Abstract
Little is known about the role of active immunization in suppressing undesirable immune responses. Because CD8α+ dendritic cells (DCs) suppress certain immune responses, we tested the hypothesis that immunization of donors with host-derived CD8α+ DCs will reduce host-specific donor T-cell responses. BALB/c T cells from the animals that were immunized with B6 CD8α+ DCs demonstrated, in vitro and in vivo, significantly reduced proliferation and secretion of inflammatory cytokines but showed enhanced secretion of interleukin-10 (IL-10). The responses against third-party and model antigens were preserved demonstrating antigen specificity. The in vivo relevance was further demonstrated by the reduction on graft-versus-host disease (GVHD) in both a major histocompatibility complex–mismatched clinically relevant BALB/c → B6 model and major histocompatibility complex–matched, minor-mismatched C3H.SW → B6 model of GVHD. Immunization of the donors that were deficient in IL-10 (IL-10−/−) or with CD8α+ DCs from B6 class II (class II−/−) failed to reduce T-cell responses, demonstrating (1) a critical role for secretion of IL-10 by donor T cells and (2) a direct contact between the T cells and the CD8α+ DCs. Together, these data may represent a novel strategy for reducing GVHD and suggest a broad counterintuitive role for vaccination strategies in mitigating undesirable immune responses in an antigen-specific manner.
Introduction
Activation of an immune response is critical for elimination of infections and certain tumors.1,2 Indeed, one of the most successful medical advances has been the development of immunization or vaccinations against infectious diseases. By contrast, persistent or unwanted activation of immune responses can result in undesirable processes, such as autoimmunity, allograft rejection, and graft-versus-host disease (GVHD). The goal of immunization strategies has generally been to stimulate and enhance antigen-specific immune responses. However, immune responses can be stimulatory as well as inhibitory in nature,3 and it is not known whether immunization or vaccination strategies can also be used to exploit the inhibitory nature of immune responses.
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative therapy for many hematologic and nonhematologic diseases.4 Acute GVHD, a major complication of allo-HCT, has limited the efficacy and application of this potent therapy.4,5 The biology of GVHD is complex. Antigen-presenting cells (APCs) are critical for GVHD.6-16 Dendritic cells (DCs) are the most potent APCs, and recent data suggest that host-type DCs are sufficient for the induction of GVHD.6,7,9,15
DC-based vaccinations, like all other immunization strategies, are generally performed to enhance antigen-specific immune responses,17,18 such as in cancer therapy.2,19 Whether or not the same strategy can be used to dampen alloantigen-specific immune responses is not known. DCs are heterogeneous with various subsets.3,20-22 Conventional DCs (cDCs) in lymphoid tissues can be separated into CD8α+ DCs, which express high levels of CD8α on the cell surface, and CD8α− DCs, which lack this marker.21,23,24 CD8α+ DCs are the main DC subsets that are capable of cross-presentation. Although they can stimulate T cells, albeit less efficiently than CD8α− DCs,25,26 they also can suppress T-cell responses and induce tolerance under certain circumstances.25,27-29
Because DCs have the potential to induce both immunity and tolerance, we tested the hypothesis that immunization of allogeneic donors with host-derived CD8α+ DCs will reduce only host-specific T-cell responses. Our data demonstrate interleukin-10 (IL-10)–dependent reduction of host alloantigen-specific responses in vitro and GVHD in vivo, but preservation of third-party responses.
Methods
Mice
Female C57BL/6 (B6, H-2b, CD45.2+), Ly5.2 (CD45.1+), C3H/HeJ (H-2k), BALB/c (H-2d), C3H.sw (H-2b, CD229.1+), B6.129IL-10 < tmlCgn > /J (IL-10−/−, H-2b, CD45.2+), and OVA-specific TCR transgenic mice OT-II (C57BL/6-Tg(TcraTcrb)425Cbn/J) were purchased from The Jackson Laboratory. H2-Ab1−/− mice (B6.129-H2-Ab1tm1Gru N12, CD45.2+) were obtained from Taconic Farms. Mice were housed in sterilized microisolator cages and received filtered water and normal chow or autoclaved hyperchlorinated drinking water for the first 3 weeks after bone marrow transplantation (BMT). All animals were cared for under regulations approved by the University Committee on Use and Care of Animals of the University of Michigan.
DC culture and isolation
To obtain DCs, bone marrow (BM) cells from recipients (B6, BALB/c, and C3H.sw) and H2-Ab1−/− mice were cultured with murine recombinant granulocyte-macrophage colony-stimulating factor (20 ng/mL; PeproTech) for 7 days and harvested as described previously.30 DCs were isolated using CD11c (N418) MicroBeads (Miltenyi Biotec) and the autoMACS (Miltenyi Biotec). CD11c+ DCs were further separated according to their CD8α expression into 2 populations, CD11c+CD8α+ and CD11c+CD8α−, by sorting on a FACSVantage SE cell sorter (BD Biosciences).31
Vaccination protocol
Donor (BALB/c or B6 or C3H.sw) mice were injected intravenously on days −8, −5 to −3, and −1 (ie, a total of 3 doses) with 2 to 3 × 105 CD11c+CD8α+ or CD11c+CD8α− DCs harvested from allogeneic host (B6 or BALB/c, respectively) BM. Splenic T cells were harvested from the vaccinated donors and used as source of T cells for both in vitro mixed lymphocyte reaction (MLR) and in vivo GVHD studies.
BMTs
BMTs were performed as described before.31 Briefly, splenic T cells from recipient DC-vaccinated donors BALB/c or B6, or C3H.sw, or IL-10−/− were enriched by autoMACS using anti-CD90.2 microbeads (Miltenyi Biotec). Recipient B6, BALB/c, and C3HHEJ mice received, respectively, 1000, 800, and 900 cGy total body irradiation (137Cs source) on day −1. Splenic T cells (4 × 106 from BALB/c or 3 × 106 from C3H.sw or 106 from WT or IL-10−/− B6 donors) and T cell–depleted (TCD) BM cells (5 × 106) from respective allogeneic or syngeneic donors were injected intravenously into recipients on day 0. Survival was monitored daily; body weight and GVHD clinical scores were measured weekly.
Assessment of acute GVHD
The degree of systemic acute GVHD was assessed by a scoring system incorporating 5 clinical parameters (weight loss, posture [hunching], activity, fur ruffling, and skin integrity), as published previously.32 Mice with a total score more than 7 were killed in accordance with University Laboratory Animal Medicine guidelines.
Induction of leukemia
Leukemia was induced for graft-versus-leukemia (GVL) experiments9 with MBL-2, a Moloney leukemia virus–induced T-cell lymphoma that is B6 origin (H-2b) as described.33 On day 0, 50 000 MBL-2 cells were injected into each recipient along with syngeneic (B6) or allogeneic (C3H.sw) BM splenic T cells. Animals were monitored twice daily for survival, and the cause of death determined by postmortem pathology examination.
FACS analysis
Fluorescence-activated cell sorter (FACS) analyses were performed as described before.31 Fluorescein isothiocynate–conjugated monoclonal antibodies (mAbs) to mouse CD4, CD8, CD45.2, and H-2Kd, and phycoerythrin (PE)–conjugated mAbs to CD25 and CD69 and IL-10 were purchased from BD Biosciences, and allophycocyanin-conjugated mAbs to CD25, CD3, and PE-conjugated mAbs to Foxp3 were purchased from eBioscience. The procedure was performed as described previously.31 IL-10 production by donor T cells on day 7 and day 14 was assessed after in vitro restimulation with 10 μg/mL CD3 and 1 ng/mL CD28 for 5 hours with brefeldin A (eBioscience). Cells were stained for cell-surface markers, fixed, permeabilized, and stained intracellularly with anti–mouse IL-10 per the manufacturer's protocol.
MLR
For measurement of MLR cultures, splenic T cells were magnetically separated from naive wild-type (WT) B6 or BALB/c mice or CD11c+CD8α+ or CD11c+CD8α− vaccinated donors (B6 or BALB/c) and by autoMACS using CD90.2 microbeads. A total of 4 × 105 of these were cultured with 1 × 105 irradiated (3000 cGy) B6 splenocytes for 72 hours. When using BM-derived DCs as stimulators, T cells (105) were cultured with B6, BALB/c, or C3HHEJ DC (2.5 × 103) for 96 hours. Supernatants were collected after 66 hours (splenocyte-stimulated) or 80 hours (BM-derived DC-stimulated). Incorporation of 3H-thymidine (1 μCi/well) by proliferating T cells during the final 6 hours (splenocyte-stimulated) or 16 hours (BM-derived DC-stimulated) of culture was measured by a Betaplate reader (Wallad). In the case of OT-II mice, 4 × 105 splenic T cells were isolated from vaccinated OT-II mice and cultured with 1 × 105 irradiated (3000 cGy) WT-B6 splenocytes, or 100 nM and 500 nM chicken ovalbumin (OVA, AnaSpec)323-339-treated B6 splenocytes for 48 hours.
Cytokine ELISA
Concentrations of tumor necrosis factor-α (TNF-α), IL-2, IL-4, IL-10, interferon-γ (IFN-γ), and transforming growth factor-β (TGF-β) were measured in serum and culture supernatants by enzyme-linked immunosorbent assay (ELISA) with specific anti–mouse mAbs for capture and detection. Appropriate standards used were purchased from BD Biosciences PharMingen (IL-2, IL-4, IL-10, and IFN-γ) and R&D Systems (TNF-α and TGF-β). Assays were performed according to the manufacturer's protocol and read at 450 nm using a microplate reader (Model 3550; Bio-Rad). All samples and standards were run in duplicate.
CFSE and apoptosis analysis
On day 8 after the first vaccination, spleens from either naive or CD11c+CD8α+ and CD11c+CD8α− vaccinated BALB/c donors were harvested and enriched for T cells by autoMACS. The cells were stained with either carboxyfluorescein diacetate succinimidyl ester (CFSE) to analyze expansion or annexin to evaluate apoptosis of divided cells.34 Analysis of cells immediately after CFSE incorporation showed a labeling efficiency that exceeded 99%. These cells were then resuspended and cultivated with or without allogeneic BALB/c T cells. After 4 days, the cells were stained with PE-conjugated anti–annexin V mAb (BD Biosciences) and allophycocyanin-conjugated anti–CD3 mAb (eBioscience) and analyzed using an Accuri C6 cytometer (Accuri). Cell apoptosis was identified based on staining for annexin V.
Histology
Formalin-preserved liver and small and large bowel were embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin for histologic examinations. Slides were coded without reference to prior treatment and examined in blinded fashion by a pathologist (C.L.). A semiquantitative scoring system was used to assess the following abnormalities known to be associated with GVHD35 : 0 indicates normal; 0.5, focal and rare; 1.0, focal and mild; 2.0, diffuse and mild; 3.0, diffuse and moderate; and 4.0, diffuse and severe. Scores were summed to provide a total score for each specimen.
Statistical analysis
The Mann-Whitney U test was used for the statistical analysis of in vitro data, and the Wilcoxon rank test was used to analyze survival data. A P value less than .05 was considered statistically significant.
Results
Immunization with allogeneic CD8α+ DCs reduces T-cell responses in vitro
We first tested the effect of CD8α+ DC immunization on in vitro T-cell responses. BALB/c mice were vaccinated with allogeneic B6 BM culture-derived CD8α+ DCs. Nonimmunized donors and those immunized with CD8α− DCs served as controls. Purified CD11c+CD8α+ DCs or CD11c+CD8α− DCs from B6 or diluent control were injected into BALB/c donors on days −8, −5 to −3, and −1 before the T cells were harvested (Figure 1). After preliminary studies, this dose and schedule of injections were chosen because they generated more robust and consistent data. We analyzed the effect of immunization on the phenotype and absolute numbers of splenic T cells. After vaccination, the mean number of splenocytes was increased, albeit in a statistically insignificant manner, in both the CD8α+ DCs and CD8α− DCs immunized donor groups compared with diluent-injected nonimmunized control mice (Table 1). Immunization with B6 CD8α− DCs significantly increased CD4+ T-cell numbers and the expression of CD25 and CD69 in both CD4+ and CD8+ T cells, but no differences were observed in the percentage of CD4+CD25+Foxp3+ cells compared with T cells from unvaccinated or CD8α+ DCs vaccinated mice (Table 1).
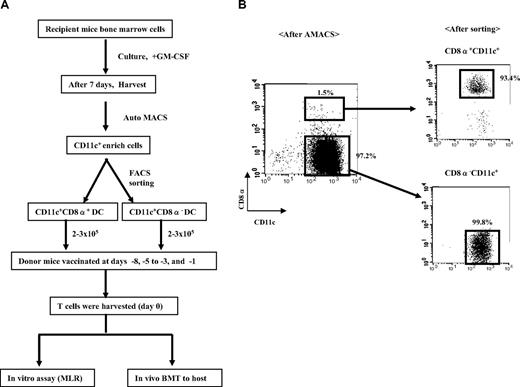
Experimental design. (A) Schema of the immunization protocol. (B) Gating strategy for FACS sorting of allogeneic BM-derived DCs into subsets of CD8α+CD11c+ DCs and CD8α−CD11c+ DCs.
Experimental design. (A) Schema of the immunization protocol. (B) Gating strategy for FACS sorting of allogeneic BM-derived DCs into subsets of CD8α+CD11c+ DCs and CD8α−CD11c+ DCs.
Effect of vaccination on the percentage of splenic cellular phenotype
| . | WT-BALB/c (n = 6) . | CD8α− DC vaccinated (n = 6) . | CD8α+ DC vaccinated (n = 6) . |
|---|---|---|---|
| Total cells ×106 (mean ± SE) | 112.3 ± 19.8 | 130.0 ± 29.5 | 145.2 ± 35.2 |
| CD4+, percentage (mean ± SE) | 23.1 ± 1.3 | 26.7 ± 1.8 | 26.0 ± 3.6 |
| CD44highCD4+, percentage (mean ± SE) | 19.9 ± 5.8 | 19.2 ± 8.4 | 23.5 ± 3.9 |
| CD25+CD4+, percentage (mean ± SE) | 11.2 ± 1.7 | 11.9 ± 2.8 | 13.7 ± 1.8* |
| CD62+CD4+, percentage (mean ± SE) | 70.8 ± 13.5 | 67.9 ± 15.3 | 69.1 ± 14.4 |
| CD69+CD4+, percentage (mean ± SE) | 9.2 ± 2.4 | 8.7 ± 0.3 | 13.9 ± 2.1* |
| CD8+, percentage (mean ± SE) | 9.4 ± 0.3 | 11.0 ± 1.0 | 9.9 ± 1.0 |
| CD44highCD8+, percentage (mean ± SE) | 14.9 ± 1.5 | 15.3 ± 1.2 | 15.8 ± 1.7 |
| CD25+CD8+, percentage (mean ± SE) | 1.9 ± 0.5 | 1.8 ± 0.9 | 5.0 ± 2.9* |
| CD62+CD8+, percentage (mean ± SE) | 87.5 ± 5.6 | 87.9 ± 12.5 | 84.3 ± 11.6 |
| CD69+CD8+, percentage (mean ± SE) | 5.1 ± 0.8 | 5.3 ± 1.6 | 10.0 ± 3.0* |
| CD4+CD25+Foxp3+, percentage (mean ± SE) | 8.1 ± 2.2 | 9.6 ± 1.6 | 9.0 ± 0.4 |
| . | WT-BALB/c (n = 6) . | CD8α− DC vaccinated (n = 6) . | CD8α+ DC vaccinated (n = 6) . |
|---|---|---|---|
| Total cells ×106 (mean ± SE) | 112.3 ± 19.8 | 130.0 ± 29.5 | 145.2 ± 35.2 |
| CD4+, percentage (mean ± SE) | 23.1 ± 1.3 | 26.7 ± 1.8 | 26.0 ± 3.6 |
| CD44highCD4+, percentage (mean ± SE) | 19.9 ± 5.8 | 19.2 ± 8.4 | 23.5 ± 3.9 |
| CD25+CD4+, percentage (mean ± SE) | 11.2 ± 1.7 | 11.9 ± 2.8 | 13.7 ± 1.8* |
| CD62+CD4+, percentage (mean ± SE) | 70.8 ± 13.5 | 67.9 ± 15.3 | 69.1 ± 14.4 |
| CD69+CD4+, percentage (mean ± SE) | 9.2 ± 2.4 | 8.7 ± 0.3 | 13.9 ± 2.1* |
| CD8+, percentage (mean ± SE) | 9.4 ± 0.3 | 11.0 ± 1.0 | 9.9 ± 1.0 |
| CD44highCD8+, percentage (mean ± SE) | 14.9 ± 1.5 | 15.3 ± 1.2 | 15.8 ± 1.7 |
| CD25+CD8+, percentage (mean ± SE) | 1.9 ± 0.5 | 1.8 ± 0.9 | 5.0 ± 2.9* |
| CD62+CD8+, percentage (mean ± SE) | 87.5 ± 5.6 | 87.9 ± 12.5 | 84.3 ± 11.6 |
| CD69+CD8+, percentage (mean ± SE) | 5.1 ± 0.8 | 5.3 ± 1.6 | 10.0 ± 3.0* |
| CD4+CD25+Foxp3+, percentage (mean ± SE) | 8.1 ± 2.2 | 9.6 ± 1.6 | 9.0 ± 0.4 |
P < .05.
We determined the effects of allogeneic B6 CD8α+ DC immunization on the functions of BALB/c T cells by performing MLR using B6 DCs as stimulators. BALB/c T cells from the B6 CD8α+ DC–vaccinated mice demonstrated significantly reduced expansion compared with the control T cells from both nonvaccinated (P < .001) and CD8α− DC–vaccinated mice (P < .001; Figure 2A). The reduction in proliferation was also associated with a significant reduction in the secretion of IL-2 and IFN-γ (Figure 2B-C; P < .05). By contrast, secretion of IL-10 was significantly increased in the CD8α+ DC–vaccinated group compared with the control groups (Figure 2D). Similar reductions were observed when freshly isolated B6 splenocytes were used as stimulators instead of B6 BM DCs demonstrating lack of DC culture–related artifacts (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, no significant difference was observed in the levels of TGF-β regardless of stimulators (data not shown).
T cells from allogeneic CD8α+ DC–vaccinated mice. Reduced expansion and proinflammatory cytokine secretion were shown in vitro in BALB/c mice that were immunized with diluent control or allogeneic B6 BM-derived CD8α+ DCs or CD8α− DCs as described in “Methods.” BALB/c CD90+ T cells (2 × 105/well) were then harvested from them and cultured for 96 hours with either syngeneic or irradiated (3000 cGy) allogeneic B6 BM DCs (1 × 104/well). During the final 16 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for (A) proliferation. Supernatants were collected at 80 hours and assayed by for (B) IL-2, (C) IFN-γ, and (D) IL-10. Error bars represent SE. *P < .05 between diluent control and allogeneic CD8α+ DC–vaccinated groups. Data are from one of 3 similar experiments. *P < .05 between diluent control and allogeneic CD8α+ DC–vaccinated groups.
T cells from allogeneic CD8α+ DC–vaccinated mice. Reduced expansion and proinflammatory cytokine secretion were shown in vitro in BALB/c mice that were immunized with diluent control or allogeneic B6 BM-derived CD8α+ DCs or CD8α− DCs as described in “Methods.” BALB/c CD90+ T cells (2 × 105/well) were then harvested from them and cultured for 96 hours with either syngeneic or irradiated (3000 cGy) allogeneic B6 BM DCs (1 × 104/well). During the final 16 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for (A) proliferation. Supernatants were collected at 80 hours and assayed by for (B) IL-2, (C) IFN-γ, and (D) IL-10. Error bars represent SE. *P < .05 between diluent control and allogeneic CD8α+ DC–vaccinated groups. Data are from one of 3 similar experiments. *P < .05 between diluent control and allogeneic CD8α+ DC–vaccinated groups.
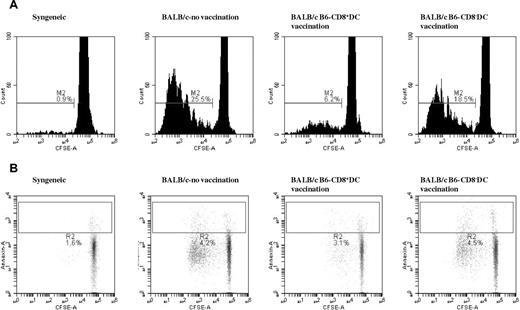
We next determined whether the reduction in proliferation of the T cells after CD8α+ DC immunization is the result of a consequence of reduced proliferation and/or enhanced apoptosis. To this end, we analyzed cell proliferation with CFSE staining and apoptosis with annexin staining in response to allogeneic B6 DC stimulation. BALB/c T cells from the B6 CD8α+ DCvaccinated group showed significantly lower proliferation than T cells from either the diluent- or the CD8α− DC–vaccinated control groups (Figure 3A). By contrast, as shown in Figure 3B, no significant differences in apoptosis were observed between any of the allogeneic groups regardless of immunization.
Immunization with allogeneic CD8α+ DCs reduces T-cell–proliferative response without altering their rate of apoptosis. T cells were harvested from BALB/c mice that were immunized with diluent or B6 BM-derived CD8α+ DC and CD8α− DC subsets as described in “Methods.” BALB/c CD90+ T cells (2 × 106/well) were then stained with CFSE and stimulated in vitro with B6 BM DCs for 96 hours and analyzed by flow cytometry. (A) Gated for fluorescein isothiocynate–conjugated CFSE and allophycocyanin-conjugated CD3+ T cells. Data are representative of 1 of 4 independent similar experiments. Combined data for the nonimmunized, CD8α+ DC–vaccinated, and CD8α− DC–vaccinated are 0.77% ± 0.15%, 24.1% ± 2.4%, 4.7% ± 1.6%, and 18.1% ± 0.6%, respectively (P < .04, nonimmunized vs CD8α+ DC). (B) PE-conjugated annexin V and allophycocyanin-conjugated anti-CD3. P = not significant between the groups. Data are representative of 1 of 3 independent similar experiments.
Immunization with allogeneic CD8α+ DCs reduces T-cell–proliferative response without altering their rate of apoptosis. T cells were harvested from BALB/c mice that were immunized with diluent or B6 BM-derived CD8α+ DC and CD8α− DC subsets as described in “Methods.” BALB/c CD90+ T cells (2 × 106/well) were then stained with CFSE and stimulated in vitro with B6 BM DCs for 96 hours and analyzed by flow cytometry. (A) Gated for fluorescein isothiocynate–conjugated CFSE and allophycocyanin-conjugated CD3+ T cells. Data are representative of 1 of 4 independent similar experiments. Combined data for the nonimmunized, CD8α+ DC–vaccinated, and CD8α− DC–vaccinated are 0.77% ± 0.15%, 24.1% ± 2.4%, 4.7% ± 1.6%, and 18.1% ± 0.6%, respectively (P < .04, nonimmunized vs CD8α+ DC). (B) PE-conjugated annexin V and allophycocyanin-conjugated anti-CD3. P = not significant between the groups. Data are representative of 1 of 3 independent similar experiments.
These data collectively demonstrate that, after immunization of BALB/c mice with allogeneic B6 CD8α+ DCs, T cells from these BALB/c animals showed significantly reduced proliferation, IL-2, and IFN-γ secretion but enhanced IL-10 secretion in response to in vitro stimulation with the same (B6) alloantigens compared with the control T cells from either B6 CD8α− DCs immunized or the nonimmunized BALB/c animals.
Immunization of donors with host-type CD8α+ DCs reduces acute GVHD
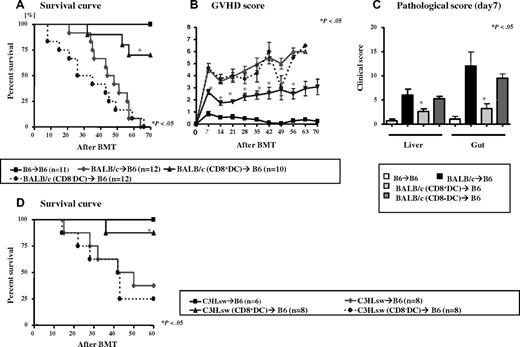
We evaluated the impact of immunization with CD8α+ DCs on in vivo allogeneic responses using a clinically relevant murine model of GVHD. Donor (BALB/c) mice were immunized 3 times as described in “Methods” before harvesting their splenic T cells for BMT. B6 animals were lethally irradiated (1000 cGy) and transplanted on day 0 with 5 × 106 TCD BM from naive syngeneic (B6) and allogeneic (BALB/c) donors along with 4 × 106 splenic T cells from naive (nonimmunized), CD8α+ DC–, or CD8α− DC–vaccinated BALB/c donors. As shown in Figure 4A-B, all of the syngeneic animals survived without showing any signs of acute GVHD. All of the allogeneic animals that received T cells from the control nonimmunized donors showed signs of severe GVHD and 100% mortality by day 70 after BMT. All of the animals that received T cells from donors immunized with B6 CD8α− DCs also showed signs of severe acute GVHD died in the observation period (Figure 4A-C). Although the kinetics of mortality was faster in this group, it was however not statistically significant compared with the animals that received allogeneic T cells from nonimmunized donors (Figure 4A). By contrast, animals that received T cells from BALB/c donors that were immunized with B6 CD8α+ DCs demonstrated significantly better survival than the other allorecipients (70% vs 0%, P < .001; Figure 4A). The improvement in survival correlated with reduced clinical severity of acute GVHD scores (Figure 4B).
Pretransplantation vaccination of donors with host-type CD8α+ DCs attenuates GVHD. Recipient B6 mice were irradiated with 1000 cGy total body irradiation (TBI) and injected with 5 × 106 TCD BM and 4 × 106 CD90+ T cells from syngeneic (squares, solid line, n = 11), allogeneic WT control (circles with dotted line, n = 12), allogeneic recipients of host-type CD8+ DC–vaccinated (triangles with solid line, n = 10), and CD8− DC–vaccinated (diamonds with dotted line, n = 12) donors. They were evaluated for (A) survival. Error bars represent SE. *P < .05 between diluent control (red line) and host-type CD8α+ DC-vaccinated (triangles) groups. (B) Clinical GVHD score. Error bars represent SE. *P < .05 between controls (circles) and host-type CD8α+ DC–vaccinated allogeneic animals (triangles). Data are combined from 2 of 4 similar experiments. (C) Small and large intestines and livers were obtained from each group (n = 4/group) for detailed histopathologic analysis on day 7 after BMT as described in “Methods.” Coded slides were scored semiquantitatively to assess GVHD-specific pathologic damage. Total GVHD score: mean ± SE of the sum of scores for gut (small and large bowels) and livers from individual animals in each group. Error bars represent SE. *P < .05 between allorecipients of diluent control and host-type CD8α+ DC–vaccinated donors. (D) Recipient B6 mice were irradiated with 1000 cGy TBI and injected with 5 × 106 TCD BM and 3 × 106 CD90+ T cells from syngeneic (black solid line, n = 6), allogeneic WT control (circles with dotted line, n = 8), allogeneic recipients CD8+ DC-vaccinated (triangles with solid line, n = 8), and host CD8− DC–vaccinated (diamonds with dotted line, n = 8) donors and evaluated for survival. Data from 2 similar experiments are combined. P < .04, CD8+ DCs vs control and CD8− DCs.
Pretransplantation vaccination of donors with host-type CD8α+ DCs attenuates GVHD. Recipient B6 mice were irradiated with 1000 cGy total body irradiation (TBI) and injected with 5 × 106 TCD BM and 4 × 106 CD90+ T cells from syngeneic (squares, solid line, n = 11), allogeneic WT control (circles with dotted line, n = 12), allogeneic recipients of host-type CD8+ DC–vaccinated (triangles with solid line, n = 10), and CD8− DC–vaccinated (diamonds with dotted line, n = 12) donors. They were evaluated for (A) survival. Error bars represent SE. *P < .05 between diluent control (red line) and host-type CD8α+ DC-vaccinated (triangles) groups. (B) Clinical GVHD score. Error bars represent SE. *P < .05 between controls (circles) and host-type CD8α+ DC–vaccinated allogeneic animals (triangles). Data are combined from 2 of 4 similar experiments. (C) Small and large intestines and livers were obtained from each group (n = 4/group) for detailed histopathologic analysis on day 7 after BMT as described in “Methods.” Coded slides were scored semiquantitatively to assess GVHD-specific pathologic damage. Total GVHD score: mean ± SE of the sum of scores for gut (small and large bowels) and livers from individual animals in each group. Error bars represent SE. *P < .05 between allorecipients of diluent control and host-type CD8α+ DC–vaccinated donors. (D) Recipient B6 mice were irradiated with 1000 cGy TBI and injected with 5 × 106 TCD BM and 3 × 106 CD90+ T cells from syngeneic (black solid line, n = 6), allogeneic WT control (circles with dotted line, n = 8), allogeneic recipients CD8+ DC-vaccinated (triangles with solid line, n = 8), and host CD8− DC–vaccinated (diamonds with dotted line, n = 8) donors and evaluated for survival. Data from 2 similar experiments are combined. P < .04, CD8+ DCs vs control and CD8− DCs.
Next we further confirmed specified GVHD protection by performing detailed histopathologic analyses of GVHD target organs, liver, and gastrointestinal tract. As shown in Figure 4C, compared with recipients of nonimmunized and CD8α− DC–vaccinated donors, allogeneic animals that received T cells from CD8α+DC-vaccinated donors demonstrated significantly reduced histopathologic damage in both the liver and gastrointestinal tract (small and large bowel) on day 7 after BMT (P < .05). The reduction in the severity of histopathologic damage was also observed on day 21 (supplemental Figure 2). The reduction in GVHD damage was also observed in the small bowel and colon when each organ was analyzed separately (data not shown). Collectively, these data demonstrate a significant and unequivocal reduction in the clinical severity, pathologic damage, and mortality from acute GVHD after immunization of donors with host-type CD8α+ DCs.
We next examined whether the reduction in GVHD is a strain-specific phenomenon. We used major histocompatibility complex (MHC)–matched, multiple minor-mismatched model of acute GVHD (C3H.sw→ B6). Donor (C3H.sw) mice were immunized with 2 to 3 × 105 recipient (B6) BM-derived CD8α+ DCs or CD8α− DCs as described in “Methods,” after which splenic T cells were harvested for BMT. Recipient B6 animals were lethally irradiated (1000 cGy) and transplanted on day 0 with TCD BM from naive syngeneic (B6) or allogeneic (C3H.sw) donors along with T cells from naive (nonimmunized), CD8α+ DC–, or CD8α− DC–vaccinated C3H.sw donors. As shown in Figure 4D, all of the syngeneic animals survived without showing any signs of acute GVHD. Allogeneic animals that received T cells from the control nonimmunized donors showed signs of severe GVHD with 70% mortality by day 60. The animals that received T cells from donors immunized with B6 CD8α− DCs also showed signs of severe acute GVHD-demonstrated 75% mortality by day 60. By contrast, animals that received T cells from C3H.sw donors that were immunized with B6 CD8α+ DCs demonstrated significantly reduced mortality from GVHD (12.5%, P < .03; Figure 4D).
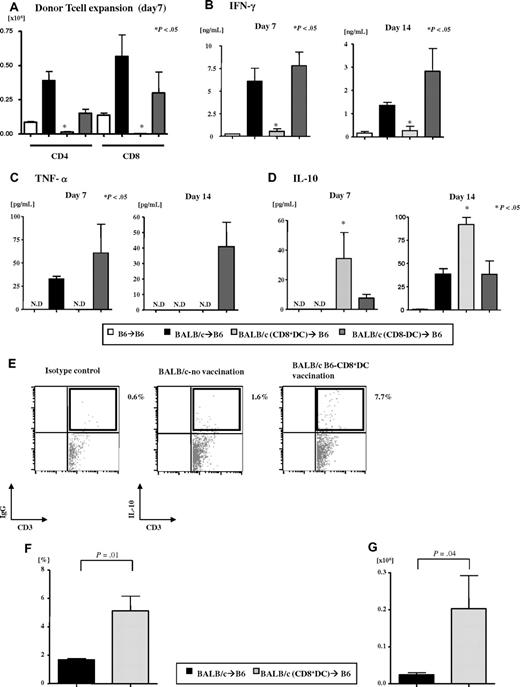
We determined next whether the reduction in GVHD was also associated with a reduction in the expansion of allogeneic T cells similar to the in vitro MLR studies. Day 7 donor T-cell expansion (both CD4+ and CD8+) in the animals that received T cells from CD8α+ DC–vaccinated donors was significantly less than the allogeneic control groups (Figure 5A). We also examined serum levels of IFN-γ, TNF-α, and IL-10 at 2 different time points, on day 7 and day 14, after BMT. As shown in Figure 5B and Figure 5C, respectively, serum levels of both IFN-γ and TNF-α were significantly reduced in the CD8+ DC allogeneic animals compared with the other allogeneic controls. By contrast, as shown in Figure 5D, significantly higher serum levels of IL-10 were observed in CD8+ DC allogeneic group on day 7 (nonimmunized, 0 pg/mL; CD8+ DC–vaccinated, 34.3 ± 17.6 pg/mL; CD8− DC–vaccinated, 7.6 ± 2.6 pg/mL) and day 14 (nonimmunized, 38.7 ± 5.6 pg/mL; CD8+ DC–vaccinated, 91.9 ± 7.6 pg/mL; CD8− DC–vaccinated, 36.4 ± 16.5 pg/mL).
Effect of host-type CD8α+ DC immunization on donor T-cell expansion and cytokines after allo-BMT. B6 animals were irradiated and transplanted after immunization of the donors as in “Vaccination protocol.” Splenocytes and serum were harvested from the recipients on day 7 and 14 after BMT. (A) Donor (CD45.2 or H2kd) CD4+ and CD8+ T-cell expansion was determined by FACS analysis. Data represent the mean ± SE. *P < .05 between allorecipients of host-type CD8α+ DC–vaccinated donors and diluent control or host-type CD8α− DC–vaccinated donors. (B-D) Serum cytokines were measured (n = 4/group) on days 7 and 14 after BMT. Serum levels of (B) IFN-γ, (C) TNF-α, and (D) IL-10. N.D indicates not detected. *P < .05 between allorecipients of host-type CD8α+ DC-vaccinated donors versus the diluent control. Data are from one of 2 similar experiments. (E-G) Representative figure of IL-10 production from donor T cells (n = 3 or 4/group) on day 14 after BMT (E). Gated on donor-specific (H-2kd) cells for the double-positive population of CD3 and IL-10. The percentage IL-10+ donor T cells (F) and the absolute cell numbers (G). P < .05.
Effect of host-type CD8α+ DC immunization on donor T-cell expansion and cytokines after allo-BMT. B6 animals were irradiated and transplanted after immunization of the donors as in “Vaccination protocol.” Splenocytes and serum were harvested from the recipients on day 7 and 14 after BMT. (A) Donor (CD45.2 or H2kd) CD4+ and CD8+ T-cell expansion was determined by FACS analysis. Data represent the mean ± SE. *P < .05 between allorecipients of host-type CD8α+ DC–vaccinated donors and diluent control or host-type CD8α− DC–vaccinated donors. (B-D) Serum cytokines were measured (n = 4/group) on days 7 and 14 after BMT. Serum levels of (B) IFN-γ, (C) TNF-α, and (D) IL-10. N.D indicates not detected. *P < .05 between allorecipients of host-type CD8α+ DC-vaccinated donors versus the diluent control. Data are from one of 2 similar experiments. (E-G) Representative figure of IL-10 production from donor T cells (n = 3 or 4/group) on day 14 after BMT (E). Gated on donor-specific (H-2kd) cells for the double-positive population of CD3 and IL-10. The percentage IL-10+ donor T cells (F) and the absolute cell numbers (G). P < .05.
We next directly examined whether there was an expansion of donor T cells that were secreting IL-10. To this end, we stained T cells with donor-specific markers and analyzed them with intracellular staining for IL-10. As shown in Figure 5E-F, both the percentage and the numbers of IL-10+CD3+ donor T cells were significantly greater in the animals that received cells from CD8α+ DC–vaccinated donors (P < .04). In contrast, there was no difference in the percentage of CD4+Foxp3+ donor T cells between all of the allogeneic groups on day 14 after BMT (nonimmunized, 6.9% ± 1.36% vs CD8+ DC-vaccinated, 9.02% ± 1.3% vs CD8− DC–vaccinated, 8% ± 0.93%, P = not significant). These data demonstrate that vaccination with allogeneic CD8α+ DCs significantly reduced alloproliferative responses but enhanced IL-10 secretion by the T cells both in vitro and in vivo.
CD8α+ DC vaccination regulates antigen-specific responses
Because vaccinations modulate antigen-specific responses, we next analyzed whether the suppressive effect of CD8α+ DC immunization is specific only to the host-type alloantigens with which the vaccination was performed. To this end, we tested B6 (H2b) T-cell responses against third-party recipients (C3H/HeJ- H2k) after immunization with BALB/c (H2d) BM-derived CD11c+CD8α+ DCs or CD11c+CD8α− DCs as described in “Methods.” The nonimmunized and those vaccinated with CD8α− DCs once again served as controls. In contrast to the data described in “Methods,” which demonstrate attenuation of T-cell responses against the same alloantigens from which CD8α+ DCs were derived, T cells from this group showed equivalent proliferation and secretion of IL-2, IL-10, and IFN-γ when stimulated with splenocytes from third-party C3H/HeJ mice (Figure 6A-B). These data show that attenuation of responses after immunization is specific to the alloantigens derived from the CD8α+ DCs.
Allogeneic CD8α+ DC immunization preserves T-cell responses to third-party antigens. B6 donor mice were immunized with diluent or recipient BALB/c BM-derived CD8α+ DC or CD8α− DCs as described in “Vaccination protocol.” T cells were harvested and used for in vitro and in vivo studies with C3H/HeJ recipients. (A) B6 splenic CD90+ T cells were stimulated with irradiated (3000 cGy) splenocytes (105/well) from C3H/HeJ. During the final 6 hours of a 72-hour culture, cells were pulsed with 3H thymidine and assayed for proliferation. (B) Supernatants were collected at 66 hours and assayed by ELISA for IL-2, IFN-γ, and IL-10. Error bars represent SE. P = not significant between diluent control and BALB/c-derived CD8α+ DC groups. Each graph represents one of 3 similar experiments. (C) C3H/HeJ mice were lethally irradiated with 9000 cGy TBI and injected with 5 × 106 TCD BM and 106 CD90+ T cells from syngeneic (black straight line, n = 3), allogeneic diluent-treated (circles, dotted line, n = 7), or BALB/c-derived CD8+ DC-vaccinated (triangles, n = 6) or CD8− DC-vaccinated (diamonds, n = 6) B6 animals and evaluated for survival. P = not significant between diluent control and the other groups. Data represent one of 2 similar experiments. (D) B6 OT-II transgenic mice were immunized with diluent or BALB/c BM-derived CD8α+ DCs or CD8α− DCs as above. Responder T cells were harvested from the OT-II B6 mice and cultured for 48 hours with B6 syngeneic splenocytes that were either not pulsed or pulsed with 100nM or 500nM OVA323-339 peptide. During the final 6 hours of a 48-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation. P = not significant between diluent control and the other immunized groups. Data are representative of one of 2 similar experiments.
Allogeneic CD8α+ DC immunization preserves T-cell responses to third-party antigens. B6 donor mice were immunized with diluent or recipient BALB/c BM-derived CD8α+ DC or CD8α− DCs as described in “Vaccination protocol.” T cells were harvested and used for in vitro and in vivo studies with C3H/HeJ recipients. (A) B6 splenic CD90+ T cells were stimulated with irradiated (3000 cGy) splenocytes (105/well) from C3H/HeJ. During the final 6 hours of a 72-hour culture, cells were pulsed with 3H thymidine and assayed for proliferation. (B) Supernatants were collected at 66 hours and assayed by ELISA for IL-2, IFN-γ, and IL-10. Error bars represent SE. P = not significant between diluent control and BALB/c-derived CD8α+ DC groups. Each graph represents one of 3 similar experiments. (C) C3H/HeJ mice were lethally irradiated with 9000 cGy TBI and injected with 5 × 106 TCD BM and 106 CD90+ T cells from syngeneic (black straight line, n = 3), allogeneic diluent-treated (circles, dotted line, n = 7), or BALB/c-derived CD8+ DC-vaccinated (triangles, n = 6) or CD8− DC-vaccinated (diamonds, n = 6) B6 animals and evaluated for survival. P = not significant between diluent control and the other groups. Data represent one of 2 similar experiments. (D) B6 OT-II transgenic mice were immunized with diluent or BALB/c BM-derived CD8α+ DCs or CD8α− DCs as above. Responder T cells were harvested from the OT-II B6 mice and cultured for 48 hours with B6 syngeneic splenocytes that were either not pulsed or pulsed with 100nM or 500nM OVA323-339 peptide. During the final 6 hours of a 48-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation. P = not significant between diluent control and the other immunized groups. Data are representative of one of 2 similar experiments.
We next reasoned that, if the suppression is specific to the CD8α+DC alloantigens, then the in vivo relevance of antigen specificity would be demonstrated by the loss of GVHD protection when third-party animals are used as recipients. Donor (B6-H2b) mice were once again immunized either with diluent alone or CD8α+ DCs or CD11c+CD8α− DCs from BALB/c mice. Third-party, C3H/HeJ (H2k) animals were lethally irradiated (900 cGy) and transplanted on day 0 with 5 × 106 TCD BM from naive syngeneic (C3H/HeJ) or allogeneic (B6) donors, along with 106 T cells from either nonimmunized or those vaccinated with BALB/c CD8α+ DCs or CD8α− DCs. As shown in Figure 6C, all of the allogeneic animals demonstrated similar kinetics of GVHD mortality and clinical severity (data not shown) regardless of whether they received T cells from immunized or nonimmunized donors.
To further confirm the specificity of the suppression, we also analyzed the effect of CD8α+ DCs immunization against the nonimmunized antigens using OT-II–specific transgenic mice. We reasoned that the OT-specific responses will be preserved regardless of immunization of these mice with allogeneic DC subsets. B6 OT-II transgenic mice were immunized with BALB/c-derived CD8α+ DCs or CD8α− DCs as described in “Methods.” Splenic T cells from these transgenic mice were harvested after immunization and cultured with irradiated (30 Gy) WT-B6 or 100nM and 500nM OVA323-339-treated B6 splenocytes for 48 hours (Figure 6D). T cells from BALB/c BM-derived CD8α+ DC–vaccinated B6 OT-II transgenic did not show any reduction in response to ova compared with either the CD8α− DC–vaccinated or the nonimmunized controls. Thus, vaccination with allogeneic CD8α+ DCs negatively regulates antigen-specific T-cell responses.
IL-10 is critical for T-cell suppression after immunization with CD8α+ DCs
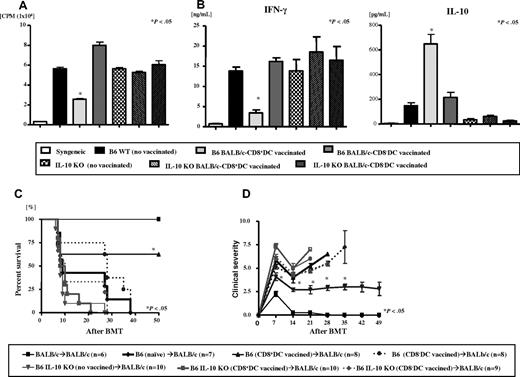
We sought to determine the mechanisms critical for the suppression of T-cell responses after immunization with CD8α+ DCs. Because secretion of IL-10, a known immunosuppressive cytokine, was enhanced after vaccination with CD8α+ DCs, we examined whether the attenuation of allospecific responses is dependent on the production of IL-10 by the donor T cells. WT and IL-10–deficient (IL-10−/−) B6 mice were immunized with either diluent or BALB/c BM-derived CD8α− DCs or CD8α+ DCs as before. The WT and IL-10−/− B6 mice that were not immunized and those vaccinated with BALB/c CD8α− DCs served as controls. T cells harvested from WT B6 animals that were immunized with BALB/c CD8α+ DCs, as expected, demonstrated significantly less proliferation and secretion of IFN-γ but greater secretion of IL-10 (P < .04) when stimulated with BALB/c splenocytes in an MLR compared with T cells from nonimmunized and CD8α− DC–vaccinated WT B6 mice (Figure 7A-B). By contrast, as shown in Figure 7A-B, this reduction in responses to BALB/c antigens after BALB/c CD8α+ DCs vaccination was not apparent in T cells harvested from IL-10−/− B6 mice demonstrating the critical requirement of IL-10 secretion by the T cells.
IL-10 is required for mediating the suppressive effects of allogeneic CD8α+ DC vaccination. T cells from WT B6 and IL-10 KO B6 donor mice were harvested after immunization with either diluent or host BALB/c BM-derived CD8α+ DCs or CD8α− DCs as described in “Vaccination protocol.” T cells were used as responders and stimulated with irradiated (3000 cGy) BALB/c splenocytes in an allogeneic MLR and assayed for (A) proliferation, and (B) supernatants were collected at 66 hours and assayed by ELISA for IFN-γ and IL-10. *P < .05 between T cells from WT B6 animals that were immunized with BALB/c BM-derived CD8α+ DC compared with diluent or CD8α− DC controls. P = not significant between for T cells from IL-10 KO B6 animals in all groups. Data represent 1 of 3 similar experiments. Recipient BALB/c mice were irradiated with 8000 cGy TBI and injected with 5 × 106 TCD BM and 106 CD90+ T cells from syngeneic (black thin solid line, n = 6) or allogeneic T cells from diluent control (black bold solid line, n = 7) or BALB/c CD8+ DC-vaccinated (triangles, n = 8) or CD8− DC-vaccinated (diamonds, n = 8) B6 WT donors or T cells from allogeneic IL-10 KO B6 donors that were immunized with diluent control (inverted triangles, n = 10), or BALB/c CD8+ DC-vaccinated (squares bold solid line, n = 10) or CD8− DC-vaccinated (circles, dotted line, n = 9) IL-10 KO donors and evaluated for (C) survival and (D) clinical GVHD score. *P < .05 between T cells from WT B6 animals that were immunized with BALB/c BM-derived CD8α+ DC versus those with diluent or CD8α− DC controls. P = not significant between for T cells from IL-10 KO B6 animals in all groups. Data represent 1 of 3 similar experiments. Data are from 2 combined experiments with similar results.
IL-10 is required for mediating the suppressive effects of allogeneic CD8α+ DC vaccination. T cells from WT B6 and IL-10 KO B6 donor mice were harvested after immunization with either diluent or host BALB/c BM-derived CD8α+ DCs or CD8α− DCs as described in “Vaccination protocol.” T cells were used as responders and stimulated with irradiated (3000 cGy) BALB/c splenocytes in an allogeneic MLR and assayed for (A) proliferation, and (B) supernatants were collected at 66 hours and assayed by ELISA for IFN-γ and IL-10. *P < .05 between T cells from WT B6 animals that were immunized with BALB/c BM-derived CD8α+ DC compared with diluent or CD8α− DC controls. P = not significant between for T cells from IL-10 KO B6 animals in all groups. Data represent 1 of 3 similar experiments. Recipient BALB/c mice were irradiated with 8000 cGy TBI and injected with 5 × 106 TCD BM and 106 CD90+ T cells from syngeneic (black thin solid line, n = 6) or allogeneic T cells from diluent control (black bold solid line, n = 7) or BALB/c CD8+ DC-vaccinated (triangles, n = 8) or CD8− DC-vaccinated (diamonds, n = 8) B6 WT donors or T cells from allogeneic IL-10 KO B6 donors that were immunized with diluent control (inverted triangles, n = 10), or BALB/c CD8+ DC-vaccinated (squares bold solid line, n = 10) or CD8− DC-vaccinated (circles, dotted line, n = 9) IL-10 KO donors and evaluated for (C) survival and (D) clinical GVHD score. *P < .05 between T cells from WT B6 animals that were immunized with BALB/c BM-derived CD8α+ DC versus those with diluent or CD8α− DC controls. P = not significant between for T cells from IL-10 KO B6 animals in all groups. Data represent 1 of 3 similar experiments. Data are from 2 combined experiments with similar results.
We further evaluated whether T-cell secretion of IL-10 was also critical in vivo by once again using the murine model of GVHD after immunization of donors with host-type CD8α+ DCs. Donor (B6) mice were vaccinated as described in “Methods.” Recipient mice were lethally irradiated (800 cGy) and transplanted on day 0 with 5 × 106 TCD BM from naive syngeneic (BALB/c) or allogeneic WT B6 donors, along with 106 T cells from WT and IL-10−/− B6 donors that were not immunized or those vaccinated with BALB/c-derived CD8α+ DCs or CD8α− DCs. Consistent with data from “Methods,” BALB/c animals that received WT T cells from CD8α+ DC–vaccinated donors demonstrated significantly better survival (62.5% vs 0%, P < .05) and reduced severity of clinical GVHD scores (Figure 7C-D) compared with the other allogeneic control groups. By contrast, all of the allogeneic animals that received T cells from IL-10−/− donors demonstrated equivalent mortality regardless of the type of vaccination (Figure 7C-D). Together, these data demonstrate a critical role for IL-10 in the regulation of both in vitro and in vivo T-cell alloresponses after immunization with CD8α+ DCs.
Regulation of T cells is dependent on direct contact with infused CD8α+ DCs
We further explored the cellular mechanisms critical for regulation of T-cell responses. We analyzed whether the regulation of T-cell function was dependent on their contact with the infused host-type CD8α+ DCs. BM DCs were generated and sorted into CD8α+ DCs and CD8α− DCs from H2-Ab1−/− B6 animals. These DCs were then infused into allogeneic BALB/c animals on days −8, −5 to −3, and −1 as before. CD4+ T cells were harvested for analysis after immunization. B6 H2-Ab1−/− animals are MHC class II–deficient and, as such, the DCs would not be able to interact directly with CD4+ T cells in the BALB/c animals. As shown in Figure 8, BALB/c CD4+ T cells from the nonimmunized and those vaccinated with both CD8α+ DCs and CD8α− DCs from MHC class II−/− B6 animals showed similar proliferation when stimulated in an MLR with allogeneic WT B6 splenocytes. These data demonstrate that direct contact between infused allogeneic CD8α+ DCs and the CD4+T cells in the animals is critical for making the T cells less responsive to subsequent alloantigen stimulation.
Regulation of T cells is dependent on contact with the infused allogeneic CD8α+ DCs. BALB/c mice were vaccinated with B6 H2-Ab1−/− BM-derived CD8α+ DCs or CD8α− DCs as in “Vaccination protocol.” T cells were harvested from these BALB/c animals and cultured for 72 hours with syngeneic or WT B6 allogeneic irradiated (3000 cGy) splenocytes and analyzed for proliferation after [3H] thymidine incorporation. P = not significant between the groups. Data are from one of 2 similar experiments.
Regulation of T cells is dependent on contact with the infused allogeneic CD8α+ DCs. BALB/c mice were vaccinated with B6 H2-Ab1−/− BM-derived CD8α+ DCs or CD8α− DCs as in “Vaccination protocol.” T cells were harvested from these BALB/c animals and cultured for 72 hours with syngeneic or WT B6 allogeneic irradiated (3000 cGy) splenocytes and analyzed for proliferation after [3H] thymidine incorporation. P = not significant between the groups. Data are from one of 2 similar experiments.
Discussion
Vaccinations are performed to enhance immunity.17-19 DCs are enhanced but can also suppress an immune response depending on the subset and context.36-40 DC-based vaccinations are being studied to enhance immune responses against cancers.2,18,19 But whether vaccinations or specific DC-based immunization can be used as a strategy to reduce an unwanted immune responses is not known.41 In this study, we showed that immunization of donors with host BM-derived CD8α+ DCs reduced host alloantigen-specific responses in vitro and GVHD mortality and severity in vivo. The suppression of host BM-derived CD8α+ DCs vaccination was observed in multiple experimental GVHD (MHC-matched but minor histocompatibility antigens (miHA)–mismatched and also in MHC-mismatched) models ruling out potential strain-dependent artifacts. The specificity of the suppression was demonstrated by the preservation of normal responses against third-party and nominal antigens. Mechanistic studies demonstrated that the immunosuppressive effect after immunization with CD8α+DC was critically dependent on (1) the secretion of IL-10 by donor T cells and (2) direct contact between the donor T cells and the infused host-type allogeneic CD8α+ DCs.
We found that the number of Foxp3+CD4+ Tregs were not significantly different between the immunized groups. Furthermore, ex vivo analysis of the BALB/c CD4+CD25+ T cells after vaccination with B6 CD8α− DCs and CD8α+ DCs demonstrated equivalent suppression of naive T-cell responses against host (B6)-specific antigens compared with CD4+CD25+ T cells from nonimmunized donors (data not shown). These data, when taken together with the critical requirement of IL-10, suggest that immunization with allogeneic CD8α+ DC suppresses alloantigen-specific responses through the generation of Tr1 type adaptive regulatory T cells.28,29,42 Future studies will determine whether the suppression of GVHD can be further enhanced by addition of adjuvants at the time of immunization. Nonetheless, our data point to a novel strategy and reveal that the vaccination strategy that had only been applied for enhancing a desirable immune response can also be applied for suppression of unwanted immune responses, such as GVHD.
Host DCs and donor T-cell responses play critical roles in the induction of acute GVHD, the most serious complication of allogeneic BMT.6-15 Recent data reported by us and others demonstrate that modulation of host DCs in the recipients can reduce the severity of GVHD.43,44 Sato et al demonstrated that injection of host-type DCs that were treated ex vivo with TGF-β generated suppressive DCs that reduced GVHD.44 However, these studies did not characterize the DC subsets and additionally were generated by ex vivo manipulation strategies. Thus, the role of unmanipulated and the specific host DC subsets that are critical for the negative regulation are not known. CD8α+ DCs can cause both cross-tolerance and cross-priming.37-40,45 We now demonstrate that a subset of host-type APCs, CD8α+ DCs, can be used for immunizing the donors to reduce severity of acute GVHD. These data confirm and extend a previous observation showing a correlation with increased numbers in host CD8α+ DCs and reduction of GVHD after treatment with Flt-3L.31
IL-10 has been shown to be significantly correlated with reduction in the severity of acute GVHD in several human and murine studies.46-49 Furthermore, treatment of both mouse and human CD4+ T cells in the presence of IL-10 induced the generation of Tr1 cells, which are important for active induction of peripheral tolerance.28,29,40 Our data demonstrating a critical requirement for the secretion of IL-10 by donor T cells in reducing acute GVHD after immunization with host-type CD8α+ DCs are thus consistent with the critical regulatory role of IL-10 and Tr1 in GVHD.
The concept of immunizing healthy donors with cells from a patient with underlying malignancy is unlikely to be clinically translatable for ethical and logistical reasons. Nonetheless, given the tight link between GVHD and GVL, we also examined whether the T cells from CD8α+ DC–vaccinated donor maintained sufficient alloreactivity for a GVL response. The GVL response in the animals that received T cells from CD8α+ DC–vaccinated donor was slightly diminished but was not statistically different from the animals that were transplanted with cells from nonvaccinated donors (supplemental Figure 3). Future studies will determine the extent of reduction in GVL magnitude. In any event, despite the preservation of sufficient alloreactivity for some GVL response, we do not anticipate this approach of immunizing healthy donors with cells from the recipient to be directly applicable to the clinical context when the hosts have active malignancy. However, this approach would be clinically feasible in cases where allogeneic BMTs are performed for nonhematologic diseases, such as aplastic anemia and inherited diseases. Furthermore, this strategy might be germane to solid organ allografting wherein the allograft recipient can be immunized with donor CD8α+ DCs.
Allograft-specific regulation of immune responses has been reported with other strategies that are similar, such as donor-specific transfusion (DST). Transfusion of BALB/c donor splenocyte DST with anti-CD154 mAb has been shown to increase skin allograft survival.50-52 The mechanism of DST-induced tolerance has been attributed to both induction of regulatory T cells and also to deletion of alloreactive CD8+ T cells.50,53 However, the critical subsets in the DST strategy are unclear.54 Our data extend these observations by suggesting that allo- CD8α+ DCs might be a critical subset. Moreover, the observation that anti-CD154mAb plus DCs induced by in vivo treatment with Flt3-L as the DST enhanced allograft tolerance47 matches well with our data suggesting that Flt3-L–induced CD8α+ DCs might be the critical subset of cells. Our data further extend these and demonstrate a novel IL-10–dependent mechanism for suppression of allograft-specific immune responses without the use of anti-CD154 mAb and/or thymectomy. In any event, because of the preservation of some alloreactivity, this strategy, if and when translated into the clinic, will probably have to be undertaken under the cover of immunosuppression. Future studies will determine whether addition of standard immunosuppression with calcineurin inhibitors or rapamycin will enhance or mitigate the protective responses afforded by the immunization strategy.
Our data also have additional ramifications for both allogeneic and nonallogeneic immunotherapeutic strategies against tumors and infections wherein DC vaccinations are being increasingly studied to enhance antigen-specific immune responses against infections55 or cancers.56-59 The promise of these strategies is yet to be realized in a clinically meaningful manner. However, our data demonstrate that vaccination with CD8α+ DCs can actively suppress an immune response; it stands to reason that one way to enhance DC vaccination strategy against infections and tumors in these nonallogeneic contexts would be to exclude the CD8α+ subset. Conversely, vaccination with CD8− DCs might exacerbate the desirable immune responses in these contexts. However, given the trend, albeit statistically not significant, for enhanced GVHD (Figure 4) with CD8− DC vaccination, it is possible that using CD8− DCs may be deleterious after allogeneic BMT.
In conclusion, we demonstrated that immunization with CD8α+ DCs can be used as an effective strategy to reduce an undesired immune response, such as GVHD. Our data also suggest that this might be a novel strategy for prevention of GVHD after allogeneic BMT for nonhematologic malignancies and also mitigating solid organ allograft rejection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health (AI-075284 and HL-090775) and the Doris Duke Clinical Scientist Development Award (P.R.).
National Institutes of Health
Authorship
Contribution: T.T. performed research, analyzed data, and wrote the paper; C.M. and I.T. performed research and analyzed data; C.L., E.N., K.P.L., and Y.S. performed research; and P.R. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavan Reddy, Department of Internal Medicine, University of Michigan Comprehensive Cancer Center, 6310 CCC, 1500 East Medical Center Dr, Ann Arbor, MI 48109-0942; e-mail: reddypr@umich.edu.

![Figure 2. T cells from allogeneic CD8α+ DC–vaccinated mice. Reduced expansion and proinflammatory cytokine secretion were shown in vitro in BALB/c mice that were immunized with diluent control or allogeneic B6 BM-derived CD8α+ DCs or CD8α− DCs as described in “Methods.” BALB/c CD90+ T cells (2 × 105/well) were then harvested from them and cultured for 96 hours with either syngeneic or irradiated (3000 cGy) allogeneic B6 BM DCs (1 × 104/well). During the final 16 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for (A) proliferation. Supernatants were collected at 80 hours and assayed by for (B) IL-2, (C) IFN-γ, and (D) IL-10. Error bars represent SE. *P < .05 between diluent control and allogeneic CD8α+ DC–vaccinated groups. Data are from one of 3 similar experiments. *P < .05 between diluent control and allogeneic CD8α+ DC–vaccinated groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2009-06-229708/4/m_zh89990947410002.jpeg?Expires=1769923727&Signature=qx9d-xcIyWgFiQNu7Qelgd2GXAYjvZTUnX8IedD3Ss6CkW79RQKObuJpgWFG4eq7WHtTQmGjxe5xryzjeol3xQngSWLsgJ~H6FFqavKCvoAzCkx7z8JqkTurq8Xg-Fi~UHsNme-Um5IFka28ae~GgKW78HuWHQIlJ--udBxbkSePDSJbZTGwKx0lu48Y2M1qWcYbb3l1k~OIcRax6tc5Os8GZsaufilvTOZ4LDaHkk3oZMAvG6WiOUv4hdRh512iJqOUJ0Eu9uBAUk~qJuutWl2Sd1xcqtDVrBMHS5JaqOuUWYxu8-~0FQqoknOHMna9WLn4ed3RxidJtV5yPQbcbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Allogeneic CD8α+ DC immunization preserves T-cell responses to third-party antigens. B6 donor mice were immunized with diluent or recipient BALB/c BM-derived CD8α+ DC or CD8α− DCs as described in “Vaccination protocol.” T cells were harvested and used for in vitro and in vivo studies with C3H/HeJ recipients. (A) B6 splenic CD90+ T cells were stimulated with irradiated (3000 cGy) splenocytes (105/well) from C3H/HeJ. During the final 6 hours of a 72-hour culture, cells were pulsed with 3H thymidine and assayed for proliferation. (B) Supernatants were collected at 66 hours and assayed by ELISA for IL-2, IFN-γ, and IL-10. Error bars represent SE. P = not significant between diluent control and BALB/c-derived CD8α+ DC groups. Each graph represents one of 3 similar experiments. (C) C3H/HeJ mice were lethally irradiated with 9000 cGy TBI and injected with 5 × 106 TCD BM and 106 CD90+ T cells from syngeneic (black straight line, n = 3), allogeneic diluent-treated (circles, dotted line, n = 7), or BALB/c-derived CD8+ DC-vaccinated (triangles, n = 6) or CD8− DC-vaccinated (diamonds, n = 6) B6 animals and evaluated for survival. P = not significant between diluent control and the other groups. Data represent one of 2 similar experiments. (D) B6 OT-II transgenic mice were immunized with diluent or BALB/c BM-derived CD8α+ DCs or CD8α− DCs as above. Responder T cells were harvested from the OT-II B6 mice and cultured for 48 hours with B6 syngeneic splenocytes that were either not pulsed or pulsed with 100nM or 500nM OVA323-339 peptide. During the final 6 hours of a 48-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation. P = not significant between diluent control and the other immunized groups. Data are representative of one of 2 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2009-06-229708/4/m_zh89990947410006.jpeg?Expires=1769923727&Signature=dzHHAgcCOLDxhz2Su-CkrVoLx38Hm9bF6XKlmfr~-Q4Dpm0FFdLxYCZQMIqnUCWpExuTiu16FrWe5i~JaD~Wp1XY4DrS9zTPEBCNNjGZ4pTq3VjrZBGOF-don2yz1QNUyQI22097r85O3H51p~qdjQsvBMA8v5skDui1jbHKg8bjwZtsgmMUH8EbYKTo58sJUZI7S1Myzz~hexfBomFoMLShR0FYKbbe1XtBtTVkGniAtGal4Rt53tccJr4isXgo9gl6V2D2T77v5bjG3LoXKDf57zljQCNrqiuTA78wDc0or8e~-B-1UkqM2B~WrRRwSM-52HtI~pdHVtTtL587Dw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 8. Regulation of T cells is dependent on contact with the infused allogeneic CD8α+ DCs. BALB/c mice were vaccinated with B6 H2-Ab1−/− BM-derived CD8α+ DCs or CD8α− DCs as in “Vaccination protocol.” T cells were harvested from these BALB/c animals and cultured for 72 hours with syngeneic or WT B6 allogeneic irradiated (3000 cGy) splenocytes and analyzed for proliferation after [3H] thymidine incorporation. P = not significant between the groups. Data are from one of 2 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2009-06-229708/4/m_zh89990947410008.jpeg?Expires=1769923727&Signature=GJaJ1nSwraPLbCNHZpNELufnbwQZEhrFV1cWTU1hfkex3ec8bcphczsEZJ6Y1bbL59PoHrhtZmyCSmco2m0LhnH9MBPBz0sm8cYL9RMydh3O-UMzn~94wLtOwd4ZFuXShkXlut0e8hUwR5wzEv8PpyQazaKbmUEg8Y8y3iHGMGp7u4lLYCbQSFQFar9fy712KBrZpGpsyK76WCvHfV~3S4NYfTk25jSA-ovNaeOIternIy5tkZbFFw6r9Mckb1DUa47hDo-bAGm3kvKONYWUTBPUy7mfh~tU84M34FVEJvJl8~ByPV0LjDqk1ufYuZmx~tnfoeOmt5vt43LUfeLGBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal