access to 6% chow diet and acidified water. All animal studies were approved by the University of Massachusetts Institutional Animal Use and Care Committee.
Antibodies and Western blot analysis
Antibodies against c-Abl (sc-131), p-Tyr (sc-508), PTEN (sc-7974), p53 (sc-6243), and actin (sc-1616-R) were purchased from Santa Cruz Biotechnology. Cre (cat. no. 69 050) antibody was ordered from Novagen. Protein lysates were prepared by lysing cells in radioimmunoprecipitation buffer, and immunoprecipitation and Western blotting were carried out as described previously.25
Construction of triple-gene coexpression plasmids
The original MSCV-IRES-GFP vector was first modified to add new cloning sites for the restriction enzymes MfeI, NotI, and MluI. To do so, the internal ribosomal entry segment (IRES) sequence was first amplified by the murine stem cell virus (MSCV) primer (CGTCTCTCCCCCTTGAACCTCCTCG) and the IRES-MfeI primer (CATGCCATGGCAATTGAGCGGCCGCTTGTGGCCATATTATCATC), which contains the new MfeI, NotI, and the existing NcoI sites (underlined). This step allowed us to synthesize a new IRES fragment containing the MfeI, NotI, and NcoI sites. To replace the original IRES sequence in the original MSCV-IRES-GFP vector with the newly synthesized IRES, this vector was cut with EcoRI and NcoI, and then the new IRES fragment was cloned into the MSCV-IRES-GFP cut with EcoRI and NcoI, forming a new MSCV-IRES-GFP vector that contains 2 additional sites, MfeI and NotI. To add the MluI site to the new MSCV-IRES-GFP vector, an IRES-GFP fragment was amplified from this vector by the MSCV primer and the GFP-MluI primer (CCATCGATACGCGTAAGCTTGGCTGCAGGTCGA), which contains the existing ClaI and the new MluI sites (underlined). The synthesized IRES-GFP fragment was digested with EcoRI and ClaI and then cloned into new MSCV-IRES-GFP vector between the EcoRI and ClaI sites to generate the final MSCV-IRES-GFP vector. Compared with the original MSCV-IRES-GFP vector, this final MSCV-IRES-GFP vector contains additional sites MfeI, NotI (before the GFP sequence), and MluI (after the GFP sequence). To clone the BCR-ABL cDNA into this final MSCV-IRES-GFP vector, BCR-ABL was cloned into it at the EcoRI site.
To make the MSCV-BCR-ABL-PTEN-GFP construct, total RNA was isolated from C57BL/6 mice liver tissue to synthesize the Pten cDNA by reverse-transcription polymerase chain reaction (RT-PCR). The Pten cDNA was amplified by PTEN-NotI (5′ AGCGGCCGCATGACAGCCATCATCAAAGAG 3′) and PTEN-MluI (5′ CGACGCGTTCAGACTTTTGTAATTTGTG 3′) primers. The cDNA was sequenced from both ends to confirm the sequence. The Pten cDNA was cloned into the MSCV-BCR-ABL-GFP vector between NotI and MluI sites. The IRES-GFP fragment was amplified by MSCV-MluI (cgacgcgtAATTCCGCCCCTCTCCCTC) and GFP-MluI (ccacgcgtTAAGCTTGGCTGCAGGTCGA) primers by use of MSCV-GFP as a template, and the IRES-GFP fragment was inserted after the Pten sequence at the MluI site.
To make MSCV-BCR-ABL-iCre-GFP construct, the iCre (improved Cre) open reading frame (ORF) was amplified by iCre-MfeI (CGCAATTGATGGTGCCCAAGAAGAAGAGG), and iCre-ClaI (CCATCGATTCAGTCCCCATCCTCGAGCAG) by use of the pBOB-CAG-iCre-SD (Addgene) as a template. The iCre ORF was cloned into the MSCV-BCR-ABL vector between NotI and MluI sites, and the IRES-GFP fragment was cloned at the MluI site after the iCre ORF.
Whitlock-Witte culture
Bone marrow cells were transduced with BCR-ABL retrovirus and then cultured in a 6-well plate in RPMI 1640 medium containing 10% FCS and 50μM 2-mercaptoethanol for 1 week. Protein lysates were collected and analyzed by Western blotting.
Bone marrow transduction/transplantation
The retroviral constructs MSCV-GFP,24 BCR-ABL-PTEN-GFP, or BCR-ABL-iCre-GFP carrying the BCR-ABL cDNA were used to make high-titer, helper-free, replication-defective ecotropic viral stocks by transient transfection of 293T cells by use of the kat system as previously described.19 Then, 6- to 10-week-old wild-type C57BL/6 and Ptenfl/fl (The Jackson Laboratory) mice were used for leukemogenesis experiments. Induction of CML and B-ALL19 was described previously. In brief, to induce CML, bone marrow cells from 5-FU–treated (200 mg/kg) donor mice were transduced twice with BCR-ABL retrovirus by cosedentation in the presence of interleukin-3, interleukin-6, and stem cell factor. To induce B-ALL, bone marrow cells from non–5-FU–treated donors were transduced with BCR-ABL in the absence of any cytokines. Wild-type recipient mice were prepared by 1150 cGy gamma irradiation. A dose of 0.5 × 106 (CML) or 1.0 × 106 (B-ALL) cells was transplanted via tail vein injection. Diseased mice were analyzed by histopathologic and biochemical analyses as described previously.25
Flow cytometry
Hematopoietic cells were collected from peripheral blood and bone marrow of the disease mice, and red blood cells were lysed with NH4Cl red blood cell lysis buffer (pH 7.4). The cells are washed with phosphate-buffered saline and stained with B220-PE for B cells and Gr1-APC for neutrophils, Sca1-APC/c-Kit-PE for hematopoietic stem cells, and Hoechst blue for DNA. After staining, the cells were washed once with phosphate-buffered saline and subjected to fluorescence-activated cell sorting (FACS) analysis.
Chip
Chromatin immunoprecipitation (Chip) assay was performed by use of the Chip-TI Express kit following the manufacturer's instructions (Active Motif). In brief, BaF3 cells were lysed and chromatin was broken from 200-bp into 500-bp fragments by sonication. Next, genomic DNA was incubated with anti-rabbit immune IgG (a negative control) or anti-p53 antibody overnight, and then protein G Sepharose beads preincubated with bovine serum albumin were added to pull down DNA fragments bound to anti-p53 antibody. Recovered bound DNA was used as templates for PCR amplification of p53 binding site on the Pten promoter with the primers 5′-CAAAGCCGGCGTAGCTC-3′ and 5′-ACAAAGAGTCCCGCCACAT-3′.
In vitro culture of LSCs
Bone marrow cells isolated from CML mice were cultured in vitro in the presence of stemspan SFEM, stem cell factor, insulin-like growth factor-2, thrombopoietin, heparin, and α-fibroblast growth factor as described previously.25
Drug treatment
Imatinib was dissolved in water directly at a concentration of 10 mg/mL and administered orally by gavage in a volume less than 0.5 mL twice a day at 100 mg/kg body weight, beginning 8 days after bone marrow transplantation (BMT) and continuing until the morbidity or death of leukemic mice. Rapamycin (Calbiochem) was dissolved in dimethyl sulfoxide (DMSO) to make 1mM stock solution before it was diluted in culture medium.
Results
PTEN expression is down-regulated by BCR-ABL
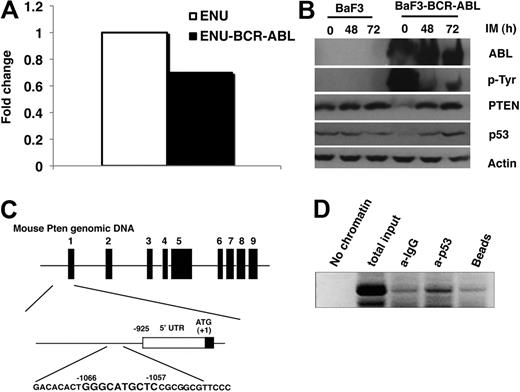
We performed a global gene expression analysis by using DNA microarray to identify genes regulated by BCR-ABL in a BCR-ABL–expressing mouse pre–B-cell line (ENU-BCR-ABL cells).26 Comparing with non–BCR-ABL–expressing parental cells, we observed a 1.43-fold decrease in the level of Pten mRNA (Figure 1A). The down-regulation of Pten by BCR-ABL was further confirmed by Western blotting in BCR-ABL–expressing Ba/F3 cells (Ba/F3-BCR-ABL; Figure 1B). We also treated Ba/F3 and Ba/F3-BCR-ABL cells with the BCR-ABL kinase inhibitor imatinib and found that imatinib did not have an effect on Pten expression in Ba/F3 cells but caused the restoration of Pten protein expression back to its endogenous level in Ba/F3-BCR-ABL cells (Figure 1B), indicating that this Pten down-regulation is dependent upon BCR-ABL kinase activity.
Down-regulation of Pten by BCR-ABL. (A) Total mRNA was isolated from parental ENU and ENU-BCR-ABL cells for DNA microarray analysis. The level of Pten mRNA was lower in BCR-ABL–expressing ENU cells than in parental ENU cells. (B) Pten protein level was also lower in Ba/F3-BCR-ABL cells than in parental Ba/F3 cells. Parental Ba/F3 and Ba/F3-BCR-ABL cells were treated with imatinib (IM; 1μM) for 48 and 72 hours, respectively. Protein lysates were analyzed by Western blot by the use of antibodies indicated. Independent experiments were repeated 3 times. (C) The mouse Pten promoter contains only one p53 binding site. (Top) Schematic representation of the mouse Pten genomics locus (GenBank accession number NM_008960). The exons are indicated by the black bars 1 to 9. (Middle) Region directly upstream of the Pten translation start site. The positions of oligonucleotide probes used for mapping the transcription stat site by PCR are indicated. (Bottom) Nucleotide sequence of the p53 binding site identified based on human p53 binding sequence.27 (D) p53 binds to Pten promoter directly. Chip was performed in BaF3 cells to show the binding of p53 to the Pten promoter as described in the Methods section.
Down-regulation of Pten by BCR-ABL. (A) Total mRNA was isolated from parental ENU and ENU-BCR-ABL cells for DNA microarray analysis. The level of Pten mRNA was lower in BCR-ABL–expressing ENU cells than in parental ENU cells. (B) Pten protein level was also lower in Ba/F3-BCR-ABL cells than in parental Ba/F3 cells. Parental Ba/F3 and Ba/F3-BCR-ABL cells were treated with imatinib (IM; 1μM) for 48 and 72 hours, respectively. Protein lysates were analyzed by Western blot by the use of antibodies indicated. Independent experiments were repeated 3 times. (C) The mouse Pten promoter contains only one p53 binding site. (Top) Schematic representation of the mouse Pten genomics locus (GenBank accession number NM_008960). The exons are indicated by the black bars 1 to 9. (Middle) Region directly upstream of the Pten translation start site. The positions of oligonucleotide probes used for mapping the transcription stat site by PCR are indicated. (Bottom) Nucleotide sequence of the p53 binding site identified based on human p53 binding sequence.27 (D) p53 binds to Pten promoter directly. Chip was performed in BaF3 cells to show the binding of p53 to the Pten promoter as described in the Methods section.
We next tested whether Pten down-regulation by BCR-ABL correlates with p53 degradation. In BCR-ABL–expressing Ba/F3 cells, the level of p53 was lower than that in parental Ba/F3 cells, and this reduced p53 level was reversed after imatinib treatment (Figure 1B). This result suggests that BCR-ABL might down-regulate Pten through P53. There are 2 p53 binding sites on human Pten promoter, and p53 positively regulates Pten by binding to these 2 sites.27 BCR-ABL causes down-regulation of p53 in leukemia cells through the up-regulation of MDM2 that inhibits p53 transcriptional activation and promotes p53 export and proteasome-dependent degradation in the cytoplasm.28 In mouse, we only found one p53 binding site on Pten promoter (Figure 1C), and we performed the Chip assay and showed that p53 bound to Pten promoter in Ba/F3 cells (Figure 1D).
PTEN deletion causes acceleration of CML development
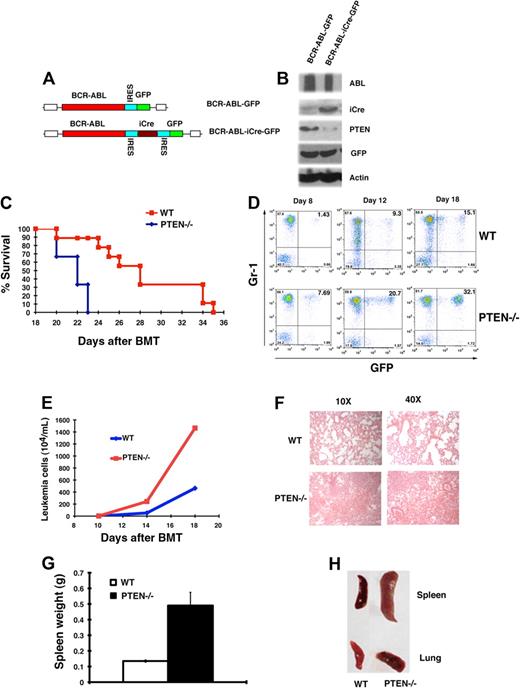
Because Pten was down-regulated by BCR-ABL (Figure 1), we tested whether Pten functions as a tumor suppressor in CML development by using Pten conditional knock mice (Ptenfl/fl). To delete Pten from bone marrow cells of Ptenfl/fl mice, we transduced the cells with BCR-ABL-iCre-GFP retrovirus or BCR-ABL-GFP retrovirus as a control (Figure 2A). Western blot analysis showed expression of iCre and a significant decrease of the Pten protein level (Figure 2B), indicating that the Pten gene was deleted from the cells. To test whether deletion of Pten affects CML development, we transduced bone marrow cells from Ptenfl/fl mice with BCR-ABL-iCre-GFP or BCR-ABL-GFP retrovirus, followed by transplantation of the transduced cells into lethal irradiated recipient mice. Mice receiving donor bone marrow cells transduced with BCR-ABL-iCre-GFP developed CML much faster than those receiving bone marrow cells transduced with BCR-ABL-GFP (Figure 2C; P < .005). In these CML disease mice, the majority of GFP cells were Gr1+ but not B220+ leukemia cells (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The accelerated death of CML mice in the absence of Pten correlated with a greater percentage of GFP+Gr1+ myeloid leukemia cells (Figure 2D) and a greater number of leukemia cells (Figure 2E) in peripheral blood of the mice. Accelerated CML development in the absence of Pten also correlated with more severe infiltration of leukemia cells in the lungs (Figure 2F,H) and splenomegaly (Figure 2G-H). These results demonstrated that Pten is a potent tumor suppressor in BCR-ABL–induced CML.
Pten deletion accelerates CML development. (A) Structure of BCR-ABL-iCre-GFP retroviral construct. (B) BCR-ABL-GFP and BCR-ABL-iCre-GFP retrovirus transduced bone marrow cells from PTENfl/fl mice were cultured under the Whitlock-Witte conditions for 1 week. Protein lysates were analyzed by Western blotting with the antibodies indicated. iCre-induced deletion of the Pten gene resulted in the removal of Pten protein. (C) Kaplan-Meier-style survival curves for recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from wild-type (WT; n = 6) or PTENfl/fl (PTEN; n = 9) mice (P < .005). (D) The percentage of leukemia cells (GFP+Gr1+) in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from Ptenfl/fl mice was greater than that in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from wild-type mice. (E) The total number of leukemia cells (total white blood cell count × percentage of GFP+Gr1+ cells) in the peripheral blood of recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from PTENfl/fl mice (PTEN) was greater than that in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from wild-type mice (WT). (F) Photomicrographs of hematoxylin and eosin-stained lung sections from recipients of bone marrow cells from PTEN-deficient CML mice (Pten−/−) showed more severe infiltration of the lungs with myeloid leukemia cells than recipients of bone marrow cells from wild-type mice (WT) at day 14 after BMT. (G) Spleen weight of recipients of wild-type (WT) or Ptenfl/fl (Pten−/−) bone marrow cells transduced with BCR-ABL-iCre-GFP retrovirus at day 14 after BMT (P = .028). (H) Gross appearance of the lungs and spleens showed severe lung hemorrhages and splenomegaly in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from PTENfl/fl CML mice (Pten−/−) than in recipients of the transduced wild-type (WT) bone marrow cells.
Pten deletion accelerates CML development. (A) Structure of BCR-ABL-iCre-GFP retroviral construct. (B) BCR-ABL-GFP and BCR-ABL-iCre-GFP retrovirus transduced bone marrow cells from PTENfl/fl mice were cultured under the Whitlock-Witte conditions for 1 week. Protein lysates were analyzed by Western blotting with the antibodies indicated. iCre-induced deletion of the Pten gene resulted in the removal of Pten protein. (C) Kaplan-Meier-style survival curves for recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from wild-type (WT; n = 6) or PTENfl/fl (PTEN; n = 9) mice (P < .005). (D) The percentage of leukemia cells (GFP+Gr1+) in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from Ptenfl/fl mice was greater than that in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from wild-type mice. (E) The total number of leukemia cells (total white blood cell count × percentage of GFP+Gr1+ cells) in the peripheral blood of recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from PTENfl/fl mice (PTEN) was greater than that in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from wild-type mice (WT). (F) Photomicrographs of hematoxylin and eosin-stained lung sections from recipients of bone marrow cells from PTEN-deficient CML mice (Pten−/−) showed more severe infiltration of the lungs with myeloid leukemia cells than recipients of bone marrow cells from wild-type mice (WT) at day 14 after BMT. (G) Spleen weight of recipients of wild-type (WT) or Ptenfl/fl (Pten−/−) bone marrow cells transduced with BCR-ABL-iCre-GFP retrovirus at day 14 after BMT (P = .028). (H) Gross appearance of the lungs and spleens showed severe lung hemorrhages and splenomegaly in recipients of BCR-ABL-iCre-GFP–transduced bone marrow cells from PTENfl/fl CML mice (Pten−/−) than in recipients of the transduced wild-type (WT) bone marrow cells.
Ptenfl/fl;Mx-1-Cre mice develop AML 20 days after PIPC treatment that initiates the deletion of Pten.24 We wondered whether the mice receiving donor bone marrow cells transduced with BCR-ABL-iCre-GFP developed AML, which may contribute to the accelerated death of CML mice in the absence of Pten (Figure 1C). We found that these mice developed typical CML (Figure 2), and we did not observe any signs for AML development (data not shown). To further rule out the possible contribution of AML to the accelerated death of CML mice in the absence of Pten, we transduced normal bone marrow cells from PTENfl/fl mice with MSCV-iCre-GFP retrovirus to delete Pten, followed by transplantation of the transduced cells into the lethal irradiated recipient mice. Although 20% white blood cells in peripheral blood of the recipient mice were GFP+, indicating that the iCre gene was expressed in the cells, none of these mice developed AML, and all mice survived (supplemental Figure 2). This result suggests that the deletion of Pten in non–BCR-ABL–expressing bone marrow cells is insufficient to induce AML in our bone marrow transduction/transplantation model system.
PTEN overexpression delays CML development
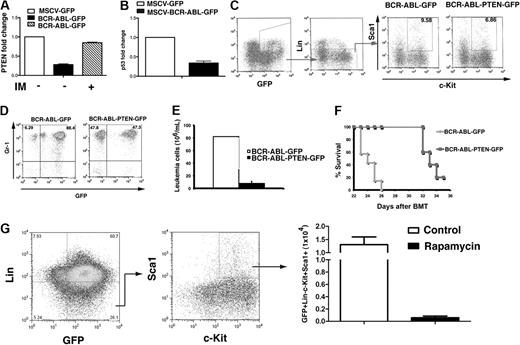
CML developed faster in the absence of Pten (Figure 2), indicating that Pten is a tumor suppressor in BCR-ABL–induced leukemia. To further test this idea, we examined whether overexpression of PTEN delays CML development. We cloned the Pten gene into the BCR-ABL-GFP construct for simultaneous expression of the 3 genes, BCR-ABL, Pten, and GFP (Figure 3A). Western blot analysis showed that this triple-gene retroviral construct allowed overexpression of Pten in cells (Figure 3B). We next transduced donor bone marrow cells from wild-type mice with BCR-ABL-PTEN-GFP or BCR-ABL-GFP retrovirus, followed by transplantation of the transduced cells into recipient mice. CML development was significantly slower in mice receiving bone marrow cells transduced with BCR-ABL-PTEN-GFP than in those receiving bone marrow cells transduced with BCR-ABL-GFP (Figure 3C, P < .001), indicating that Pten overexpression caused a delay of CML development. The delayed CML development correlated with a less percentage and number of leukemia cells in peripheral blood (Figures 3D,E), and also with less severe splenomegaly (Figure 3F) and infiltration of leukemia cells in the lungs (Figure 3G). These results further support the role of Pten as a tumor suppressor in CML development.
Overexpression of Pten delays CML development. (A) Structure of BCR-ABL-PTEN-GFP retroviral construct. (B) Western blot analysis shows expression of BCR-ABL, PTEN, and GFP from BCR-ABL-PTEN-GFP retrovirus. NIH3T3 cells were transduced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP retrovirus for 3 hours. Then, 2 days later, protein lysates were analyzed be Western blotting by the use of the antibodies indicated. (C) Overexpression of Pten alone or in combination imatinib treatment prolongs survival of CML mice. Mice with CML induced with BCR-ABL-GFP (n = 20) or BCR-ABL-PTEN-GFP (n = 20) were treated with a placebo (n = 7) or imatinib (n = 7, 100 mg/kg, twice a day by gavage), beginning at day 8 after transplantation. (D) Flow cytometry analysis showed a slower accumulation of GFP+Gr1+ leukemia cells in peripheral blood of recipients of BCR-ABL-PTEN-GFP–transduced bone marrow cells than that in recipients of BCR-ABL-GFP–transduced bone marrow cells. (E) CML was induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP, and the difference in peripheral blood leukemia cell counts (white blood cell count × the percentage of GFP+Gr1+ cells) in CML mice treated with a placebo or imatinib was determined at day 20 after BMT. (F) Spleen weight of CML mice induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP. (G) Photomicrographs of hematoxylin and eosin–stained lung sections from mice with CML induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP at day 20 after transplantation. (H) At day 20 after BMT, peripheral blood cells were stained with Gr1 and Hoechst blue. The S + G2M phase of leukemia cells (GFP+Gr1+) was represented by the percentage of Hoechst blue–positive cells. Mean percentage for each cell population (n = 3) was shown. (I) At day 20 after BMT, peripheral blood cells were stained with Gr1, annexin V, and propidium iodide (PI). Apoptotic leukemia cells were represented by the GFP+Gr1+annexinV+PI+ population. Mean percentage for each cell population (n = 3) was shown.
Overexpression of Pten delays CML development. (A) Structure of BCR-ABL-PTEN-GFP retroviral construct. (B) Western blot analysis shows expression of BCR-ABL, PTEN, and GFP from BCR-ABL-PTEN-GFP retrovirus. NIH3T3 cells were transduced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP retrovirus for 3 hours. Then, 2 days later, protein lysates were analyzed be Western blotting by the use of the antibodies indicated. (C) Overexpression of Pten alone or in combination imatinib treatment prolongs survival of CML mice. Mice with CML induced with BCR-ABL-GFP (n = 20) or BCR-ABL-PTEN-GFP (n = 20) were treated with a placebo (n = 7) or imatinib (n = 7, 100 mg/kg, twice a day by gavage), beginning at day 8 after transplantation. (D) Flow cytometry analysis showed a slower accumulation of GFP+Gr1+ leukemia cells in peripheral blood of recipients of BCR-ABL-PTEN-GFP–transduced bone marrow cells than that in recipients of BCR-ABL-GFP–transduced bone marrow cells. (E) CML was induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP, and the difference in peripheral blood leukemia cell counts (white blood cell count × the percentage of GFP+Gr1+ cells) in CML mice treated with a placebo or imatinib was determined at day 20 after BMT. (F) Spleen weight of CML mice induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP. (G) Photomicrographs of hematoxylin and eosin–stained lung sections from mice with CML induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP at day 20 after transplantation. (H) At day 20 after BMT, peripheral blood cells were stained with Gr1 and Hoechst blue. The S + G2M phase of leukemia cells (GFP+Gr1+) was represented by the percentage of Hoechst blue–positive cells. Mean percentage for each cell population (n = 3) was shown. (I) At day 20 after BMT, peripheral blood cells were stained with Gr1, annexin V, and propidium iodide (PI). Apoptotic leukemia cells were represented by the GFP+Gr1+annexinV+PI+ population. Mean percentage for each cell population (n = 3) was shown.
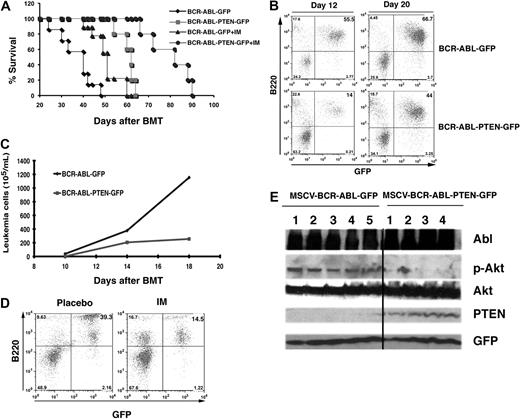
To evaluate whether PTEN overexpression in BCR-ABL–expressing cells synergizes with the therapeutic effect of imatinib on CML, we treated mice receiving bone marrow cells transduced with BCR-ABL-PTEN-GFP or BCR-ABL-GFP retrovirus with imatinib. As expected, imatinib treatment prolonged survival of CML mice receiving bone marrow cells transduced with BCR-ABL-GFP (Figure 3C; P < .001). However, imatinib-treated CML mice receiving bone marrow cells transduced with BCR-ABL-PTEN-GFP lived significantly longer than those not treated with imatinib (Figure 3C; P < .001). The synergistic effect of Pten overexpression with imatinib treatment correlated with less leukemia cells in peripheral blood of the mice (Figure 3E). To explain how Pten reduced proliferation of leukemia cells, we performed the DNA content analysis to examine the effect of Pten overexpression on cell-cycle progression of these cells. We showed that the percentage of leukemia cells in the S + G2M phase was much lower in leukemia cells with Pten overexpression than in those without Pten overexpression (Figure 3H; P < .01), indicating that Pten inhibits the proliferation of leukemia cells by inducing a cell-cycle arrest. Furthermore, we examined whether Pten induces apoptosis of leukemia cells by staining the cells with PI and annexin V. Corresponding to the result in the cell-cycle analysis, apoptosis in leukemia cells with Pten overexpression was more severe than in those without Pten overexpression (Figure 3I; P < .05).
PTEN suppresses CML stem cells
CML is derived from hematopoietic stem cells harboring the BCR-ABL oncogene.29 It is possible that Pten suppresses CML stem cells, resulting in acceleration of CML when deleted (Figure 2) and delay of CML when overexpressed (Figure 3). We have previously identified BCR-ABL–expressing Lin−c-kit+Sca1+ cells as LSCs in CML induced by BCR-ABL in mice.26 To test whether Pten expression is affected by BCR-ABL in LSCs, GFP+Lin−c-kit+Sca1+ cells were sorted by FACS from CML mice treated with a placebo or imatinib, and total RNA was isolated for DNA microarray analysis. The microarray study showed that Pten mRNA was significantly down-regulated approximately 3.59-fold by BCR-ABL, and this down-regulation was restored upon imatinib treatment (Figure 4A; P < .001). Correlating with Pten down-regulation in LSCs, p53 was also down-regulated approximately 2.9-fold by BCR-ABL in LSCs (data not shown). These results further support our observations in BaF3-BCR-ABL cells (Figure 1).
Pten suppresses leukemia stem cells. (A) BCR-ABL down-regulates Pten expression, and this down-regulation is abolished upon imatinib treatment. Bone marrow cells were transduced with GFP or BCR-ABL-GFP retrovirus, followed by transplantation into recipient mice. Some recipients of BCR-ABL-GFP–transduced bone marrow cells were treated with imatinib (100 mg/kg, twice a day by gavage), beginning at day 8 after BMT. At 24 hours later, GFP+Lin−c-Kit+Sca1+ cells in bone marrow were sorted from these mice by FACS, and total RNA was isolated for DNA microarray assay. (B) DNA microarray assay shows that the mRNA level of p53 was down-regulated by BCR-ABL in LSCs. (C) Bone marrow cells were isolated from mice with CML induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP. The percentage of GFP+Lin−c-Kit+Sca1+ cells in bone marrow was analyzed by FACS. (D) At day 20 after BMT, the percentages of GFP+Gr1+ leukemia cells in peripheral blood of recipients of bone marrow cells transduced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP were analyzed by FACS. (E) Total numbers of leukemia cells in peripheral blood of recipients of bone marrow cells transduced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP were analyzed by FACS. (F) Pten overexpression reduces the ability of leukemia stem cells to induce CML. Bone marrow cells from mice with CML induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP were sorted by lineage-depletion MACS columns (Miltenyi Biotec), followed by FACS analysis for the percentages of c-Kit+Sca1+ cells. After normalization, the same number (3 × 104) of GFP+Lin−c-Kit+Sca1+ cells from each group was transferred into recipient mice (BCR-ABL-GFP, n = 7; BCR-ABL-PTEN-GFP, n = 5) to induce CML. (G) Rapamycin inhibits leukemia stem cells from CML mice in vitro. Bone marrow cells isolated from mice with CML induced by BCR-ABL-GFP were cultured (2 × 106 cells/6-cm plate) under the stem cell conditions (see “Methods”) in the presence of DMSO or rapamycin (10μM) for 3 days, followed by FACS analysis of leukemia stem cells (GFP+Lin−c-Kit+Sca1+).
Pten suppresses leukemia stem cells. (A) BCR-ABL down-regulates Pten expression, and this down-regulation is abolished upon imatinib treatment. Bone marrow cells were transduced with GFP or BCR-ABL-GFP retrovirus, followed by transplantation into recipient mice. Some recipients of BCR-ABL-GFP–transduced bone marrow cells were treated with imatinib (100 mg/kg, twice a day by gavage), beginning at day 8 after BMT. At 24 hours later, GFP+Lin−c-Kit+Sca1+ cells in bone marrow were sorted from these mice by FACS, and total RNA was isolated for DNA microarray assay. (B) DNA microarray assay shows that the mRNA level of p53 was down-regulated by BCR-ABL in LSCs. (C) Bone marrow cells were isolated from mice with CML induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP. The percentage of GFP+Lin−c-Kit+Sca1+ cells in bone marrow was analyzed by FACS. (D) At day 20 after BMT, the percentages of GFP+Gr1+ leukemia cells in peripheral blood of recipients of bone marrow cells transduced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP were analyzed by FACS. (E) Total numbers of leukemia cells in peripheral blood of recipients of bone marrow cells transduced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP were analyzed by FACS. (F) Pten overexpression reduces the ability of leukemia stem cells to induce CML. Bone marrow cells from mice with CML induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP were sorted by lineage-depletion MACS columns (Miltenyi Biotec), followed by FACS analysis for the percentages of c-Kit+Sca1+ cells. After normalization, the same number (3 × 104) of GFP+Lin−c-Kit+Sca1+ cells from each group was transferred into recipient mice (BCR-ABL-GFP, n = 7; BCR-ABL-PTEN-GFP, n = 5) to induce CML. (G) Rapamycin inhibits leukemia stem cells from CML mice in vitro. Bone marrow cells isolated from mice with CML induced by BCR-ABL-GFP were cultured (2 × 106 cells/6-cm plate) under the stem cell conditions (see “Methods”) in the presence of DMSO or rapamycin (10μM) for 3 days, followed by FACS analysis of leukemia stem cells (GFP+Lin−c-Kit+Sca1+).
To test whether PTEN functions as a tumor suppressor in LSCs, we transduced bone marrow cells with BCR-ABL-PTEN-GFP or BCR-ABL-GFP retrovirus, followed by transplantation of the transduced cells into recipient mice. At 14 days after the transplantation, bone marrow cells were isolated from CML mice, and LSCs (GFP+Lin−c-kit+Sca1+) were analyzed by flow cytometry. The percentage of LSCs in mice with CML induced by BCR-ABL-PTEN-GFP was significantly lower than that in mice with CML induced by BCR-ABL-GFP (Figure 4B), indicating that Pten suppresses LSCs.
To determine whether Pten affects the function of LSCs, we compared the ability to induce CML between LSCs that expressed BCR-ABL-PTEN-GFP and those that expressed BCR-ABL-GFP. At 14 days after BMT, the same number (3 × 104) of GFP+Lin−cKit+Sca1+ cells sorted from CML mice receiving BCR-ABL-GFP or BCR-ABL-pTen-GFP transduced bone marrow cells were transferred into recipient mice. The percentages and numbers of leukemia cells in peripheral blood were monitored at day 25 after BMT. The total number of leukemia cells in CML mice receiving BCR-ABL-PTEN-GFP transduced bone marrow cells was 4-fold lower than that in CML mice receiving BCR-ABL-GFP–transduced bone marrow cells (Figure 4D), correlating with a lower percentage of leukemia cells in peripheral blood (Figure 4C). Consistent with less-severe CML induced by BCR-ABL-PTEN-GFP, the survival of mice receiving LSCs transduced with BCR-ABL-PTEN-GFP was significantly longer than that of mice receiving LSCs transduced with BCR-ABL-GFP (Figure 4E; P < .001). These results indicate that Pten suppresses the function of LSCs.
Because rapamycin suppresses AML cells in vitro and prolongs the survival of Ptenfl/fl;Mx-1-Cre AML mice, likely through inhibiting AML stem cells,24 we tested whether rapamycin also inhibits leukemia stem cells in CML. We isolated bone marrow cells from CML mice and cultured the cells under the conditions that support survival and growth of leukemia stem cells from CML mice.25 During the culture, the cells were treated with rapamycin. At 3 days after the treatment, we calculated the numbers of GFP+Lin−c-Kit+Sca-1+ cells that remained in the culture based on FACS analysis and total cell counts (Figure 4F). We showed that inhibition of mTOR by rapamycin also significantly inhibited CML stem cells in vitro.
Pten overexpression delays B-ALL development
We showed previously in this report that Pten functions as a tumor suppressor in CML development. We determined to examine whether Pten also plays a suppressive role in the development of B-ALL induced by BCR-ABL. To induce B-ALL in mice, donor bone marrow cells were transduced with BCR-ABL-PTEN-GFP or BCR-ABL-GFP retrovirus, followed by transplantation of the transduced cells into lethally irradiated recipient mice, as described previously.30 All mice receiving bone marrow cells transduced with BCR-ABL-GFP developed and died of B-ALL within 4 to 5 weeks after transplantation (Figure 5A), whereas mice receiving bone marrow cells transduced with BCR-ABL-PTEN-GFP developed B-ALL with much longer disease latency (Figure 5A, P = .004). The delayed B-ALL development correlated with a lower percentage and number of BCR-ABL–expressing B-lymphoid cells (GFP+B220+) in peripheral blood of the mice (Figures 5B,C), in which GFP+Gr1+ cells were almost undetectable (supplemental Figure 3).
Pten overexpression delays B-ALL development. (A) Overexpression of Pten alone or in combination with imatinib treatment prolonged survival of B-ALL mice. Mice with B-ALL induced with BCR-ABL-GFP (n = 10) or BCR-ABL-PTEN-GFP (n = 10) were treated with a placebo (n = 5) or imatinib (n = 5, 100 mg/kg, twice a day by gavage), beginning at day 8 after BMT. (B) FACS analysis showed a slower accumulation of GFP+B220+ leukemia cells in peripheral blood of recipients of BCR-ABL-PTEN-GFP transduced bone marrow cells than that in recipients of BCR-ABL-GFP–transduced bone marrow cells. (C) The difference in peripheral blood leukemia cell counts (white blood cell count × percentage of GFP+B220 cells) in B-ALL mice induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP was determined at day 12 or 20 after BMT. (D) Mice with B-ALL induced with BCR-ABL-PTEN-GFP were treated with a placebo or imatinib (IM). Peripheral blood leukemia cells were analyzed by FACS at day 35 after BMT. (E) Western blot analysis of spleen cell lysates for Pten overexpression and Akt phosphorylation in mice with B-ALL induced by BCR-ABL-PTEN-GFP or by BCR-ABL-GFP mice. The protein lysates were isolated from the mice at day 20 after BMT. The black line indicates that the lanes not adjacent on the same original sodium dodecyl sulfate–polyacrylamide gel electrophoresis were brought together to generate this figure.
Pten overexpression delays B-ALL development. (A) Overexpression of Pten alone or in combination with imatinib treatment prolonged survival of B-ALL mice. Mice with B-ALL induced with BCR-ABL-GFP (n = 10) or BCR-ABL-PTEN-GFP (n = 10) were treated with a placebo (n = 5) or imatinib (n = 5, 100 mg/kg, twice a day by gavage), beginning at day 8 after BMT. (B) FACS analysis showed a slower accumulation of GFP+B220+ leukemia cells in peripheral blood of recipients of BCR-ABL-PTEN-GFP transduced bone marrow cells than that in recipients of BCR-ABL-GFP–transduced bone marrow cells. (C) The difference in peripheral blood leukemia cell counts (white blood cell count × percentage of GFP+B220 cells) in B-ALL mice induced with BCR-ABL-GFP or BCR-ABL-PTEN-GFP was determined at day 12 or 20 after BMT. (D) Mice with B-ALL induced with BCR-ABL-PTEN-GFP were treated with a placebo or imatinib (IM). Peripheral blood leukemia cells were analyzed by FACS at day 35 after BMT. (E) Western blot analysis of spleen cell lysates for Pten overexpression and Akt phosphorylation in mice with B-ALL induced by BCR-ABL-PTEN-GFP or by BCR-ABL-GFP mice. The protein lysates were isolated from the mice at day 20 after BMT. The black line indicates that the lanes not adjacent on the same original sodium dodecyl sulfate–polyacrylamide gel electrophoresis were brought together to generate this figure.
To evaluate whether PTEN overexpression synergizes with imatinib in treating B-ALL mice, we treated mice receiving bone marrow cells transduced with BCR-ABL-PTEN-GFP or BCR-ABL-GFP retrovirus with imatinib. As expected, imatinib treatment prolonged the survival of B-ALL mice receiving bone marrow cells transduced with BCR-ABL-GFP (Figure 5A). However, imatinib-treated B-ALL mice receiving bone marrow cells transduced with BCR-ABL-PTEN-GFP lived significantly longer than those not treated with imatinib (Figure 5A; P = .001). The synergistic effect of Pten overexpression and imatinib treatment correlated with fewer leukemia cells in peripheral blood of the mice (Figure 5D). To examine whether Pten is overexpressed in vivo, leading to the inhibition of Akt phosphorylation, Western blot analysis of spleen cell lysates from mice with B-ALL induced by BCR-ABL-GFP or BCR-ABL-PTEN-GFP was performed. We found that Pten was undetectable and that the levels of Akt phosphorylation were high in the majority of mice with B-ALL induced by BCR-ABL-GFP. In contrast, Pten was detected and the levels of Akt phosphorylation were low in mice with B-ALL induced by BCR-ABL-PTEN-GFP (Figure 5E).
Pten delays B-ALL development through its downstream gene Akt1
The Akt pathway is downstream of Pten because Pten inactivation often results in Akt activation in human cancers.31,32 There are 3 mammalian Akt genes that share greater than 85% sequence similarity and encode the Akt isoforms 1 to 3.23 It is still unclear whether the 3 Akt isoforms possess different functional specificities in vivo. A recent study has shown that the deletion of the Akt1 gene has a dramatic inhibitory effect on the development of endometrium carcinoma, prostate cancer, thyroid tumor, and adrenal medulla tumors.23 Akt1 deficiency also inhibits the proliferation of lymphoid hyperplasia and expansion of both B- and T-cell populations in Pten+/− mice.23 In addition, the first transforming point mutation in Akt1 (E17K) has been discovered in human breast, colorectal, and ovarian cancers.8
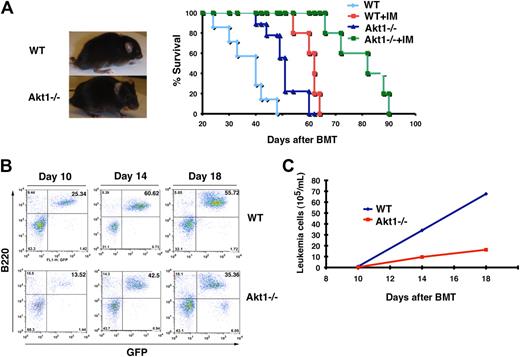
Furthermore, fetal liver cells from Eu-Myc transgenic mouse were transduced with this Akt1 (E17K), followed by transplantation into recipient mice. After 16 weeks, 6 of 10 recipients developed pre–pro-B-cell leukemia.8 Here we determined to test whether Akt1 is functionally involved in the development of B-ALL induced by BCR-ABL by using Akt1−/− mice as donors in our B-ALL mouse model. The majority of recipients of BCR-ABL–transduced wild-type bone marrow cells developed and died of B-ALL within 5 to 7 weeks (Figure 6A), whereas recipients of BCR-ABL–transduced Akt1−/− bone marrow cells developed and died of B-ALL with a significantly longer disease delay (Figure 6A; P < .005). This delayed B-ALL development caused by the Akt1 deficiency correlated with a lesser percentage and number of B-leukemia cells (B220+GFP+) in the peripheral blood of the mice (Figure 6B,C). We examined whether Akt1−/− have a defect in B-cell development because a reduction of bone marrow pro-B cells, the target cells for BCR-ABL to induce B-ALL,26 could lead to a delayed disease development. To rule out this possibility, we analyzed bone marrow cells of Akt1−/− mice by FACS and found that Akt1−/− mice have a normal percentage of pro-B cells (CD43+B220+) in bone marrow compared with wild-type mice (supplemental Figure 4).
Loss of Akt1 delays B-ALL development. (A) Gross appearance and Kaplan-Meier–style survival curves for recipients of BCR-ABL–transduced bone marrow cells from wild-type (WT) or Akt1−/− mice. B-ALL mice transduced from wild-type mice are treated with placebo (n = 20) or imatinib (IM; n = 10, 100 mg/kg, twice a day) and B-ALL mice transduced from Akt1−/− mice are also treated with placebo (n = 25) or imatinib (n = 10, 100 mg/kg, twice a day). (B) Bone marrow cells were harvested from recipients of BCR-ABL transduced wild-type or Akt1−/− bone marrow cells and were stained with antibodies against CD43 and B220 (representing pro-B cells) for FACS analysis. (C) FACS analysis showed the numbers of peripheral blood leukemia cells (GFP+B220+) in recipients of BCR-ABL-GFP–transduced wild-type or Akt1−/− bone marrow cells.
Loss of Akt1 delays B-ALL development. (A) Gross appearance and Kaplan-Meier–style survival curves for recipients of BCR-ABL–transduced bone marrow cells from wild-type (WT) or Akt1−/− mice. B-ALL mice transduced from wild-type mice are treated with placebo (n = 20) or imatinib (IM; n = 10, 100 mg/kg, twice a day) and B-ALL mice transduced from Akt1−/− mice are also treated with placebo (n = 25) or imatinib (n = 10, 100 mg/kg, twice a day). (B) Bone marrow cells were harvested from recipients of BCR-ABL transduced wild-type or Akt1−/− bone marrow cells and were stained with antibodies against CD43 and B220 (representing pro-B cells) for FACS analysis. (C) FACS analysis showed the numbers of peripheral blood leukemia cells (GFP+B220+) in recipients of BCR-ABL-GFP–transduced wild-type or Akt1−/− bone marrow cells.
Because Pten overexpression synergizes with imatinib in treating B-ALL mice (Figure 5A), we examined whether the Akt1 deficiency also synergizes with imatinib in treating B-ALL. We treated mice receiving wild-type or Akt1−/− bone marrow cells transduced with BCR-ABL-GFP retrovirus with imatinib. As expected, imatinib treatment prolonged survival of B-ALL mice receiving BCR-ABL–transduced wild-type bone marrow cells, whereas imatinib treatment more significantly improved survival of B-ALL mice receiving BCR-ABL-GFP–transduced Akt1−/− bone marrow cells (Figure 6A; P < .001).
Rapamycin inhibits proliferation and induces apoptosis of human CML cells
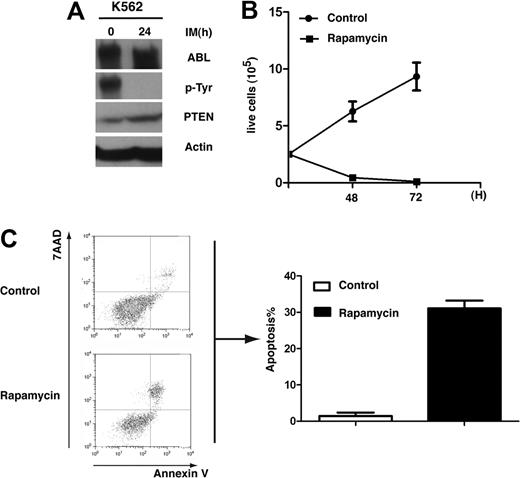
Recently, down-regulation of PTEN mRNA in CD34+ bone marrow cells of CML patients has been reported,33 supporting our finding that PTEN plays a tumor suppressor role in CML development. We further tested the inhibitory role of PTEN in human K562 CML cells. Western blot analysis showed that the level of PTEN was significantly lower in DMSO-treated K562 cells than in K562 cells treated with imatinib for 24 hours (Figure 7A). We also treated K562 cells with rapamycin and found that rapamycin significantly inhibited survival of the cells (Figure 7B). Rapamycin also significantly induced the apoptosis of K562 cells (Figure 7C).
PTEN down-regulation in K562 cells and inhibition of K562 cells by rapamycin. (A) PTEN protein level was elevated by K562 cells treated with imatinib (IM). K562 cells were treated with imatinib (1μM) for 24 hours, and protein lysates were analyzed by the use of Western blotting with the antibodies indicated. (B) Rapamycin inhibits proliferation of K562 cells. K562 cells were treated with DMSO or rapamycin (10μM) for 48 and 72 hours, and live cells were counted. (C) Rapamycin induces apoptosis of K562 cells. K562 cells were treated with DMSO or rapamycin (10μM) for 48 hours. Apoptotic cells (annexin V+/7AAD+) cells were analyzed by FACS.
PTEN down-regulation in K562 cells and inhibition of K562 cells by rapamycin. (A) PTEN protein level was elevated by K562 cells treated with imatinib (IM). K562 cells were treated with imatinib (1μM) for 24 hours, and protein lysates were analyzed by the use of Western blotting with the antibodies indicated. (B) Rapamycin inhibits proliferation of K562 cells. K562 cells were treated with DMSO or rapamycin (10μM) for 48 and 72 hours, and live cells were counted. (C) Rapamycin induces apoptosis of K562 cells. K562 cells were treated with DMSO or rapamycin (10μM) for 48 hours. Apoptotic cells (annexin V+/7AAD+) cells were analyzed by FACS.
Discussion
Some tumor suppressor genes have been shown to be inactivated or down-regulated by BCR-ABL in Ph+ leukemia, including PP2A,34 p53,35 RB,35 and interferon consensus sequence-binding protein.36 In this study, we show that the tumor suppressor Pten is also down-regulated by BCR-ABL and that overexpression of Pten delays the development of CML and B-ALL induced by BCR-ABL. Our DNA microarray study shows that Pten mRNA level was decreased in BCR-ABL–expressing LSCs, indicating that BCR-ABL regulates Pten at a transcriptional level. Our finding that both Pten and p53 are simultaneously down-regulated in BCR-ABL–expressing cells suggests that the Pten down-regulation by BCR-ABL may be mediated by P53, as PTEN transcription is regulated by p53.37,38 p53 has been shown to up-regulate Pten by binding to its promoter27 ; in p53−/− MEF cells, the level of Pten is only 30% of that in the wild-type cells.39
Besides p53, other mechanisms might also be involved in the down-regulation of Pten by BCR-ABL. An analysis of the Pten promoter sequence shows potential binding sites for early growth-regulated transcriptional factor 1 (EGR1), and Pten is up-regulated by EGR1 in response to radiation treatment.40 EGR1 also up-regulates Pten, which likely mediates the apoptotic effect of the phosphatase inhibitor calyculin A. There are also pathways that negatively regulate Pten expression. For example, mitogen-activated protein kinase kinase 4 inhibits Pten transcription by activating nuclear factorκB that binds to the Pten promoter.41 In pancreatic cancer cells42 or mesangial cells,43 Pten is down-regulated by transforming growth factor-β. Pten is also regulated at a posttranscriptional level. Phosphorylation of Pten at specific residues in its C-terminal tail is associated with an increase in its stability,44-46 whereas phosphorylation at other sites decreases the protein stability.47 Ubiquitin-dependent degradation of PTEN occurs when human bronchial cells were exposed to zinc ions,48 and the finding of 2 major conserved ubiquitination sites on PTEN supports this regulation.49 BCR-ABL may regulate these pathways to down-regulate Pten expression, and these potential mechanisms need to be explored further.
PTEN maintains normal hematopoietic stem cells in lineage choice and prevents the leukemia development from leukemia stem cells.22,24 Our microarray data show that Pten is down-regulated in BCR-ABL–expressing LSCs, suggesting that BCR-ABL regulates the functions of LSCs through regulating Pten expression. This idea is supported by our finding that LSCs in CML mice grew significantly slower when Pten was overexpressed. The role of Pten in LSCs provides a potential strategy for targeting the Pten and its related PI3K/AKT pathways in eradication of LSCs.
In this study, we also show that overexpression of PTEN delays B-ALL development and that Akt1 is a major downstream signaling molecule of Pten. Moreover, the inhibition of mTOR by rapamycin significantly inhibits proliferation of human CML leukemia cells K562 and leukemia stem cells from CML mice. These findings support the use of the PTEN-PI3K-AKT-mTOR pathway as a target in treating B-ALL, which is not sensitive to imatinib therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the grants from the Leukemia & Lymphoma Society and the National Institutes of Health (R01-CA122142, R01-CA114199) to S.L. S.L. is a Scholar of the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: C.P. and S.L. designed the experiments, analyzed data, and wrote the paper; C.P., Y.C., Z.Y., and S.L. performed the experiments; and H.Z., A. R., and L.P. helped with the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaoguang Li, 364 Plantation St, LRB 315, Worcester, MA 01604; e-mail: Shaoguang.Li@umassmed.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal