In this issue of Blood, White and colleagues demonstrate that expression of the anticoagulant protein TFPI by ECs limits thrombus growth in a mouse model of carotid artery injury.1
In blood, the tissue factor (TF) pathway inhibitor (TFPI) is primarily associated with lipoproteins: one of its original names was lipoprotein-associated coagulation inhibitor. TFPI is a Kunitz-type serine protease inhibitor that contains 3 Kunitz domains and inhibits the procoagulant TF–factor VIIa complex in a factor Xa–dependent manner.2 The first Kunitz domain binds factor VIIa whereas the second binds factor Xa. Endothelial cells (ECs) are thought to be the primary site of TFPI expression, although TFPI is also expressed by megakaryocytes, monocytes, and vascular smooth muscle cells (VSMCs).3 One study found that stimulated platelets secrete TFPI and may be a significant source of plasma TFPI.3 The majority of TFPI associates with cell surfaces via a glycosyl-phosphatidylinositol anchor. In addition, a small amount of TFPI is bound to glycosaminoglycans through an interaction with the C-terminal region. This pool is released after administration of heparin.
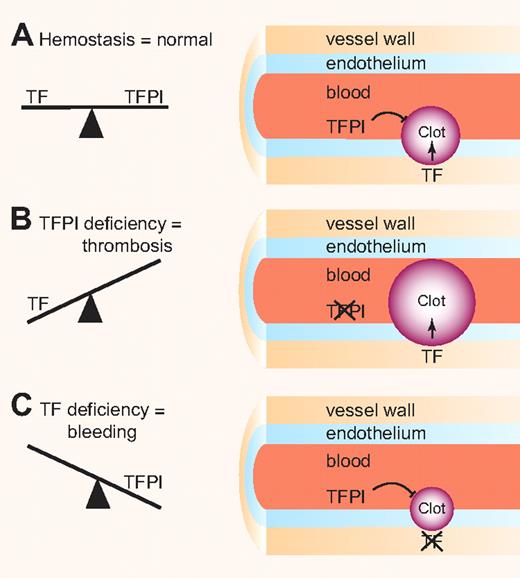
Importantly, a complete deficiency of TFPI in mice results in embryonic lethality and there are no reports of humans lacking TFPI.4 Consistent with its primary role as an inhibitor of the TF–factor VIIa complex, we found that lowering the level of TF rescued TFPI-null embryos.5 This result indicated that levels of TF and TFPI must be balanced to allow hemostasis and prevent thrombosis or bleeding (see figure). Interestingly, TFPI inhibitors are being developed as a new class of drugs to treat hemophilia patients.6
Balance clotting. (A) Levels of the procoagulant protein TF and the anticoagulant protein TFPI must be balanced to maintain normal hemostasis. (B) TFPI deficiency in the endothelium results in increased clot formation. (C) TF deficiency in the vessel wall results in decreased clot formation. Professional illustration by Paulette Dennis.
Balance clotting. (A) Levels of the procoagulant protein TF and the anticoagulant protein TFPI must be balanced to maintain normal hemostasis. (B) TFPI deficiency in the endothelium results in increased clot formation. (C) TF deficiency in the vessel wall results in decreased clot formation. Professional illustration by Paulette Dennis.
White and colleagues1 used gene targeting to generate mice that contained a floxed TFPI gene (TFPIFlox). It should be noted that the targeting strategy only removed the first Kunitz domain. The mutant form of TFPI expressed in the TFPIflox/Cre mice is unable to inhibit the TF–factor VIIa complex but retains the last 2 Kunitz domains and the C-terminal region. This is important because the functions of the third Kunitz domain and the C-terminal region have not been fully elucidated. The TFPI gene was deleted in either ECs plus hematopoietic cells by crossing TFPIFlox mice with Tie2-Cre mice (referred to as TFPITie2 mice), or in myeloid cells by crossing TFPIFlox mice with LysM-Cre mice (referred to as TFPILysM mice). TFPITie2 and TFPILysM mice survived embryonic development indicating that other cellular sources of TFPI provide sufficient anticoagulation in the embryo to inhibit the TF–factor VIIa complex. Plasma TFPI activity was reduced 71% and 19% in adult TFPITie2 and TFPILysM mice, respectively. Hematopoietic stem cell transplantation of wild-type mice with TFPI−/− fetal liver cells resulted in a 20% reduction in plasma TFPI activity levels. These results indicated that EC contributed approximately 50% of the plasma TFPI activity whereas 20% of plasma TFPI was provided by myeloid cells. Although it appears that monocytes are the major hematopoietic cell type that contribute to the pool of plasma TFPI, these results do not exclude the possibility that platelets also express TFPI. This can be analyzed in mice specifically lacking the TFPI gene in platelets. TFPITie2 and TFPILysM mice have “normal” hemostasis in a tail-clip model and a cuticle-bleeding model, suggesting that other cellular sources of TFPI are important for hemostasis. Clearly, determining the effect on hemostasis of deleting the TFPI gene in VSMCs will be interesting.
A major finding of the current study is that TFPITie2 exhibited a significant shortening of the occlusion time in a ferric chloride thrombosis model of the carotid artery. In contrast, a deficiency of TFPI in myeloid cells had no effect. White and colleagues1 concluded that EC TFPI regulates arterial thrombosis. However, a contribution from myeloid cell TFPI cannot be excluded in this experiment. Examining the phenotype of TFPITie2 containing wild-type bone marrow would be interesting and is important for 2 reasons. (1) The endothelium may be denuded in the ferric chloride model so it is unclear how anticoagulants on the surface of the endothelium regulate thrombosis in this model; and (2) an earlier study found that a 50% decrease in TFPI levels in all cell types was not associated with a difference in occlusion times in a Rose Bengal thrombosis model of the carotid artery.7 Further studies with atherosclerotic vessels found that TFPI+/− mice had shorter occlusion times than mice with wild-type TFPI levels. Therefore, the differences may be due to the use of different thrombosis models and/or a contribution of myeloid TFPI to the ferric chloride model. We observed reduced thrombosis in mice lacking the TF gene in VSMCs using a ferric chloride injury model of the carotid artery.8 Determining whether a deficiency of TFPI in other cell types, such as myeloid cells or platelets, increases thrombosis in other models will be exciting. For instance, leukocyte-derived, TF-positive microparticles play a role in thrombosis in the laser injury model and TFPI expression by monocytes and/or platelets may be important in regulating this source of blood-borne TF.
Generation of TFPIFlox mice is an important advance that will allow new investigations into the role of TFPI expressed by different cell types in a variety of processes. For instance, TFPI has been shown to contribute to intimal hyperplasia, angiogenesis, and lipid metabolism.9,10 Interestingly, a 23-amino acid peptide from the C-terminal region has both antitumor and antiangiogenic activity.9 Further studies are needed to determine how different cellular sources of TFPI regulate thrombosis in different models and to better define its role in angiogenesis and lipid metabolism.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal